Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sharof M. Tugizov | -- | 1755 | 2023-05-24 17:37:44 | | | |
| 2 | Rita Xu | Meta information modification | 1755 | 2023-05-25 03:15:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tugizov, S.M. Oropharyngeal Mucosal Epithelium in HIV-1 Transmission. Encyclopedia. Available online: https://encyclopedia.pub/entry/44792 (accessed on 08 February 2026).
Tugizov SM. Oropharyngeal Mucosal Epithelium in HIV-1 Transmission. Encyclopedia. Available at: https://encyclopedia.pub/entry/44792. Accessed February 08, 2026.
Tugizov, Sharof M.. "Oropharyngeal Mucosal Epithelium in HIV-1 Transmission" Encyclopedia, https://encyclopedia.pub/entry/44792 (accessed February 08, 2026).
Tugizov, S.M. (2023, May 24). Oropharyngeal Mucosal Epithelium in HIV-1 Transmission. In Encyclopedia. https://encyclopedia.pub/entry/44792
Tugizov, Sharof M.. "Oropharyngeal Mucosal Epithelium in HIV-1 Transmission." Encyclopedia. Web. 24 May, 2023.
Copy Citation
The oropharyngeal mucosal epithelia have a polarized organization, which is critical for maintaining a highly efficient barrier as well as innate immune functions. In human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) disease, the barrier and innate immune functions of the oral mucosa are impaired via a number of mechanisms.
human immunodeficiency virus
oropharyngeal mucosal epithelium
reactivation of opportunistic infections
1. Introduction
Four decades ago, the HIV/AIDS pandemic began. Global spread led to 75 million infections and 32 million deaths. Today, highly effective prevention strategies are available to reduce the likelihood of HIV transmission via sexual intercourse. Furthermore, antiretroviral therapy for people living with HIV/AIDS can reduce viral loads to levels that cannot be detected or transmitted. However, despite the availability of these highly effective agents, HIV/AIDS continues to be a lethal disease. HIV/AIDS was responsible for one death each minute in 2021; every two minutes, a young woman becomes newly infected with HIV. Likewise, approximately 200,000 cases of mother-to-child transmission are reported each year. Thus, HIV/AIDS remains an unsolved problem; additional new knowledge may help improve treatment and prophylaxis.
The surface of the oropharyngeal cavity is covered with a multilayer stratified squamous epithelium supported by the lamina propria, which is a layer of fibrous connective tissue [1][2]. Stratified oropharyngeal epithelial cells from the parabasal to the granulosum layers have well-developed lateral adherens and tight junctions, indicating a polarized organization [3][4][5][6]. The lateral localization of adherens and tight junctions between neighboring cells of oral epithelium contribute to a physical barrier that protects the body from penetration by viruses and other pathogens [4][5].
The oropharyngeal mucosa also contains a broad population of adaptive and innate immune cells, including T and B cells, macrophages, dendritic/Langerhans cells (DC/LCs), and natural killer (NK) cells that are distributed within the epithelium and lamina propria [2][7][8][9][10][11][12][13][14][15][16]. Intraepithelial DC/LCs and macrophages in the oral mucosa are critical components of the innate immune system. These cells defend against pathogens that enter the body via the oral cavity [2][13][14][16][17][18][19][20][21][22]. Intraepithelial macrophages and DC/LCs are also antigen-presenting cells that are capable of activating an adaptive immune response. Thus, these cells may serve as “bridges” between the innate and adaptive immune systems [2][14][23][24][25]. In addition, oral mucosal epithelial cells express toll-like receptors (TLRs) 2, 3, 4, 5, 6, and 9, which are critical facilitators of innate immune responses against numerous pathogens [26][27].
Intraepithelial oral mucosal DC/LC and macrophages originate from peripheral blood CD14+ monocytes [28][29]. Recruitment of circulating monocytes into the mucosal epithelium is mediated by monocyte chemotactic protein-1 (MCP-1), MCP-2, macrophage inflammatory protein-1 alpha (MIP-1α), and MIP-1β [30][31][32]. Expression and secretion of these mediators in the mucosal epithelium are modulated by multiple chemokine/cytokines, including interferon-ɣ (IFN-ɣ), tumor necrosis factor-α (TNF-α), and interleukins (ILs), including IL-1, IL-1β, IL-4, IL-6, IL-8, IL10, IL-13, and IL-15 [14][32][33][34][35][36]. MCP-1 is secreted from the basolateral membranes of polarized epithelial cells [37][38]; the polarized release of MCP-1 may generate a gradient toward blood vessels that serve to recruit monocytes into the epithelium. Once they have transited across the endothelial layer, monocytes differentiate into macrophages and DC/LCs [11]. Further traffic of monocytes/macrophages, DCs, and T lymphocytes within the mucosal epithelium is coordinated by the formation of transient tight junctions between immune and epithelial cells [39][40][41][42]. Migrating immune cells, particularly DC/LCs, express the tight junction proteins known as claudin-1 and occludin. Transient association of these proteins with cell junctions promotes the migration of immune cells without disrupting epithelial barrier functions [39][41][43][44]. This allows DC/LCs to reach the mucosal surface [39][43]. The absence of epithelial junctions may reduce the efficiency of epithelial–lymphocyte interactions, thereby leading to dysfunction (i.e., little to no retention of interepithelial lymphocytes and DC/LCs and thus their depletion) [45][46][47]. Interactions of polarized mucosal epithelial cells with DC/LCs and other cells of the adaptive and innate immune systems are critical for maintaining oral mucosal immune homeostasis.
The oropharyngeal mucosal epithelium and its intraepithelial and subepithelial adaptive and innate immune cells play critical roles in promoting protection against numerous pathogens, including viruses, bacteria, and fungi. However, in HIV/AIDS, various chronic disorders can develop with a significant impact on the oral mucosal epithelium. These include inflammation, necrotizing mucosal ulcers, and malignant and nonmalignant lesions that may impair the barrier as well as the innate and acquired immune functions of the oral mucosa [48][49][50][51][52][53][54][55].
Systemic HIV/AIDS is accompanied by the spread of cell-free and cell-associated HIV-1 in the oral mucosal environment. There are several reports of viral DNA/RNA, cell-free HIV-1 virions, and tat and gp120 proteins isolated from oral mucosal tissue and saliva of HIV/AIDS patients [6][56][57][58][59][60][61][62][63][64]. HIV-infected DC/LCs, lymphocytes, and macrophages were also detected in the mucosal and submucosal layers of the oropharyngeal epithelium [6][56][57][62][63][65]. Electron microscopy revealed that HIV virions could be found within the tight junctions of the oral epithelium [62]. The presence of both cell-free and cell-associated HIV-1 both around and within oropharyngeal mucosa may result in numerous changes that impair its innate immune and barrier functions.
2. Role of Oropharyngeal Mucosal Epithelium in HIV-1 Transmission in Adults and Children
Oral HIV-1 transmission in the adult population may occur during oral sex, in newborn children and infants during delivery and breastfeeding [66][67][68][69][70][71][72][73]. The rate of adult oral HIV-1 transmission has been estimated at ~0.004% per exposure (i.e., not a highly efficient process) [66][67][68]. By contrast, the rate of mother-to-child transmission (MTCT) of HIV-1 in the absence of antiretroviral therapy (ART) may be as high as 15% in Europe and 25–30% in Asian and African countries [74][75][76].
The oropharyngeal and tonsillar mucosal epithelium may express one or more co-receptors or non-canonical HIV-1 receptors that may facilitate virus binding and entry, including CC chemokine receptor type 5 (CCR5), CXC chemokine receptor type 4 (CXCR4), heparan sulfate proteoglycans (HSPGs), mannose receptor, galactosylceramide (GalCer), and T-cell immunoglobulin and mucin domain 1 (TIM-1) [3][77][78][79][80][81][82][83][84].
Results of studies featuring ex vivo adult and fetal/infant tissue explants revealed that HIV-1 transmission through the adult oral epithelium was less efficient than fetal/infant epithelial tissues, which supported rapid viral transmigration through the mucosal epithelium and infection of virus-susceptible intraepithelial and subepithelial cells [3][85]. The resistance provided by the adult tissues was primarily due to the presence of multiple epithelial layers and tissue stratification (20–30) with highly-effective tight junctions (Figure 1). The highly stratified adult oral epithelial cells limit viral penetration more efficiently than the less stratified fetal/neonatal/infant counterparts (i.e., with 3–5 layers) [3].
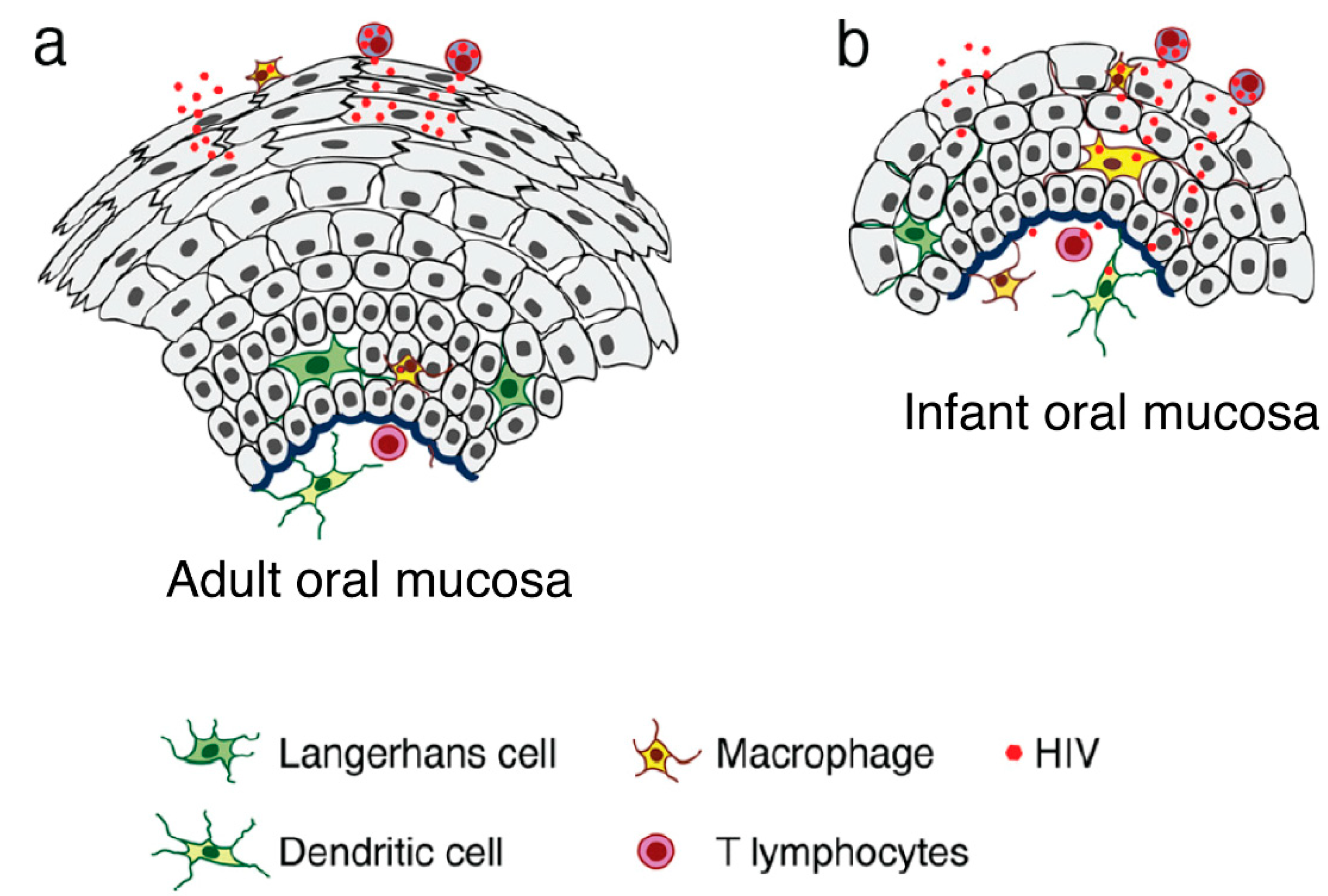
Figure 1. Model of HIV transmigration in the infant/fetal and adult oral epithelium. (a) Adult oral epithelial cells are stratified into numerous layers. While cell-free HIV can transmigrate to some extent across the upper regions of the intact adult oral epithelium (across two to five layers), the virus is unable to spread to the lower layers or the lamina propria. (b) By contrast, the infant/fetal oral mucosal epithelium contains only two to five epithelial layers and is not completely stratified. The spread of HIV-infected macrophages and cell-free virions across infant/fetal oral epithelial cells may result in the infection of HIV-susceptible epithelial and submucosal cells, including macrophages, LC/DCs, and T lymphocytes. Thus, fetal/infant oropharyngeal epithelial cells may serve as a critical portal for HIV entry during MTCT. Increased rates of HIV transmission across the fetal/infant oral epithelium compared to that of adults may represent both reduced barrier function (associated with pauci-stratification) as well as lower levels of innate immune proteins.
Furthermore, adult oral epithelial cells express high levels of anti-HIV-1 innate proteins, including human beta-defensin (hBD)2 and hBD3. These cells also express secretory leukocyte protease inhibitor that inactivates intraepithelial virions and reduces oral transmission of HIV-1 [79][82][85]. By contrast, fetal/infant oral epithelial cells do not express high levels of these proteins, which may be among the factors contributing to the comparatively high rate of HIV-1 MTCT [79][82][85]. HBDs tagged with the HIV-1 Tat's protein transduction domain were delivered to HIV-1-infected infant tonsillar epithelial cells [85]. PTD-mediated internalization of these proteins in infant tonsillar epithelial cells was followed by their penetration into multivesicular bodies (MVBs) and vacuoles containing HIV-1. PTD also promoted the fusion of HIV-containing vesicles with lysosomes which led to the degradation of gp120 and p24 and viral inactivation [85]. Ex vivo PTD-mediated internalization of hBD2 and hBD3 into tonsillar tissue explants from infants also reduced virus spread from epithelial cells to CD68+ macrophages, CD4+ T lymphocytes, and CD1c+ DCs [85].
HIV-1 internalization through the apical surface into infant tonsillar epithelial cells can be initiated by multiple entry pathways, including micropinocytosis as well as clathrin and caveolin/lipid raft-associated endocytosis [83]. An evaluation of HIV-1 transmission through polarized tonsillar epithelial cells revealed that approximately 0.05% of inoculated virions underwent transcytosis across the epithelium [86]. More than 90% of the internalized virions were sequestered in epithelial endosomes that included MVBs and vacuoles. Sequestration of HIV-1 in the endosomal compartment of tonsillar epithelial cells was observed both in the single layer of polarized cells as well as ex vivo in explants of tonsillar epithelial tissue [83][85][86]. Intraepithelial HIV-1 remained infectious for several days, although no virion release was observed [86]. Interactions of HIV-1-containing epithelial cells with activated peripheral blood mononuclear cells and CD4+ T lymphocytes led to the disruption of epithelial cortical actin and the spread of the virus from epithelial cells to the lymphocytes. IFN-ɣ and TNF-α treatment of tonsillar epithelial cells also induced reorganization of cortical actin and intracellular virion release [86].
The release of HIV-1 from oropharyngeal mucosal epithelial cells may result in the virus spreading into intraepithelial and subepithelial macrophages, DC/LCs, and CD4 T+ lymphocytes. This is the first step in establishing systemic HIV-1 infection. Mucosal macrophages, DC/LCs, and intraepithelial T lymphocytes, DC/LCs may then transmit HIV-1 across the mucosal epithelium into regional lymph nodes [87][88][89][90][91][92].
Oral transmission of HIV-1 may also result from paracellular virus penetration if the integrity of the oral mucosal epithelium is impaired. HIV-1 gp120 binding to GalCer can result in elevated levels of intracellular calcium and activation of mitogen-activated protein kinase (MAPK) and PI3K signaling [93][94][95]. In addition, HIV-1 envelope protein gp120-induced activation of MAPK and NF-κB signaling reduced the expression of ZO-1, occludin, and claudin-1 in oral epithelial cells, leading to the disruption of tight junctions [96][97][98]; this may facilitate paracellular penetration of HIV-1 virions. HIV/AIDS-associated production and release of proinflammatory cytokines, including TNF-α and IFN-γ, may also disrupt tight junctions of oral epithelial cells and lead to paracellular penetration of HIV-1 [4][5].
Oral epithelial cells may also support non-replicative HIV-1 infection and virus transfer to CD4+ T lymphocytes [99] and immobilize the infectious virions on their surfaces to facilitate their transfer to permissive cells [100]. Likewise, ex vivo HIV-1 infection of tonsillar explants can lead to a productive infection of intraepithelial and submucosal macrophages and lymphocytes [65][96][101][102].
References
- Winning, T.A.; Townsend, G.C. Oral mucosal embryology and histology. Clin. Dermatol. 2000, 18, 499–511.
- Groeger, S.; Meyle, J. Oral Mucosal Epithelial Cells. Front. Immunol. 2019, 10, 208.
- Tugizov, S.M.; Herrera, R.; Veluppillai, P.; Greenspan, D.; Soros, V.; Greene, W.C.; Levy, J.A.; Palefsky, J.M. Differential transmission of HIV traversing fetal oral/intestinal epithelia and adult oral epithelia. J. Virol. 2012, 86, 2556–2570.
- Tugizov, S. Human immunodeficiency virus-associated disruption of mucosal barriers and its role in HIV transmission and pathogenesis of HIV/AIDS disease. Tissue Barriers 2016, 4, e1159276.
- Tugizov, S. Virus-associated disruption of mucosal epithelial tight junctions and its role in viral transmission and spread. Tissue Barriers 2021, 9, 1943274.
- Tugizov, S.M.; Herrera, R.; Chin-Hong, P.; Veluppillai, P.; Greenspan, D.; Michael Berry, J.; Pilcher, C.D.; Shiboski, C.H.; Jay, N.; Rubin, M.; et al. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology 2013, 446, 378–388.
- Jotwani, R.; Palucka, A.K.; Al-Quotub, M.; Nouri-Shirazi, M.; Kim, J.; Bell, D.; Banchereau, J.; Cutler, C.W. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: In situ, in vivo, and in vitro studies. J. Immunol. 2001, 167, 4693–4700.
- Nave, H.; Gebert, A.; Pabst, R. Morphology and immunology of the human palatine tonsil. Anat. Embryol. 2001, 204, 367–373.
- Zhao, Z.Z.; Savage, N.W.; Sugerman, P.B.; Walsh, L.J. Mast cell/T cell interactions in oral lichen planus. J. Oral. Pathol. Med. 2002, 31, 189–195.
- Allam, J.P.; Novak, N.; Fuchs, C.; Asen, S.; Berge, S.; Appel, T.; Geiger, E.; Kochan, J.P.; Bieber, T. Characterization of dendritic cells from human oral mucosa: A new Langerhans′ cell type with high constitutive FcepsilonRI expression. J. Allergy Clin. Immunol. 2003, 112, 141–148.
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811.
- Banchereau, J.; Paczesny, S.; Blanco, P.; Bennett, L.; Pascual, V.; Fay, J.; Palucka, A.K. Dendritic cells: Controllers of the immune system and a new promise for immunotherapy. Ann. N. Y. Acad. Sci. 2003, 987, 180–187.
- Challacombe, S.J.; Sweet, S.P. Oral mucosal immunity and HIV infection: Current status. Oral. Dis. 2002, 8 (Suppl. S2), 55–62.
- Caputo, V.; Libera, M.; Sisti, S.; Giuliani, B.; Diotti, R.A.; Criscuolo, E. The initial interplay between HIV and mucosal innate immunity. Front. Immunol. 2023, 14, 1104423.
- Challacombe, S.J.; Naglik, J.R. The effects of HIV infection on oral mucosal immunity. Adv. Dent. Res. 2006, 19, 29–35.
- Pelaez-Prestel, H.F.; Sanchez-Trincado, J.L.; Lafuente, E.M.; Reche, P.A. Immune Tolerance in the Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 12149.
- Lombardi, T.; Hauser, C.; Budtz-Jorgensen, E. Langerhans cells: Structure, function and role in oral pathological conditions. J. Oral. Pathol. Med. 1993, 22, 193–202.
- Bilsborough, J.; Viney, J.L. Gastrointestinal dendritic cells play a role in immunity, tolerance, and disease. Gastroenterology 2004, 127, 300–309.
- Hon, H.; Jacob, J. Tracking dendritic cells in vivo: Insights into DC biology and function. Immunol. Res. 2004, 29, 69–80.
- Wilson, N.S.; Villadangos, J.A. Lymphoid organ dendritic cells: Beyond the Langerhans cells paradigm. Immunol. Cell Biol. 2004, 82, 91–98.
- Walsh, L.J. Mast cells and oral inflammation. Crit. Rev. Oral Biol. Med. 2003, 14, 188–198.
- Mowat, A.M.; Parker, L.A.; Beacock-Sharp, H.; Millington, O.R.; Chirdo, F. Oral tolerance: Overview and historical perspectives. Ann. N. Y. Acad. Sci. 2004, 1029, 1–8.
- Ozbilgin, M.K.; Polat, S.; Mete, U.O.; Tap, O.; Kaya, M. Antigen-presenting cells in the hypertrophic pharyngeal tonsils: A histochemical, immunuhistochemical and ultrastructural study. J. Investig. Allergol. Clin. Immunol. 2004, 14, 320–328.
- Foti, M.; Granucci, F.; Ricciardi-Castagnoli, P. A central role for tissue-resident dendritic cells in innate responses. Trends Immunol. 2004, 25, 650–654.
- Hoebe, K.; Janssen, E.; Beutler, B. The interface between innate and adaptive immunity. Nat. Immunol. 2004, 5, 971–974.
- Sarah, S.M.; Tamilselvan, S.; Kamatchiammal, S.; Suresh, R. Expression of Toll-like receptors 2 and 4 in gingivitis and chronic periodontitis. Indian J. Dent. Res. 2006, 17, 114–116.
- Lange, M.J.; Lasiter, J.C.; Misfeldt, M.L. Toll-like receptors in tonsillar epithelial cells. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 613–621.
- Palucka, A.K.; Banchereau, J. Langerhans cells: Daughters of monocytes. Nat. Immunol. 2006, 7, 223–224.
- Wacleche, V.S.; Tremblay, C.L.; Routy, J.P.; Ancuta, P. The Biology of Monocytes and Dendritic Cells: Contribution to HIV Pathogenesis. Viruses 2018, 10, 65.
- Eugenin, E.A.; Berman, J.W. Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods 2003, 29, 351–361.
- Muller, W.A. New mechanisms and pathways for monocyte recruitment. J. Exp. Med. 2001, 194, F47–F51.
- Imhof, B.A.; Aurrand-Lions, M. Adhesion mechanisms regulating the migration of monocytes. Nat. Rev. Immunol. 2004, 4, 432–444.
- Lundien, M.C.; Mohammed, K.A.; Nasreen, N.; Tepper, R.S.; Hardwick, J.A.; Sanders, K.L.; Van Horn, R.D.; Antony, V.B. Induction of MCP-1 expression in airway epithelial cells: Role of CCR2 receptor in airway epithelial injury. J. Clin. Immunol. 2002, 22, 144–152.
- Nasreen, N.; Mohammed, K.A.; Galffy, G.; Ward, M.J.; Antony, V.B. MCP-1 in pleural injury: CCR2 mediates haptotaxis of pleural mesothelial cells. Am. J. Physiol. Lung. Cell Mol. Physiol. 2000, 278, L591–L598.
- Lugering, N.; Kucharzik, T.; Maaser, C.; Kraft, M.; Domschke, W. Interleukin-15 strongly inhibits interleukin-8 and monocyte chemoattractant protein-1 production in human colonic epithelial cells. Immunology 1999, 98, 504–509.
- Kucharzik, T.; Lugering, N.; Pauels, H.G.; Domschke, W.; Stoll, R. IL-4, IL-10 and IL-13 down-regulate monocyte-chemoattracting protein-1 (MCP-1) production in activated intestinal epithelial cells. Clin. Exp. Immunol. 1998, 111, 152–157.
- Holtkamp, G.M.; De Vos, A.F.; Peek, R.; Kijlsta, A. Analysis of the secretion pattern of monocyte chemotactic protein-1 (MCP-1) and transforming growth factor-beta 2 (TGF-beta2) by human retinal pigment epithelial cells. Clin. Exp. Immunol. 1999, 118, 35–40.
- Holtkamp, G.M.; Van Rossem, M.; de Vos, A.F.; Willekens, B.; Peek, R.; Kijlstra, A. Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clin. Exp. Immunol. 1998, 112, 34–43.
- Rimoldi, M.; Chieppa, M.; Vulcano, M.; Allavena, P.; Rescigno, M. Intestinal epithelial cells control dendritic cell function. Ann. N. Y. Acad. Sci. 2004, 1029, 66–74.
- Takano, K.; Kojima, T.; Go, M.; Murata, M.; Ichimiya, S.; Himi, T.; Sawada, N. HLA-DR- and CD11c-positive dendritic cells penetrate beyond well-developed epithelial tight junctions in human nasal mucosa of allergic rhinitis. J. Histochem. Cytochem. 2005, 53, 611–619.
- Zen, K.; Parkos, C.A. Leukocyte-epithelial interactions. Curr. Opin. Cell Biol. 2003, 15, 557–564.
- Edens, H.A.; Parkos, C.A. Modulation of epithelial and endothelial paracellular permeability by leukocytes. Adv. Drug. Deliv. Rev. 2000, 41, 315–328.
- Rescigno, M.; Rotta, G.; Valzasina, B.; Ricciardi-Castagnoli, P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology 2001, 204, 572–581.
- Alexander, J.S.; Dayton, T.; Davis, C.; Hill, S.; Jackson, T.H.; Blaschuk, O.; Symonds, M.; Okayama, N.; Kevil, C.G.; Laroux, F.S.; et al. Activated T-lymphocytes express occludin, a component of tight junctions. Inflammation 1998, 22, 573–582.
- Edelblum, K.L.; Shen, L.; Weber, C.R.; Marchiando, A.M.; Clay, B.S.; Wang, Y.; Prinz, I.; Malissen, B.; Sperling, A.I.; Turner, J.R. Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proc. Natl. Acad. Sci. USA 2012, 109, 7097–7102.
- Ali, A.; Tan, H.; Kaiko, G.E. Role of the Intestinal Epithelium and Its Interaction With the Microbiota in Food Allergy. Front. Immunol. 2020, 11, 604054.
- Sheridan, B.S.; Lefrancois, L. Intraepithelial lymphocytes: To serve and protect. Curr. Gastroenterol. Rep. 2010, 12, 513–521.
- Aguirre-Urizar, J.M.; Echebarria-Goicouria, M.A.; Eguia-del-Valle, A. Acquired immunodeficiency syndrome: Manifestations in the oral cavity. Med. Oral. Patol. Oral. Cir. Bucal. 2004, 9 (Suppl. S153–S157), 148–153.
- Leigh, J.E.; Shetty, K.; Fidel, P.L., Jr. Oral opportunistic infections in HIV-positive individuals: Review and role of mucosal immunity. AIDS Patient Care STDS 2004, 18, 443–456.
- Hille, J.J.; Webster-Cyriaque, J.; Palefski, J.M.; Raab-Traub, N. Mechanisms of expression of HHV8, EBV and HPV in selected HIV-associated oral lesions. Oral. Dis. 2002, 8 (Suppl. S2), 161–168.
- Syrjanen, S.; Leimola-Virtanen, R.; Schmidt-Westhausen, A.; Reichart, P.A. Oral ulcers in AIDS patients frequently associated with cytomegalovirus (CMV) and Epstein-Barr virus (EBV) infections. J. Oral. Pathol. Med. 1999, 28, 204–209.
- Reichart, P.A. Oral ulcerations in HIV infection. Oral. Dis. 1997, 3 (Suppl. S1), S180–S182.
- Greenspan, D.; Greenspan, J.S. Oral manifestations of HIV infection. AIDS Clin. Care 1997, 9, 29–33.
- Challacombe, S. Revised classification of HIV--associated oral lesions. Br. Dent. J. 1991, 170, 305–306.
- Lomeli-Martinez, S.M.; Gonzalez-Hernandez, L.A.; Ruiz-Anaya, A.J.; Lomeli-Martinez, M.A.; Martinez-Salazar, S.Y.; Mercado Gonzalez, A.E.; Andrade-Villanueva, J.F.; Varela-Hernandez, J.J. Oral Manifestations Associated with HIV/AIDS Patients. Medicina 2022, 58, 1214.
- Rodriguez-Inigo, E.; Jimenez, E.; Bartolome, J.; Ortiz-Movilla, N.; Bartolome Villar, B.; Jose Arrieta, J.; Manzarbeitia, F.; Carreno, V. Detection of human immunodeficiency virus type 1 RNA by in situ hybridization in oral mucosa epithelial cells from anti-HIV-1 positive patients. J. Med. Virol. 2005, 77, 17–22.
- Chou, L.L.; Epstein, J.; Cassol, S.A.; West, D.M.; He, W.; Firth, J.D. Oral mucosal Langerhans′ cells as target, effector and vector in HIV infection. J. Oral Pathol. Med. 2000, 29, 394–402.
- Goto, Y.; Yeh, C.K.; Notkins, A.L.; Prabhakar, B.S. Detection of proviral sequences in saliva of patients infected with human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 1991, 7, 343–347.
- Kakizawa, J.; Ushijima, H.; Oka, S.; Ikeda, Y.; Schroder, H.C.; Muller, W.E. Detection of human immunodeficiency virus-1 DNA, RNA and antibody, and occult blood in inactivated saliva: Availability of the filter paper disk method. Acta. Paediatr. Jpn. 1996, 38, 218–223.
- Liuzzi, G.; Chirianni, A.; Clementi, M.; Bagnarelli, P.; Valenza, A.; Cataldo, P.T.; Piazza, M. Analysis of HIV-1 load in blood, semen and saliva: Evidence for different viral compartments in a cross-sectional and longitudinal study. Aids 1996, 10, F51–F56.
- Maticic, M.; Poljak, M.; Kramar, B.; Tomazic, J.; Vidmar, L.; Zakotnik, B.; Skaleric, U. Proviral HIV-1 DNA in gingival crevicular fluid of HIV-1-infected patients in various stages of HIV disease. J. Dent. Res. 2000, 79, 1496–1501.
- Qureshi, M.N.; Barr, C.E.; Hewlitt, I.; Boorstein, R.; Kong, F.; Bagasra, O.; Bobroski, L.E.; Joshi, B. Detection of HIV in oral mucosal cells. Oral. Dis. 1997, 3 (Suppl. S1), S73–S78.
- Qureshi, M.N.; Barr, C.E.; Seshamma, T.; Reidy, J.; Pomerantz, R.J.; Bagasra, O. Infection of oral mucosal cells by human immunodeficiency virus type 1 in seropositive persons. J. Infect. Dis. 1995, 171, 190–193.
- Zuckerman, R.A.; Whittington, W.L.; Celum, C.L.; Collis, T.; Lucchetti, A.; Sanchez, J.L.; Hughes, J.P.; Coombs, R.W. Factors associated with oropharyngeal human immunodeficiency virus shedding. J. Infect. Dis. 2003, 188, 142–145.
- Jayakumar, P.; Berger, I.; Autschbach, F.; Weinstein, M.; Funke, B.; Verdin, E.; Goldsmith, M.A.; Keppler, O.T. Tissue-resident macrophages are productively infected ex vivo by primary X4 isolates of human immunodeficiency virus type 1. J. Virol. 2005, 79, 5220–5226.
- del Romero, J.; Marincovich, B.; Castilla, J.; Garcia, S.; Campo, J.; Hernando, V.; Rodriguez, C. Evaluating the risk of HIV transmission through unprotected orogenital sex. Aids 2002, 16, 1296–1297.
- Page-Shafer, K.; Shiboski, C.H.; Osmond, D.H.; Dilley, J.; McFarland, W.; Shiboski, S.C.; Klausner, J.D.; Balls, J.; Greenspan, D.; Greenspan, J.S. Risk of HIV infection attributable to oral sex among men who have sex with men and in the population of men who have sex with men. Aids 2002, 16, 2350–2352.
- Tudor-Williams, G.; Lyall, E.G. Mother to infant transmission of HIV. Curr. Opin. Infect Dis. 1999, 12, 21–26.
- Semba, R.D.; Kumwenda, N.; Hoover, D.R.; Taha, T.E.; Quinn, T.C.; Mtimavalye, L.; Biggar, R.J.; Broadhead, R.; Miotti, P.G.; Sokoll, L.J.; et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J. Infect. Dis. 1999, 180, 93–98.
- Kuhn, L.; Kim, H.Y.; Walter, J.; Thea, D.M.; Sinkala, M.; Mwiya, M.; Kankasa, C.; Decker, D.; Aldrovandi, G.M. HIV-1 concentrations in human breast milk before and after weaning. Sci. Transl. Med. 2013, 5, 181ra151.
- Satomi, M.; Shimizu, M.; Shinya, E.; Watari, E.; Owaki, A.; Hidaka, C.; Ichikawa, M.; Takeshita, T.; Takahashi, H. Transmission of macrophage-tropic HIV-1 by breast-milk macrophages via DC-SIGN. J. Infect. Dis. 2005, 191, 174–181.
- Lewis, P.; Nduati, R.; Kreiss, J.K.; John, G.C.; Richardson, B.A.; Mbori-Ngacha, D.; Ndinya-Achola, J.; Overbaugh, J. Cell-free human immunodeficiency virus type 1 in breast milk. J. Infect. Dis. 1998, 177, 34–39.
- Koulinska, I.N.; Villamor, E.; Chaplin, B.; Msamanga, G.; Fawzi, W.; Renjifo, B.; Essex, M. Transmission of cell-free and cell-associated HIV-1 through breast-feeding. J. Acquir. Immune. Defic. Syndr. 2006, 41, 93–99.
- De Cock, K.M.; Fowler, M.G.; Mercier, E.; de Vincenzi, I.; Saba, J.; Hoff, E.; Alnwick, D.J.; Rogers, M.; Shaffer, N. Prevention of mother-to-child HIV transmission in resource-poor countries: Translating research into policy and practice. JAMA 2000, 283, 1175–1182.
- Luzuriaga, K. Mother-to-child Transmission of HIV: A Global Perspective. Curr. Infect Dis. Rep. 2007, 9, 511–517.
- UNAIDS. Full Report—In Danger: UNAIDS Global AIDS Update 2022. Available online: https://www.unaids.org/en/resources/documents/2022/in-danger-global-aids-update (accessed on 13 March 2023).
- Bobardt, M.D.; Chatterji, U.; Selvarajah, S.; Van der Schueren, B.; David, G.; Kahn, B.; Gallay, P.A. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J. Virol. 2007, 81, 395–405.
- Howell, A.L.; Asin, S.N.; Yeaman, G.R.; Wira, C.R. HIV-1 infection of the female reproductive tract. Curr. HIV/AIDS Rep. 2005, 2, 35–38.
- Tugizov, S.M.; Herrera, R.; Veluppillai, P.; Greenspan, D.; Soros, V.; Greene, W.C.; Levy, J.A.; Palefsky, J.M. HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology 2011, 409, 211–222.
- Dwinell, M.B.; Eckmann, L.; Leopard, J.D.; Varki, N.M.; Kagnoff, M.F. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology 1999, 117, 359–367.
- Liu, X.; Zha, J.; Chen, H.; Nishitani, J.; Camargo, P.; Cole, S.W.; Zack, J.A. Human immunodeficiency virus type 1 infection and replication in normal human oral keratinocytes. J. Virol. 2003, 77, 3470–3476.
- Herrera, R.; Morris, M.; Rosbe, K.; Feng, Z.; Weinberg, A.; Tugizov, S. Human beta-defensins 2 and -3 cointernalize with human immunodeficiency virus via heparan sulfate proteoglycans and reduce infectivity of intracellular virions in tonsil epithelial cells. Virology 2016, 487, 172–187.
- Yasen, A.; Herrera, R.; Rosbe, K.; Lien, K.; Tugizov, S.M. HIV internalization into oral and genital epithelial cells by endocytosis and macropinocytosis leads to viral sequestration in the vesicles. Virology 2018, 515, 92–107.
- Kumar, R.B.; Maher, D.M.; Herzberg, M.C.; Southern, P.J. Expression of HIV receptors, alternate receptors and co-receptors on tonsillar epithelium: Implications for HIV binding and primary oral infection. Virol. J. 2006, 3, 25.
- Herrera, R.; Rosbe, K.; Tugizov, S.M. Inactivation of HIV-1 in Polarized Infant Tonsil Epithelial Cells by Human Beta-Defensins 2 and 3 Tagged with the Protein Transduction Domain of HIV-1 Tat. Viruses 2021, 13, 2043.
- Yasen, A.; Herrera, R.; Rosbe, K.; Lien, K.; Tugizov, S.M. Release of HIV-1 sequestered in the vesicles of oral and genital mucosal epithelial cells by epithelial-lymphocyte interaction. PLoS Pathog 2017, 13, e1006247.
- Pope, M. Mucosal dendritic cells and immunodeficiency viruses. J. Infect. Dis. 1999, 179 (Suppl. S3), S427–S430.
- Steinman, R.M.; Granelli-Piperno, A.; Pope, M.; Trumpfheller, C.; Ignatius, R.; Arrode, G.; Racz, P.; Tenner-Racz, K. The interaction of immunodeficiency viruses with dendritic cells. Curr. Top Microbiol. Immunol. 2003, 276, 1–30.
- Turville, S.; Wilkinson, J.; Cameron, P.; Dable, J.; Cunningham, A.L. The role of dendritic cell C-type lectin receptors in HIV pathogenesis. J. Leukoc. Biol. 2003, 2, 2.
- Geijtenbeek, T.B.; van Kooyk, Y. DC-SIGN: A novel HIV receptor on DCs that mediates HIV-1 transmission. Curr. Top Microbiol. Immunol. 2003, 276, 31–54.
- Gurney, K.B.; Elliott, J.; Nassanian, H.; Song, C.; Soilleux, E.; McGowan, I.; Anton, P.A.; Lee, B. Binding and transfer of human immunodeficiency virus by DC-SIGN+ cells in human rectal mucosa. J. Virol. 2005, 79, 5762–5773.
- Arrighi, J.F.; Pion, M.; Garcia, E.; Escola, J.M.; van Kooyk, Y.; Geijtenbeek, T.B.; Piguet, V. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J. Exp. Med. 2004, 200, 1279–1288.
- Lee, C.; Liu, Q.H.; Tomkowicz, B.; Yi, Y.; Freedman, B.D.; Collman, R.G. Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways. J. Leukoc. Biol. 2003, 74, 676–682.
- Del Corno, M.; Liu, Q.H.; Schols, D.; de Clercq, E.; Gessani, S.; Freedman, B.D.; Collman, R.G. HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pertussis toxin-insensitive chemokine receptor signaling. Blood 2001, 98, 2909–2916.
- Dayanithi, G.; Yahi, N.; Baghdiguian, S.; Fantini, J. Intracellular calcium release induced by human immunodeficiency virus type 1 (HIV-1) surface envelope glycoprotein in human intestinal epithelial cells: A putative mechanism for HIV-1 enteropathy. Cell Calcium 1995, 18, 9–18.
- Sufiawati, I.; Herrera, R.; Mayer, W.; Cai, X.; Borkakoti, J.; Lin, V.; Rosbe, K.; Tugizov, S.M. Human Immunodeficiency Virus (HIV) and Human Cytomegalovirus (HCMV) Coinfection of Infant Tonsil Epithelium May Synergistically Promote both HIV-1 and HCMV Spread and Infection. J. Virol. 2021, 95, e0092121.
- Sufiawati, I.; Tugizov, S.M. HIV-Associated Disruption of Tight and Adherens Junctions of Oral Epithelial Cells Facilitates HSV-1 Infection and Spread. PLoS ONE 2014, 9, e88803.
- Sufiawati, I.; Tugizov, S.M. HIV-induced matrix metalloproteinase-9 activation through mitogen-activated protein kinase signalling promotes HSV-1 cell-to-cell spread in oral epithelial cells. J. Gen. Virol. 2018, 99, 937–947.
- Vacharaksa, A.; Asrani, A.C.; Gebhard, K.H.; Fasching, C.E.; Giacaman, R.A.; Janoff, E.N.; Ross, K.F.; Herzberg, M.C. Oral keratinocytes support non-replicative infection and transfer of harbored HIV-1 to permissive cells. Retrovirology 2008, 5, 66.
- Kohli, A.; Islam, A.; Moyes, D.L.; Murciano, C.; Shen, C.; Challacombe, S.J.; Naglik, J.R. Oral and vaginal epithelial cell lines bind and transfer cell-free infectious HIV-1 to permissive cells but are not productively infected. PLoS ONE 2014, 9, e98077.
- Maher, D.M.; Zhang, Z.Q.; Schacker, T.W.; Southern, P.J. Ex vivo modeling of oral HIV transmission in human palatine tonsil. J. Histochem. Cytochem. 2005, 53, 631–642.
- Maher, D.; Wu, X.; Schacker, T.; Larson, M.; Southern, P. A model system of oral HIV exposure, using human palatine tonsil, reveals extensive binding of HIV infectivity, with limited progression to primary infection. J. Infect. Dis. 2004, 190, 1989–1997.
More
Information
Subjects:
Virology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
25 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




