Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sharof M. Tugizov and Version 2 by Rita Xu.
The oropharyngeal mucosal epithelia have a polarized organization, which is critical for maintaining a highly efficient barrier as well as innate immune functions. In human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) disease, the barrier and innate immune functions of the oral mucosa are impaired via a number of mechanisms.
- human immunodeficiency virus
- oropharyngeal mucosal epithelium
- reactivation of opportunistic infections
1. Introduction
Four decades ago, the HIV/AIDS pandemic began. Global spread led to 75 million infections and 32 million deaths. Today, highly effective prevention strategies are available to reduce the likelihood of HIV transmission via sexual intercourse. Furthermore, antiretroviral therapy for people living with HIV/AIDS can reduce viral loads to levels that cannot be detected or transmitted. However, despite the availability of these highly effective agents, HIV/AIDS continues to be a lethal disease. HIV/AIDS was responsible for one death each minute in 2021; every two minutes, a young woman becomes newly infected with HIV. Likewise, approximately 200,000 cases of mother-to-child transmission are reported each year. Thus, HIV/AIDS remains an unsolved problem; additional new knowledge may help improve treatment and prophylaxis.
The surface of the oropharyngeal cavity is covered with a multilayer stratified squamous epithelium supported by the lamina propria, which is a layer of fibrous connective tissue [1][2][1,2]. Stratified oropharyngeal epithelial cells from the parabasal to the granulosum layers have well-developed lateral adherens and tight junctions, indicating a polarized organization [3][4][5][6][3,4,5,6]. The lateral localization of adherens and tight junctions between neighboring cells of oral epithelium contribute to a physical barrier that protects the body from penetration by viruses and other pathogens [4][5][4,5].
The oropharyngeal mucosa also contains a broad population of adaptive and innate immune cells, including T and B cells, macrophages, dendritic/Langerhans cells (DC/LCs), and natural killer (NK) cells that are distributed within the epithelium and lamina propria [2][7][8][9][10][11][12][13][14][15][16][2,7,8,9,10,11,12,13,14,15,16]. Intraepithelial DC/LCs and macrophages in the oral mucosa are critical components of the innate immune system. These cells defend against pathogens that enter the body via the oral cavity [2][13][14][16][17][18][19][20][21][22][2,13,14,16,17,18,19,20,21,22]. Intraepithelial macrophages and DC/LCs are also antigen-presenting cells that are capable of activating an adaptive immune response. Thus, these cells may serve as “bridges” between the innate and adaptive immune systems [2][14][23][24][25][2,14,23,24,25]. In addition, oral mucosal epithelial cells express toll-like receptors (TLRs) 2, 3, 4, 5, 6, and 9, which are critical facilitators of innate immune responses against numerous pathogens [26][27][26,27].
Intraepithelial oral mucosal DC/LC and macrophages originate from peripheral blood CD14+ monocytes [28][29][28,29]. Recruitment of circulating monocytes into the mucosal epithelium is mediated by monocyte chemotactic protein-1 (MCP-1), MCP-2, macrophage inflammatory protein-1 alpha (MIP-1α), and MIP-1β [30][31][32][30,31,32]. Expression and secretion of these mediators in the mucosal epithelium are modulated by multiple chemokine/cytokines, including interferon-ɣ (IFN-ɣ), tumor necrosis factor-α (TNF-α), and interleukins (ILs), including IL-1, IL-1β, IL-4, IL-6, IL-8, IL10, IL-13, and IL-15 [14][32][33][34][35][36][14,32,33,34,35,36]. MCP-1 is secreted from the basolateral membranes of polarized epithelial cells [37][38][37,38]; the polarized release of MCP-1 may generate a gradient toward blood vessels that serve to recruit monocytes into the epithelium. Once they have transited across the endothelial layer, monocytes differentiate into macrophages and DC/LCs [11]. Further traffic of monocytes/macrophages, DCs, and T lymphocytes within the mucosal epithelium is coordinated by the formation of transient tight junctions between immune and epithelial cells [39][40][41][42][39,40,41,42]. Migrating immune cells, particularly DC/LCs, express the tight junction proteins known as claudin-1 and occludin. Transient association of these proteins with cell junctions promotes the migration of immune cells without disrupting epithelial barrier functions [39][41][43][44][39,41,43,44]. This allows DC/LCs to reach the mucosal surface [39][43][39,43]. The absence of epithelial junctions may reduce the efficiency of epithelial–lymphocyte interactions, thereby leading to dysfunction (i.e., little to no retention of interepithelial lymphocytes and DC/LCs and thus their depletion) [45][46][47][45,46,47]. Interactions of polarized mucosal epithelial cells with DC/LCs and other cells of the adaptive and innate immune systems are critical for maintaining oral mucosal immune homeostasis.
The oropharyngeal mucosal epithelium and its intraepithelial and subepithelial adaptive and innate immune cells play critical roles in promoting protection against numerous pathogens, including viruses, bacteria, and fungi. However, in HIV/AIDS, various chronic disorders can develop with a significant impact on the oral mucosal epithelium. These include inflammation, necrotizing mucosal ulcers, and malignant and nonmalignant lesions that may impair the barrier as well as the innate and acquired immune functions of the oral mucosa [48][49][50][51][52][53][54][55][48,49,50,51,52,53,54,55].
Systemic HIV/AIDS is accompanied by the spread of cell-free and cell-associated HIV-1 in the oral mucosal environment. There are several reports of viral DNA/RNA, cell-free HIV-1 virions, and tat and gp120 proteins isolated from oral mucosal tissue and saliva of HIV/AIDS patients [6][56][57][58][59][60][61][62][63][64][6,56,57,58,59,60,61,62,63,64]. HIV-infected DC/LCs, lymphocytes, and macrophages were also detected in the mucosal and submucosal layers of the oropharyngeal epithelium [6][56][57][62][63][65][6,56,57,62,63,65]. Electron microscopy revealed that HIV virions could be found within the tight junctions of the oral epithelium [62]. The presence of both cell-free and cell-associated HIV-1 both around and within oropharyngeal mucosa may result in numerous changes that impair its innate immune and barrier functions.
2. Role of Oropharyngeal Mucosal Epithelium in HIV-1 Transmission in Adults and Children
Oral HIV-1 transmission in the adult population may occur during oral sex, in newborn children and infants during delivery and breastfeeding [66][67][68][69][70][71][72][73][66,67,68,69,70,71,72,73]. The rate of adult oral HIV-1 transmission has been estimated at ~0.004% per exposure (i.e., not a highly efficient process) [66][67][68][66,67,68]. By contrast, the rate of mother-to-child transmission (MTCT) of HIV-1 in the absence of antiretroviral therapy (ART) may be as high as 15% in Europe and 25–30% in Asian and African countries [74][75][76][74,75,76]. The oropharyngeal and tonsillar mucosal epithelium may express one or more co-receptors or non-canonical HIV-1 receptors that may facilitate virus binding and entry, including CC chemokine receptor type 5 (CCR5), CXC chemokine receptor type 4 (CXCR4), heparan sulfate proteoglycans (HSPGs), mannose receptor, galactosylceramide (GalCer), and T-cell immunoglobulin and mucin domain 1 (TIM-1) [3][77][78][79][80][81][82][83][84][3,77,78,79,80,81,82,83,84]. Results of studies featuring ex vivo adult and fetal/infant tissue explants revealed that HIV-1 transmission through the adult oral epithelium was less efficient than fetal/infant epithelial tissues, which supported rapid viral transmigration through the mucosal epithelium and infection of virus-susceptible intraepithelial and subepithelial cells [3][85][3,85]. The resistance provided by the adult tissues was primarily due to the presence of multiple epithelial layers and tissue stratification (20–30) with highly-effective tight junctions (Figure 1). The highly stratified adult oral epithelial cells limit viral penetration more efficiently than the less stratified fetal/neonatal/infant counterparts (i.e., with 3–5 layers) [3].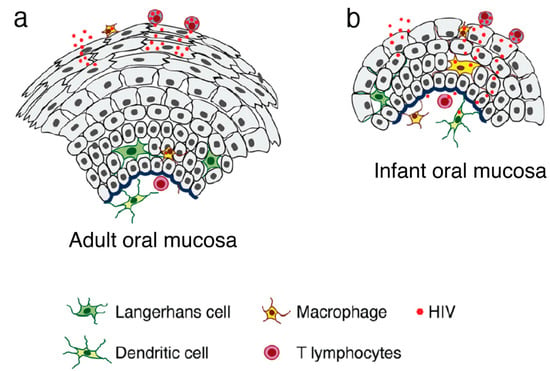
Figure 1. Model of HIV transmigration in the infant/fetal and adult oral epithelium. (a) Adult oral epithelial cells are stratified into numerous layers. While cell-free HIV can transmigrate to some extent across the upper regions of the intact adult oral epithelium (across two to five layers), the virus is unable to spread to the lower layers or the lamina propria. (b) By contrast, the infant/fetal oral mucosal epithelium contains only two to five epithelial layers and is not completely stratified. The spread of HIV-infected macrophages and cell-free virions across infant/fetal oral epithelial cells may result in the infection of HIV-susceptible epithelial and submucosal cells, including macrophages, LC/DCs, and T lymphocytes. Thus, fetal/infant oropharyngeal epithelial cells may serve as a critical portal for HIV entry during MTCT. Increased rates of HIV transmission across the fetal/infant oral epithelium compared to that of adults may represent both reduced barrier function (associated with pauci-stratification) as well as lower levels of innate immune proteins.
