Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexander E. Berezin | -- | 3945 | 2023-05-24 14:19:58 | | | |
| 2 | Peter Tang | Meta information modification | 3945 | 2023-05-25 03:43:13 | | | | |
| 3 | Peter Tang | -2 word(s) | 3943 | 2023-05-25 08:34:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Demirel, O.; Berezin, A.E.; Mirna, M.; Boxhammer, E.; Gharibeh, S.X.; Hoppe, U.C.; Lichtenauer, M. Biomarkers of Atrial Fibrillation Recurrence. Encyclopedia. Available online: https://encyclopedia.pub/entry/44781 (accessed on 08 February 2026).
Demirel O, Berezin AE, Mirna M, Boxhammer E, Gharibeh SX, Hoppe UC, et al. Biomarkers of Atrial Fibrillation Recurrence. Encyclopedia. Available at: https://encyclopedia.pub/entry/44781. Accessed February 08, 2026.
Demirel, Ozan, Alexander E. Berezin, Moritz Mirna, Elke Boxhammer, Sarah X. Gharibeh, Uta C. Hoppe, Michael Lichtenauer. "Biomarkers of Atrial Fibrillation Recurrence" Encyclopedia, https://encyclopedia.pub/entry/44781 (accessed February 08, 2026).
Demirel, O., Berezin, A.E., Mirna, M., Boxhammer, E., Gharibeh, S.X., Hoppe, U.C., & Lichtenauer, M. (2023, May 24). Biomarkers of Atrial Fibrillation Recurrence. In Encyclopedia. https://encyclopedia.pub/entry/44781
Demirel, Ozan, et al. "Biomarkers of Atrial Fibrillation Recurrence." Encyclopedia. Web. 24 May, 2023.
Copy Citation
Atrial fibrillation (AF) is associated with atrial remodeling, cardiac dysfunction, and poor clinical outcomes. External direct electrical cardioversion is a well-developed urgent treatment strategy for patients presenting with recent-onset AF.
atrial fibrillation
electrical cardioversion
post-procedural complications
biomarkers
1. Introduction
Atrial fibrillation (AF) is the most common form of sustained cardiac arrhythmia in the world [1]. The prevalence of AF advances with increasing age. After the age of 80, atrial fibrillation affects 10–17% of the population [2]. The morbidity is increased and mortality rises up to 3.5-fold in men and women [3]. Along with it, AF frequently occurs in patients at higher risk of cardiovascular diseases (CVD) as well as among individuals with known CVD [4]. Unfortunately, AF and CVD exacerbate each other and mutually intervene in prognosis. Indeed, patients with any form of AF demonstrated poorer clinical outcomes if there is concomitant heart failure (HF), coronary artery disease (CAD), type 2 diabetes mellitus (T2DM), obesity, obstructive sleep apnea, chronic kidney disease (CKD), or peripheral artery disease [5][6][7]. Further, the prognosis of patients with AF is poorer than the prognosis of patients with various CVD and comorbid conditions (i.e., HF, CKD) without AF [8]. Multi-morbidity among patients with AF seems to play a pivotal role in natural evolution of primary and secondary AF through direct and indirect impact on the structural and/or electrophysiological abnormalities that occur in AF [9][10]. AF influences electrical remodeling, i.e., shortening of refractoriness due to the high atrial rate itself, resulting in adverse cardiac remodeling [11]. Yet, the persistence of AF itself modulates the risk of cerebrovascular and cardiovascular events [12][13].
The management of AF includes either rhythm restoration or rate control along with comorbidity management, prevention of stroke, and systemic thromboembolism [14]. Synchronized electrical cardioversion can terminate AF. Combined with sedation, it is a safe procedure and highly effective, restoring sinus rhythm in more than 90% [15][16][17]. It is important to detect AF recurrence after successful electrical cardioversion. In this case, early cardioversion could prolong the subsequent duration of sinus rhythm and slow disease progression compared to delayed sinus rhythm restoration [18].
Although the current clinical protocol of initial AF management seems to be very useful in practice [1], it poses challenges in predicting incidental AF and early detection of AF-related complications [19][20]. There are many factors associated with AF recurrence, such as duration of AF, higher age, sex, HF, LA volume index, chronic obstructive pulmonary disease, hypertension, obstructive sleep apnea, hyperthyroidism, smoking, and obesity [21][22].
2. Promoting Factors and Electrophysiological/Anatomical Substrates of AF
Vulnerable substrates for the occurrence, support, and recurrence of AF are electrophysiological and adverse cardiac remodeling, along with structural remodeling, mechanical dysfunction, and trigger activity, which are mediated by genetic ion channel alterations, concomitant cardiovascular (CV) diseases (acute and chronic coronary syndromes, multifocal atherosclerosis, primary and secondary cardiomyopathy, etc.), CV risk factors (hypertension, smoking, obesity, diabetes mellitus, resistance to insulin, and dyslipidemia), and comorbidities (chronic obstructive pulmonary disease, bronchial asthma, chronic kidney disease) (Figure 1). In addition, concomitant hemodynamic factors as a result of numerous diseases (heart failure, atrial cardiomyopathy, pulmonary hypertension, inherited and acquired heart diseases, myocarditis) and conditions (chemotherapy, cardiac toxicity) play a crucial role in secondary structural remodeling of the heart [23][24][25]. These factors contribute to AF occurrence by maintaining afterdepolarization-induced triggered ectopic activity, focal enhanced automaticity, altered function of ion channels, micro-reentrant circus rotor, less dynamic head–tail interactions during re-entry in cardiac tissue, altered ion accumulation on the dynamics of re-entry and electrical heterogeneity [26]. Indeed, head–tail interactions have previously been known to have a causative impact on the dynamics of the reentrant action potential, which plays a pivotal role in inducing AF [26]. To note, intracellular ions, mainly Ca2+ and Na+, accumulated during reentrant arrhythmia through the rapid repetitive cellular excitation may lead to spontaneous termination of re-entry or break-up of the re-entry loop into multiple pathways resulting in AF. Along with it, the initiation and persistence of AF are controlled by both parasympathetic and sympathetic stimulation, as well as hormonal influences, which also seem to play a role in AF recurrence [27]. However, the continuous interaction between electrophysiological, structural, and anatomical remodeling leads to intercellular uncoupling and a pro-fibrotic response, which is crucial for trigger activity, the presence of AF, and the transformation of cardiac dysfunction into HF [28].
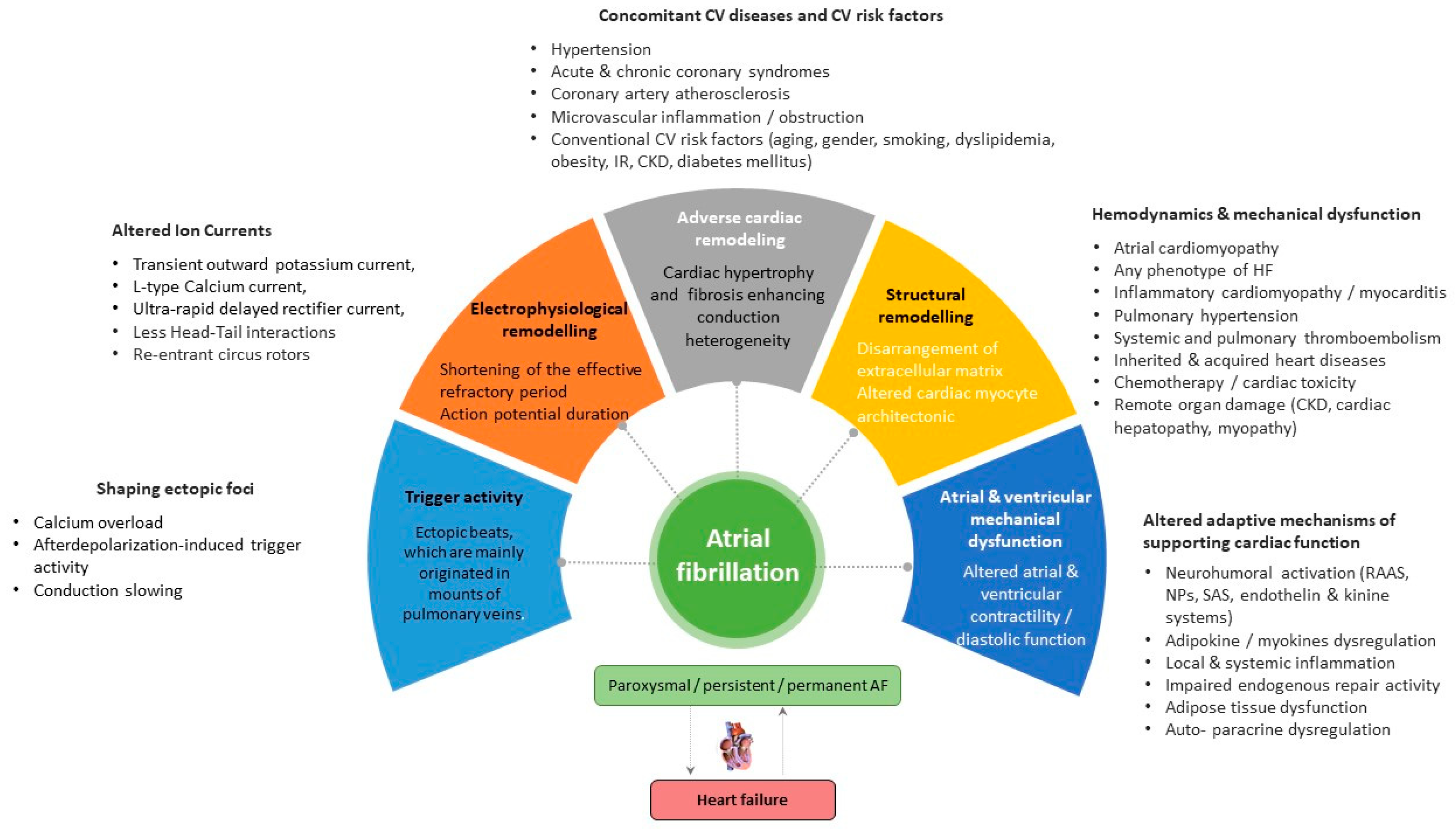
Figure 1. Promoting factors and plausible pathogenetic mechanisms of AF. Abbreviations: AF, atrial fibrillation; CV, cardiovascular; CKD, chronic kidney disease; SAS, sympathoadrenal system; IR, insulin resistance; HF, heart failure; RAAS, renin-angiotensin-aldosterone system.
3. Electrical Cardioversion of AF: Safety and Outcomes
It seems that standard external direct current electrical cardioversion is a well-developed urgent treatment strategy for patients presenting recent-onset AF [1][29]. Numerous retrospective one-center studies and multicenter trials yielded 86–88% efficacy of the approach in restoring sinus rhythm along with 6–10% relapse of AF in a short-term perspective (7–28 days) [30][31][32]. Overall, electrical cardioversion in AF patients who required emergency department transportation was associated with infrequent hospital admission and few mild-to-moderate complications [30]. However, the duration of AF in the majority of studies was less than 48 h in 99% of the patients. Burton JH et al. (2004) [30] observed in a retrospective multicenter study that electrical cardioversion had an 86% success rate, and only 10% of the patients returned to the emergency department within 7 days. Fried AM et al. (2021) [16] reported that the efficacy of this procedure, defined as restoration of sinus rhythm, reached 88% in routine clinical practice, whereas major complications (post-cardioversion stroke, thromboembolic events, jaw thrust maneuver for hypoxia, and overnight observation for hypotension) and predefined minor adverse events (frequently related to general anesthesia, skin burns) were detected in 0.3% and 14%, respectively. In addition, electrical cardioversion was about 2.5 times more effective than conventional pharmacological treatment in restoring sinus rhythm [31][32]. Although there are numerous potential complications of electrical cardioversion (i.e., ventricular fibrillation, thromboembolism due to inadequate anticoagulant therapy, nonsustained ventricular tachycardia, various forms of atrial arrhythmias, bradycardia, transient left bundle branch block, myocardial necrosis, asymptomatic myocardial dysfunction, acute HF, transient hypotension, pulmonary edema, and stroke), they occur less frequently than recurrent AF. Further, 6.4% of patients revisited the emergency department within 30 days, and 4.8% returned with AF or atrial flutter. It is noteworthy that the return visit rate for patients with relapsed AF varies between 3% and 17% [33].
Overall, 30-day all-cause mortality among AF patients undergoing direct-current electrical cardioversion was 0.8% [34]. Data received from the FIRE (Atrial Fibrillation/flutter Italian Registry) registry showed that predictors of unsuccessful electrical cardioversion were onset of AF > 48 h, concomitant HF, increasing age, syncope, transient ischemic attack (TIA)/stroke as well as previous admission to a non-cardiology department [35]. The investigators also found several predictors of in-hospital mortality in this patient population, including age, HF, diabetes mellitus, previous admission to a non-cardiology department, and TIA/stroke [35]. Thus, patients at low risk for thromboembolic complications, including stroke and heart failure, seem to benefit more from electric cardioversion than other individuals with recent-onset AF [36].
Another reason for physicians to use this approach may be cost savings and a short period of emergency department admission [36][37]. Houghton AR et al. (2000) [38] and Boriani G. et al. (2007) [39] did not identify concise hemodynamic predictors of successful external electrical cardioversion or relapses after electrical cardioversion among patients with persistent AF or atrial flutter. However, only two predictors (duration of arrhythmia ≥1 year and previous cardioversion) were found to be powerful for this matter [38][39], whereas, in previous investigations, relapse of AF was associated with reduced left ventricular ejection fraction [40]. Along with it, standard external biphasic direct current electrical cardioversion has better efficacy than monophasic electrical cardioversion (360-J) for restoration of sinus rhythm in AF patients, although dual external monophasic 360-J cardioversion may increase the success rate as a rescue technique after failing standard external direct current cardioversion [41][42]. In this concept, the prediction of plausible cardiovascular events, including relapsed AF, with a biomarker strategy seems promising in patients with recent-onset AF.
4. Predictors for AF Recurrence Following Electrical Cardioversion
Biomarkers reflecting the complex pathophysiological mechanisms underlying AF seem to be an effective tool to predict rhythm status after cardioversion as well as other AF-related complications, which can intervene in mortality, hospital admission, cardiovascular (CV), and non-CV outcomes.
4.1. Natriuretic Peptides
Natriuretic peptides (brain natriuretic peptide [BNP], N-terminal pro-B-type natriuretic peptide (NT-proBNP), mid-regional pro-A type natriuretic peptide (MR-proANP)) serve as circulating cardiac biomarkers of biomechanical stress, adverse cardiac remodeling and fluid overload with established diagnostic and predictive values for acute and chronic HF involving any phenotypes [43][44]. Along with it, elevated levels of NPs were strongly associated with all-cause and CV mortality and urgent hospitalization among patients with AF, T2DM, CKD, hypertension, and cardiac hypertrophy [45][46]. Moreover, NT-proBNP and BNP were found to be predictors for AF [47][48][49]. However, it has been suggested that restoration of sinus rhythm through effective electric cardioversion may associate with a reduction in NP concentrations and thereby predict the recurrence of new episodes of arrhythmia. Xu X et al. (2017) [50] observed in a meta-analysis that low levels of BNP and NT-proBNP were associated with the maintenance of sinus rhythm and that the baseline concentrations of both biomarkers may be a predictor of AF recurrence after successful electrical cardioversion. Ari H. et al. (2008) [51] reported that a significant decrease in BNP levels 30 min after electric cardioversion corresponded to six-month maintenance of sinus rhythm in follow-up.
In the GAPP-AF (The gene expression patterns for the prediction of atrial fibrillation) study, Meyre PB (2022) [52] investigated 21 conventional and new circulating biomarkers reflecting inflammation, myocardial injury, cardiac biomechanical stress, and renal dysfunction before and 30 days after electrical cardioversion and evaluated plausible associations of changes in circulating biomarker levels with rhythm status at 30-day follow-up. The patients included in the study had no acute HF, severe valvular disease, or life-limiting active or chronic serious concomitant diseases. The authors found that low levels of NT-proBNP were independently associated with sinus rhythm restoration after electric cardioversion. On the other hand, initial levels of BNP and NT-proBNP in patients with persistent AF without established CVD did not predict long-term sinus rhythm maintenance, although conversion to sinus rhythm related to a significant decrease in circulating BNP but not NT-proBNP level [53]. In contrast, NT-proBNP levels were found to be a predictor of AF recurrence 30 days after successful electric cardioversion among patients with persistent AF and CV risk factors, including hypertension and dyslipidemia [54]. In another study, pre-procedural NT-proBNP levels, but not post-procedural levels of the peptide, independently predicted the relapse of AF after successful electrical cardioversion [55]. These controversial issues perhaps may relate to the presence of concomitant HF. Indeed, in the CAPRAF (Candesartan in the Prevention of Relapsing Atrial Fibrillation) trial, plasma NT-proBNP concentrations measured before electrical cardioversion did not predict cardioversion success nor the relapse of AF in patients without HF [56]. Mabuchi N et al. (2000) [57] noticed that low atrial natriuretic peptide (ANP) and high BNP levels before electric cardioversion were independent predictors of recurrent AF in mild chronic HF patients. Moreover, the authors established that ANP to BNP ratio <0.44 was a significant risk factor for AF recurrence [57]. The BNP level of 700 fmol/mL or higher on day 7 after cardioversion was most predictive for AF recurrence (sensitivity, 78%; specificity, 71%), whereas ANP did not predict the relapse of AF [58]. Buccelletti F. et al. (2011) [59] measured the levels of NT-proBNP in 200 patients admitted to the emergency department due to new-onset AF (<2 weeks) regardless of HF presence. The authors found that NT-proBNP levels of either ≤450 pg/mL or >1800 pg/mL seem to show positive and negative predictive values for cardioversion in rate-control and rhythm-control strategies, respectively. In the range of 450 to 1800 pg/mL, NT-proBNP did not exhibit serious clinical utility [59]. However, it remained unclear whether continuous monitoring of the dynamic changes of NPs after sinus rhythm restoration predicts recurrent AF [60]. Overall, the restoration of sinus rhythm after electric cardioversion in AF patients is associated with a decrease in circulating levels of NPs and low levels of NT-proBNP predicts a sustainable maintains of sinus rhythm in follow-up.
4.2. Biomarkers of Fibrosis
Cardiac fibrosis was found to be closely associated with AF. Circulating biomarkers of fibrosis have already been proposed as a promising tool in its evaluation, but which biomarkers are most appropriate for AF remains unclear [61]. There are a large number of circulating biomarkers, which characterize the accumulation of extracellular matrix components and fibrosis, such as soluble suppressor tumorigenicity-2 (sST2), galectin-3 (Gal-3), procollagen type III N terminal peptide (PIIINP), type I collagen carboxyl telopeptide (ICTP), and fibroblast growth factor 23 (FGF-23) [62].
4.3. Biomarkers of Inflammation
4.3.1. GDF15
GDF15 is a member of the TGF-beta superfamily whose expression is increased in response to biomechanical myocardial stress, inflammation, or ischemia/hypoxia [63]. GDF15 is involved in the regulation of energy homeostasis, thermogenesis, and eating behavior [64]. Yet, GDF15 also exerts anti-inflammatory and anti-proliferative properties, although the underlying molecular mechanisms are still unclear [65]. Elevated GDF15 levels were found in patients with any phenotypes of chronic HF, stroke, AF, and T2DM [66][67][68][69][70]. In the general population, GDF-15 did not show a positive association with the prevalence of AF and the risk of AF occurrence [71]. The suitability of GDF15 for predicting bleeding and/or atrial thrombosis during anticoagulant therapy remains questionable [72]. Clinical evidence for the discriminative value of GDF15 for AF relapse or sinus rhythm maintenance is extremely limited. There is one small study that prospectively included 82 patients with persistent AF [73]. Although log10 serum GDF-15 levels correlated positively with the CHA2DS2-VASc score, there was no close association between GDF-15 levels and sinus rhythm maintenance in patients after successful electric cardioversion [73]. Thus, a discriminative potency of GDF15 for the prediction of clinical efficacy of electrical cardioversion among patients with nonvalvular/valvular AF is not completely understood and requires scrutiny in large clinical studies.
4.3.2. hs-CRP
High-sensitivity C-reactive protein (hs-CRP) is a classic biomarker of inflammation and is a component of the inflammatory profile observed in AF patients. Elevated hs-CRP levels were found in patients with all forms of nonvalvular/valvular AF, regardless of etiology and concomitant comorbidities [74][75]. hs-CRP predicted new-onset AF both in the general population as well as in patients with established cardiovascular or metabolic diseases, such as HF, acute myocardial infarction, T2DM, and metabolic syndrome [76][77][78]. Among patients with AF complicated by systemic thromboembolism, the levels of hs-CRP correlated positively with the CHA2DS2-VASc score [79].
Loricchio ML et al. (2007) [80] investigated plausible predictors for a 1-year risk of AF recurrence after electrical cardioversion. In a Cox regression analysis, the authors found that age, gender, hypertension, T2DM, LVEF, left atrial diameter, use of various antiarrhythmic and antihypertensive (including angiotensin-converting enzyme inhibitors or angiotensin II antagonists) drugs, and statins were not associated with relapsing AF. On the contrary, a low quartile of hs-CRP levels was found to be a strong predictor for this outcome [80]. Lombardi F. et al. (2008) [81] did not find any changes in hs-CRP levels after cardioversion in patients with persistent AF and preserved LVEF, regardless of the post-procedural underlying rhythm. However, NT-proBNP levels decreased significantly in patients who maintained sinus rhythm but not in those who had AF. Yet, baseline hs-CRP levels, but not echocardiographic features of atrial dysfunction and initial NT-proBNP levels, predicted recurrences of AF after cardioversion in patients without pre-existing left ventricular dysfunction [81]. Barassi A et al. (2012) [82] and Korantzopoulos P et al. (2008) [83] confirmed that in patients with persistent AF and preserved LVEF, elevated hs-CRP levels independently predicted subacute AF recurrence rate, whereas NT-proBNP concentrations were not associated with arrhythmic outcome but corresponded to the alterations of cardiac hemodynamics secondary to the presence of AF.
Overall, there is ample strong evidence that elevated preprocedural hs-CRP levels may provide independent predictive information for both successful electrical cardioversion of AF and maintenance of sinus rhythm after conversion [84][85]. The meta-analysis by Liu et al. [86], which included six prospective observational studies (n = 366 patients), showed that peripheral blood CRP levels were higher in patients with failed electric cardioversion than in those with successful restoration of sinus rhythm. In another meta-analysis by Yo CH et al. (2014) [87], a cut-off value of 1.9 mg/L hs-CRP predicted long-term AF recurrence (77% sensitivity, 65% specificity), and more than 3 mg/L predicted short-term AF relapse (73% sensitivity, 71% specificity). Thus, the measurement of CRP levels before the procedure may provide additional prognostic information about the success of sinus rhythm maintenance.
In addition, there are data illustrating that hs-CRP levels measured shortly after electrical cardioversion may be a powerful biomarker for assessing the risk of relapsing AF in the long-term. In particular, Celebi OO et al. (2011) [88] reported that hs-CRP levels measured before and 2 days after electrical cardioversion predicted the 1-year risk of AF relapse. Whether postprocedural hs-CRP provides more information to predict the event than preprocedural hs-CRP is still unclear. However, elevated levels of hs-CRP predicted new-onset AF in the general population and among patients with known cardiovascular diseases, while their role as a marker of sustainable sinus rhythm control places under question.
4.4. Myokines and Adipocytokines
Several interdependent canonic signaling pathways, such as the renin-angiotensin-aldosterone system; TGF-beta pathway, inflammatory chemokines, and cytokines lead to cardiac fibrosis through modulation of oxidative stress and inflammation. However, the direct mechanical stretch may act as a modulator of extracellular matrix remodeling by attenuating the expression of matrix metalloproteinases and their inhibitors. Recently, another signaling pathway has been identified that induces atrial fibrosis via the secretion of adipokines from epicardial, perivascular, and adipose tissue white adipocytes. In addition, recent studies have shown that myokines derived from cardiac and skeletal muscle myocytes may act as adaptive regulators of extracellular matrix remodeling and can attenuate fibrosis [89][90]. Depending on their origin, adipokines and myokines may modulate myofibroblast capabilities, regulate myocyte energy homeostasis and protect against inflammation and fibrosis [91][92]. However, some pro-fibrotic adipokines and myokines can switch a generation of reactive oxygen species to pro-inflammatory and pro-fibrotic stimuli, stimulate myofibroblast differentiation through JAK/STAT3 and JNK/c-Jun signaling, interfere with myocyte electrophysiology, and promote fibrosis in the myocardium [93][94][95]. Numerous previous studies have shown that resistin, apelin, and adiponectin are adipokines associated with several known risk factors for AF and risk of AF [96][97][98][99]. A recent meta-analysis of 34 studies (total number of patients = 31,479) showed that some adipokines, mainly adiponectin, apelin, and resistin, were associated with the risk of AF in the pooled univariate data, whereas the associations were not apparent after multivariate adjustment [100]. However, there is limited evidence of the relation between adipokine and myokine signatures and the risk of AF-related outcomes after electric cardioversion.
4.5. Biomarkers of Oxidative Stress and Endothelial Dysfunction
4.5.1. Cell-Free Circulating DNA
Cell-free circulating DNA (cfcDNA) circulates in two main pools: circular and single-stranded molecules belonging to mitochondrial-derived and nuclear-derived subpopulations, reflecting patterns of DNA methylation and a variety of neutrophil extracellular traps (NETosis) [101][102]. The cfcDNA are determined in subdetectable concentrations under certain physiological conditions, such as physical exercise, whereas increased circulating levels of these fragments are strongly associated with cardiovascular, autoimmune, rheumatic diseases, infections, and malignancy [103][104][105][106][107]. The main causes of cfcDNA production are mitochondrial dysfunction and inflammation, which are powerful drivers of numerous diseases and conditions, including AF [108].
Wiersma M. et al. (2020) [109] reported that levels of cell-free circulating mitochondrial DNA (cfc-mtDNA) were significantly increased in patients with paroxysmal AF undergoing AF treatment, especially in men and in patients with AF recurrence after electrical cardioversion or pulmonary vein isolation. In contrast, cfc-mtDNA levels gradually decreased in patients with persistent AF and long-standing persistent AF. Nevertheless, the authors suggested that cfc-mtDNA levels might be associated with the stage of AF and the risk of AF recurrence after treatment, especially in men. Gender differences in descriptive values of cfc-mtDNA for AF recurrence remain poorly understood but could be related to different comorbidities in both subpopulations. However, another study found no significant changes in mtDNA copy number in the peripheral blood of AF patients of different sex and age [110]. Perhaps, cfcDNA may be included in the multiple biomarker models with the aim of improving their predictive potency in AF patients with low levels of NT-proBNP or in AF patients with malignancy who are treated with chemotherapy.
4.5.2. mRNA
MicroRNAs (miRNAs) participate in atrial remodeling and cardiac fibrosis, contributing to the development of AF [111]. Garcia-Elias A et al. (2021) [112] established that circulating levels of miR-199a-5p and miR-22-5p, which regulate fibrogenic response in the myocardium, were higher in HFrEF patients with AF than in those without AF [112]. MiR-21, which corresponds to atrial fibrosis, is associated with the risk of persistent AF in patients with left atrial enlargement [113]. Interestingly, increased circulating levels of miR-1-3p, which is a myosine gene regulator involved in hypertrophy, myocardial infarction, and cardiac arrhythmogenesis, predicted a high risk of subclinical AF [114]. MiR423, which downregulates fibrosis-related genes such as collagen I, collagen III, fibronectin, and TGF-beta, may be a pivotal factor in stratifying patients at risk of AF occurrence and persistence [115]. Moreover, differences in miRNA expression in the atrial myocardium of men and women may mediate a sex-specific association between circulating miRNAs in plasma and AF at the population level [111]. In addition, there is evidence that epigenetic regulation of NETosis may participate in the development of AF susceptibility. As a matter of fact, miR-146a and miR21 may provide prognostic information in patients with AF [116][117] due to its direct effects on NETosis. In a study by da Silva AMG (2018) [118], miR-21, miR-133b, and miR-499, which are directly involved in the downregulation of apoptosis and fibrosis, were found to be directly involved in AF. However, it remains to be determined whether a signature of mi-Rs can be used to predict poor response to AF treatment, including electrical cardioversion. At the same time, Zhou Q et al. (2018) [118] reported that among 123 miRs affecting cardiac fibrosis, hypertrophy, and inflammation by relation with the SMAD7 and FASLG genes, only miR-21 demonstrated a positive correlation with left atrial low-voltage areas in patients with persistent AF and was associated with post-ablation outcome. Overall, the signature of miRs appears to be a more promising tool for higher AF risk than for outcomes after treatment, although this conjecture needs to be further investigated in the future.
4.5.3. Asymmetric Dimethylarginine
Asymmetric dimethylarginine (ADMA) is a well-known biomarker of endothelial dysfunction that indirectly reflects vascular NO production and exhibits certain predictive information for mortality and morbidity of cardiovascular diseases, including AF [119][120]. In the population-based Gutenberg Health Study (n = 5000), ADMA levels were correlated with left ventricular hypertrophy and AF prevalence [121]. An ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) substudy showed that elevated ADMA levels exhibited a weak association with thromboembolic events in AF patients treated with anticoagulants (warfarin or apixaban) for a median of 1.9 years [122]. The investigators found that tertile groups of ADMA levels were sufficiently associated with death, stroke, and systemic embolism and that incorporating ADMA into CHA2DS2-VASc or HAS-BLED predictive models significantly improved C-indices for those clinical outcomes [122].
There is strong evidence that acute and persistent episodes of AF seem to show elevated ADMA levels accompanied by increased biomarkers of ischemic myocardial injury like cardiac troponins [123]. In the animal AF model, ADMA concentrations in peripheral blood returned to normal within 24 h after successful electrical cardioversion [123]. Along with it, increased circulating levels of ADMA in AF may be reduced by a Mediterranean diet and statin treatment [124][125]. Thus, being closely associated with thrombus formation and CHADS2/CHA2DS2-VASc score, ADMA is a biomarker for predicting pro-thrombotic risk in AF [126][127].
There are controversial data for ADMA’s predictive ability regarding AF recurrence after electrical cardioversion. Xia W et al. (208) [128] reported that elevated ADMA levels were strongly associated with an increased risk of AF relapse within 1 month after electrical cardioversion. On the contrary, Tveit A et al. (2010) [129] found that the levels of ADMA and the L-arginine/ADMA ratio did not exert predictive ability for sinus rhythm maintenance after electrical cardioversion, while the L-arginine/ADMA ratio remained elevated in patients with sinus rhythm for 6 months compared with patients with AF recurrence. The discriminative potency of ADMA may be strongly related to comorbidities. Indeed, serum ADMA levels were not associated with incident AF in the general population after adjusting for other cardiovascular risk factors [129]. Overall, the utility of ADMA refines clinical risk stratification in AF regardless of the treatment strategy.
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498.
- Zoni-Berisso, M.; Lercari, F.; Carazza, T.; Domenicucci, S. Epidemiology of Atrial Fibrillation: European Perspective. Clin. Epidemiol. 2014, 6, 213–220.
- Magnussen, C.; Niiranen, T.J.; Ojeda, F.M.; Gianfagna, F.; Blankenberg, S.; Njølstad, I.; Vartiainen, E.; Sans, S.; Pasterkamp, G.; Hughes, M.; et al. Sex Differences and Similarities in Atrial Fibrillation Epidemiology, Risk Factors, and Mortality in Community Cohorts: Results from the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation 2017, 136, 1588–1597.
- Wachter, R. Vorhofflimmern als Komorbidität bei Herzinsuffizienz. Internist 2018, 59, 415–419.
- Alonso, A.; Almuwaqqat, Z.; Chamberlain, A. Mortality in Atrial Fibrillation: Is It Changing? Trends Cardiovasc. Med. 2021, 31, 469–473.
- Walczak-Galezewska, M.; Markowska, M.; Braszak, A.; Bryl, W.; Bogdanski, P. Atrial Fibrillation and Obesity: Should Doctors Focus on This Comorbidity? Minerva Med. 2019, 110, 175–176.
- Hong, K.L.; Glover, B.M. The Impact of Lifestyle Intervention on Atrial Fibrillation. Curr. Opin. Cardiol. 2018, 33, 14–19.
- Shaikh, F.; Pasch, L.B.; Newton, P.J.; Bajorek, B.V.; Ferguson, C. Addressing Multimorbidity and Polypharmacy in Individuals with Atrial Fibrillation. Curr. Cardiol. Rep. 2018, 20, 32.
- Heijman, J.; Linz, D.; Schotten, U. Dynamics of Atrial Fibrillation Mechanisms and Comorbidities. Annu. Rev. Physiol. 2021, 83, 83–106.
- Schoonderwoerd, B.A.; Van Gelder, I.C.; Van Veldhuisen, D.J.; Van den Berg, M.P.; Crijns, H.J.G.M. Electrical and Structural Remodeling: Role in the Genesis and Maintenance of Atrial Fibrillation. Prog. Cardiovasc. Dis. 2005, 48, 153–168.
- Cha, T.-J.; Ehrlich, J.R.; Zhang, L.; Shi, Y.-F.; Tardif, J.-C.; Leung, T.K.; Nattel, S. Dissociation between Ionic Remodeling and Ability to Sustain Atrial Fibrillation during Recovery from Experimental Congestive Heart Failure. Circulation 2004, 109, 412–418.
- Vanbeselaere, V.; Truyers, C.; Elli, S.; Buntinx, F.; De Witte, H.; Degryse, J.; Henrard, S.; Vaes, B. Association between Atrial Fibrillation, Anticoagulation, Risk of Cerebrovascular Events and Multimorbidity in General Practice: A Registry-Based Study. BMC Cardiovasc. Disord. 2016, 16, 61.
- Bernard, M.L. Atrial Fibrillation and Multimorbidity. Mayo Clin. Proc. 2019, 94, 2381–2382.
- Kwok, C.S.; Lip, G.Y.H. The Patient Pathway Review for Atrial Fibrillation. Crit. Pathw. Cardiol. 2022, 21, 96–102.
- Furniss, S.S.; Sneyd, J.R. Safe Sedation in Modern Cardiological Practice. Heart 2015, 101, 1526–1530.
- Fried, A.M.; Strout, T.D.; Perron, A.D. Electrical Cardioversion for Atrial Fibrillation in the Emergency Department: A Large Single-Center Experience. Am. J. Emerg. Med. 2021, 42, 115–120.
- Brandes, A.; Crijns, H.J.G.M.; Rienstra, M.; Kirchhof, P.; Grove, E.L.; Pedersen, K.B.; Van Gelder, I.C. Cardioversion of Atrial Fibrillation and Atrial Flutter Revisited: Current Evidence and Practical Guidance for a Common Procedure. EP Eur. 2020, 22, 1149–1161.
- Voskoboinik, A.; Kalman, E.; Plunkett, G.; Knott, J.; Moskovitch, J.; Sanders, P.; Kistler, P.M.; Kalman, J.M. A Comparison of Early versus Delayed Elective Electrical Cardioversion for Recurrent Episodes of Persistent Atrial Fibrillation: A Multi-Center Study. Int. J. Cardiol. 2019, 284, 33–37.
- Yoon, M.; Yang, P.-S.; Jang, E.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Kim, J.-Y.; Sung, J.-H.; Pak, H.-N.; Lee, M.-H.; et al. Improved Population-Based Clinical Outcomes of Patients with Atrial Fibrillation by Compliance with the Simple ABC (Atrial Fibrillation Better Care) Pathway for Integrated Care Management: A Nationwide Cohort Study. Thromb. Haemost. 2019, 119, 1695–1703.
- Cheung, C.C.; Nattel, S.; Macle, L.; Andrade, J.G. Management of Atrial Fibrillation in 2021: An Updated Comparison of the Current CCS/CHRS, ESC, and AHA/ACC/HRS Guidelines. Can. J. Cardiol. 2021, 37, 1607–1618.
- Toufan, M.; Kazemi, B.; Molazadeh, N. The Significance of the Left Atrial Volume Index in Prediction of Atrial Fibrillation Recurrence after Electrical Cardioversion. J. Cardiovasc. Thorac. Res. 2017, 9, 54–59.
- Ecker, V.; Knoery, C.; Rushworth, G.; Rudd, I.; Ortner, A.; Begley, D.; Leslie, S.J. A Review of Factors Associated with Maintenance of Sinus Rhythm after Elective Electrical Cardioversion for Atrial Fibrillation. Clin. Cardiol. 2018, 41, 862–870.
- Conte, M.; Petraglia, L.; Cabaro, S.; Valerio, V.; Poggio, P.; Pilato, E.; Attena, E.; Russo, V.; Ferro, A.; Formisano, P.; et al. Epicardial Adipose Tissue and Cardiac Arrhythmias: Focus on Atrial Fibrillation. Front. Cardiovasc. Med. 2022, 9, 932262.
- Abe, I.; Teshima, Y.; Kondo, H.; Kaku, H.; Kira, S.; Ikebe, Y.; Saito, S.; Fukui, A.; Shinohara, T.; Yufu, K.; et al. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm 2018, 15, 1717–1727.
- Michniewicz, E.; Mlodawska, E.; Lopatowska, P.; Tomaszuk-Kazberuk, A.; Malyszko, J. Patients with atrial fibrillation and coronary artery disease—Double trouble. Adv. Med. Sci. 2018, 63, 30–35.
- Huang, T.; Nairn, D.; Chen, J.; Mueller-Edenborn, B.; Pilia, N.; Mayer, L.; Eichenlaub, M.; Moreno-Weidmann, Z.; Allgeier, J.; Trenk, D.; et al. Structural and electrophysiological determinants of atrial cardiomyopathy identify remodeling discrepancies between paroxysmal and persistent atrial fibrillation. Front. Cardiovasc. Med. 2023, 9, 1101152.
- Sánchez-Quintana, D.; López-Mínguez, J.R.; Pizarro, G.; Murillo, M.; Cabrera, J.A. Triggers and anatomical substrates in the genesis and perpetuation of atrial fibrillation. Curr. Cardiol. Rev. 2012, 8, 310–326.
- Gomez, J.F.; Cardona, K.; Martinez, L.; Saiz, J.; Trenor, B. Electrophysiological and structural remodeling in heart failure modulate arrhythmogenesis. 2D simulation study. PLoS ONE 2014, 9, e103273.
- Wolfes, J.; Ellermann, C.; Frommeyer, G.; Eckardt, L. Evidence-based treatment of atrial fibrillation around the globe: Comparison of the latest ESC, AHA/ACC/HRS, and CCS guidelines on the management of atrial fibrillation. Rev. Cardiovasc. Med. 2022, 23, 56.
- Burton, J.H.; Vinson, D.R.; Drummond, K.; Strout, T.D.; Thode, H.C.; McInturff, J.J. Electrical cardioversion of emergency department patients with atrial fibrillation. Ann. Emerg. Med. 2004, 44, 20–30.
- Michael, J.A.; Stiell, I.G.; Agarwal, S.; Mandavia, D.P. Cardioversion of paroxysmal atrial fibrillation in the emergency department. Ann. Emerg. Med. 1999, 33, 379–387.
- Dankner, R.; Shahar, A.; Novikov, I.; Agmon, U.; Ziv, A.; Hod, H. Treatment of stable atrial fibrillation in the emergency department: A population-based comparison of electrical direct-current versus pharmacological cardioversion or conservative management. Cardiology 2009, 112, 270–278.
- Von Besser, K.; Mills, A.M. Is discharge to home after emergency department cardioversion safe for the treatment of recent-onset atrial fibrillation? Ann. Emerg. Med. 2011, 58, 517–520.
- Xavier Scheuermeyer, F.; Grafstein, E.; Stenstrom, R.; Innes, G.; Poureslami, I.; Sighary, M. Thirty-day outcomes of emergency department patients undergoing electrical cardioversion for atrial fibrillation or flutter. Acad. Emerg. Med. 2010, 17, 408–415.
- Santini, M.; De Ferrari, G.M.; Pandozi, C.; Alboni, P.; Capucci, A.; Disertori, M.; Gaita, F.; Lombardi, F.; Maggioni, A.P.; Mugelli, A.; et al. Atrial fibrillation requiring urgent medical care. Approach and outcome in the various departments of admission. Data from the atrial Fibrillation/flutter Italian REgistry (FIRE). Ital. Heart J. 2004, 5, 205–213.
- Cohn, B.G.; Keim, S.M.; Yealy, D.M. Is emergency department cardioversion of recent-onset atrial fibrillation safe and effective? J. Emerg. Med. 2013, 45, 117–127.
- Cristoni, L.; Tampieri, A.; Mucci, F.; Iannone, P.; Venturi, A.; Cavazza, M.; Lenzi, T. Cardioversion of acute atrial fibrillation in the short observation unit: Comparison of a protocol focused on electrical cardioversion with simple antiarrhythmic treatment. Emerg. Med. J. 2011, 28, 932–937.
- Houghton, A.R.; Sharman, A.; Pohl, J.E. Determinants of successful direct current cardioversion for atrial fibrillation and flutter: The importance of rapid referral. Br. J. Gen. Pract. 2000, 50, 710–711.
- Boriani, G.; Diemberger, I.; Biffi, M.; Domenichini, G.; Martignani, C.; Valzania, C.; Branzi, A. Electrical cardioversion for persistent atrial fibrillation or atrial flutter in clinical practice: Predictors of long-term outcome. Int. J. Clin. Pract. 2007, 61, 748–756.
- Larsen, M.T.; Lyngborg, K.; Pedersen, F.; Corell, P. Predictive factors of maintenance of sinus rhythm after direct current (DC) cardioversion of atrial fibrillation/atrial flutter. Ugeskr. Laeger 2005, 167, 3408–3412.
- Mittal, S.; Ayati, S.; Stein, K.M.; Schwartzman, D.; Cavlovich, D.; Tchou, P.J.; Markowitz, S.M.; Slotwiner, D.J.; Scheiner, M.A.; Lerman, B.B. Transthoracic cardioversion of atrial fibrillation: Comparison of rectilinear biphasic versus damped sine wave monophasic shocks. Circulation 2000, 101, 1282–1287.
- Inácio, J.F.; da Rosa Mdos, S.; Shah, J.; Rosário, J.; Vissoci, J.R.; Manica, A.L.; Rodrigues, C.G. Monophasic and biphasic shock for transthoracic conversion of atrial fibrillation: Systematic review and network meta-analysis. Resuscitation 2016, 100, 66–75.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726.
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032.
- Cui, K.; Huang, W.; Fan, J.; Lei, H. Midregional pro-atrial natriuretic peptide is a superior biomarker to N-terminal pro-B-type natriuretic peptide in the diagnosis of heart failure patients with preserved ejection fraction. Medicine 2018, 97, e12277.
- Okutucu, S.; Gorenek, B. Current Recommendations on Atrial Fibrillation: A Comparison of the Recent European and Canadian Guidelines. Cardiology 2022, 147, 81–89.
- Schnabel, R.B.; Larson, M.G.; Yamamoto, J.F.; Sullivan, L.M.; Pencina, M.J.; Meigs, J.B.; Tofler, G.H.; Selhub, J.; Jacques, P.F.; Wolf, P.A.; et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation 2010, 121, 200–207.
- Fan, J.; Cao, H.; Su, L.; Ling, Z.; Liu, Z.; Lan, X.; Xu, Y.; Chen, W.; Yin, Y. NT-proBNP, but not ANP and C-reactive protein, is predictive of paroxysmal atrial fibrillation in patients undergoing pulmonary vein isolation. J. Interv. Card. Electrophysiol. 2012, 33, 93–100.
- Beck-da-Silva, L.; de Bold, A.; Fraser, M.; Williams, K.; Haddad, H. Brain natriuretic peptide predicts successful cardioversion in patients with atrial fibrillation and maintenance of sinus rhythm. Can. J. Cardiol. 2004, 20, 1245–1248.
- Xu, X.; Tang, Y. Relationship between Brain Natriuretic Peptide and Recurrence of Atrial Fibrillation after Successful Electrical Cardioversion: An Updated Meta-Analysis. Braz. J. Cardiovasc. Surg. 2017, 32, 530–535.
- Ari, H.; Binici, S.; Ari, S.; Akkaya, M.; Koca, V.; Bozat, T.; Gürdoğan, M. The predictive value of plasma brain natriuretic peptide for the recurrence of atrial fibrillation six months after external cardioversion. Turk. Kardiyol. Dern. Ars. 2008, 36, 456–460.
- Meyre, P.B.; Aeschbacher, S.; Blum, S.; Voellmin, G.; Kastner, P.M.; Hennings, E.; Kaufmann, B.A.; Kühne, M.; Osswald, S.; Conen, D. Biomarkers associated with rhythm status after cardioversion in patients with atrial fibrillation. Sci. Rep. 2022, 12, 1680.
- Wozakowska-Kapłon, B.; Bartkowiak, R.; Grabowska, U.; Janiszewska, G. B-type natriuretic peptide level after sinus rhythm restoration in patients with persistent atrial fibrillation—clinical significance. Kardiol. Pol. 2010, 68, 781–786.
- Andersson, J.; Rosenqvist, M.; Tornvall, P.; Boman, K. NT-proBNP predicts maintenance of sinus rhythm after electrical cardioversion. Thromb. Res. 2015, 135, 289–291.
- Kallergis, E.M.; Manios, E.G.; Kanoupakis, E.M.; Mavrakis, H.E.; Goudis, C.A.; Maliaraki, N.E.; Saloustros, I.G.; Milathianaki, M.E.; Chlouverakis, G.I.; Vardas, P.E. Effect of sinus rhythm restoration after electrical cardioversion on apelin and brain natriuretic Peptide prohormone levels in patients with persistent atrial fibrillation. Am. J. Cardiol. 2010, 105, 90–94.
- Tveit, A.; Seljeflot, I.; Grundvold, I.; Abdelnoor, M.; Arnesen, H.; Smith, P. Candesartan, NT-proBNP and recurrence of atrial fibrillation after electrical cardioversion. Int. J. Cardiol. 2009, 131, 234–239.
- Mabuchi, N.; Tsutamoto, T.; Maeda, K.; Kinoshita, M. Plasma cardiac natriuretic peptides as biochemical markers of recurrence of atrial fibrillation in patients with mild congestive heart failure. Jpn. Circ. J. 2000, 64, 765–771.
- Lewicka, E.; Dudzińska-Gehrmann, J.; Dąbrowska-Kugacka, A.; Zagożdżon, P.; Stepnowska, E.; Liżewska, A.; Kozłowski, D.; Raczak, G. Plasma biomarkers as predictors of recurrence of atrial fibrillation. Pol. Arch. Med. Wewn. 2015, 125, 424–433.
- Buccelletti, F.; Gilardi, E.; Marsiliani, D.; Carroccia, A.; Silveri, N.G.; Franceschi, F. Predictive value of NT-proBNP for cardioversion in a new onset atrial fibrillation. Eur. J. Emerg. Med. 2011, 18, 157–161.
- Koniari, I.; Artopoulou, E.; Velissaris, D.; Ainslie, M.; Mplani, V.; Karavasili, G.; Kounis, N.; Tsigkas, G. Biomarkers in the clinical management of patients with atrial fibrillation and heart failure. J. Geriatr. Cardiol. 2021, 18, 908–951.
- Scalise, R.F.M.; De Sarro, R.; Caracciolo, A.; Lauro, R.; Squadrito, F.; Carerj, S.; Bitto, A.; Micari, A.; Bella, G.D.; Costa, F.; et al. Fibrosis after Myocardial Infarction: An Overview on Cellular Processes, Molecular Pathways, Clinical Evaluation and Prognostic Value. Med. Sci. 2021, 9, 16.
- Kawamura, M.; Ito, H.; Onuki, T.; Miyoshi, F.; Watanabe, N.; Asano, T.; Tanno, K.; Kobayashi, Y. Candesartan decreases type III procollagen-N-peptide levels and inflammatory marker levels and maintains sinus rhythm in patients with atrial fibrillation. J. Cardiovasc. Pharmacol. 2010, 55, 511–517.
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jørgensen, S.B.; Steinberg, G.R. GDF15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 2021, 17, 592–607.
- Breit, S.N.; Brown, D.A.; Tsai, V.W. The GDF15-GFRAL Pathway in Health and Metabolic Disease: Friend or Foe? Annu. Rev. Physiol. 2021, 83, 127–151.
- Perrone, M.A.; Aimo, A.; Bernardini, S.; Clerico, A. Inflammageing and Cardiovascular System: Focus on Cardiokines and Cardiac-Specific Biomarkers. Int. J. Mol. Sci. 2023, 24, 844.
- Aulin, J.; Hijazi, Z.; Lindbäck, J.; Alexander, J.H.; Gersh, B.J.; Granger, C.B.; Hanna, M.; Horowitz, J.; Lopes, R.D.; McMurray, J.J.V.; et al. Biomarkers and heart failure events in patients with atrial fibrillation in the ARISTOTLE trial evaluated by a multi-state model. Am. Heart J. 2022, 251, 13–24.
- Hijazi, Z.; Wallentin, L.; Lindbäck, J.; Alexander, J.H.; Connolly, S.J.; Eikelboom, J.W.; Ezekowitz, M.D.; Granger, C.B.; Lopes, R.D.; Pol, T.; et al. Screening of Multiple Biomarkers Associated with Ischemic Stroke in Atrial Fibrillation. J. Am. Heart Assoc. 2020, 9, e018984.
- Charafeddine, K.; Zakka, P.; Bou Dargham, B.; Abdulhai, F.; Zakka, K.; Zouein, F.A.; Refaat, M. Potential Biomarkers in Atrial Fibrillation: Insight into Their Clinical Significance. J. Cardiovasc. Pharmacol. 2021, 78, 184–191.
- Shao, Q.; Liu, H.; Ng, C.Y.; Xu, G.; Liu, E.; Li, G.; Liu, T. Circulating serum levels of growth differentiation factor-15 and neuregulin-1 in patients with paroxysmal non-valvular atrial fibrillation. Int. J. Cardiol. 2014, 172, e311–e313.
- Eddy, A.C.; Trask, A.J. Growth differentiation factor-15 and its role in diabetes and cardiovascular disease. Cytokine Growth Factor Rev. 2021, 57, 11–18.
- Lyngbakken, M.N.; Rønningen, P.S.; Solberg, M.G.; Berge, T.; Brynildsen, J.; Aagaard, E.N.; Kvisvik, B.; Røsjø, H.; Steine, K.; Tveit, A.; et al. Prediction of incident atrial fibrillation with cardiac biomarkers and left atrial volumes. Heart 2023, 109, 356–363.
- Matusik, P.T.; Małecka, B.; Lelakowski, J.; Undas, A. Association of NT-proBNP and GDF-15 with markers of a prothrombotic state in patients with atrial fibrillation off anticoagulation. Clin. Res. Cardiol. 2020, 109, 426–434.
- Cichoń, M.; Mizia-Szubryt, M.; Olszanecka-Glinianowicz, M.; Bożentowicz-Wikarek, M.; Owczarek, A.J.; Michalik, R.; Mizia-Stec, K. Biomarkers of left atrial overload in obese and nonobese patients with atrial fibrillation qualified for electrical cardioversion. Kardiol. Pol. 2021, 79, 269–276.
- Ding, B.; Liu, P.; Zhang, F.; Hui, J.; He, L. Predicting Values of Neutrophil-to-Lymphocyte Ratio (NLR), High-Sensitivity C-Reactive Protein (hs-CRP), and Left Atrial Diameter (LAD) in Patients with Nonvalvular Atrial Fibrillation Recurrence After Radiofrequency Ablation. Med. Sci. Monit. 2022, 28, e934569.
- Li, X.; Peng, S.; Wu, X.; Guan, B.; Tse, G.; Chen, S.; Zhou, G.; Wei, Y.; Gong, C.; Lu, X.; et al. C-reactive protein and atrial fibrillation: Insights from epidemiological and Mendelian randomization studies. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1519–1527.
- Fu, Y.; Pan, Y.; Gao, Y.; Yang, X.; Chen, M. Predictive value of CHA2DS2-VASc score combined with hs-CRP for new-onset atrial fibrillation in elderly patients with acute myocardial infarction. BMC Cardiovasc. Disord. 2021, 21, 175.
- Sinning, C.; Kempf, T.; Schwarzl, M.; Lanfermann, S.; Ojeda, F.; Schnabel, R.B.; Zengin, E.; Wild, P.S.; Lackner, K.J.; Munzel, T.; et al. Biomarkers for characterization of heart failure–Distinction of heart failure with preserved and reduced ejection fraction. Int. J. Cardiol. 2017, 227, 272–277.
- Georgakopoulos, C.; Vlachopoulos, C.; Lazaros, G.; Tousoulis, D. Biomarkers of Atrial Fibrillation in Metabolic Syndrome. Curr. Med. Chem. 2019, 26, 898–908.
- Sun, J.; Xu, J.; Yang, Q. Expression and predictive value of NLRP3 in patients with atrial fibrillation and stroke. Am. J. Transl. Res. 2022, 14, 3104–3112.
- Loricchio, M.L.; Cianfrocca, C.; Pasceri, V.; Bianconi, L.; Auriti, A.; Calo, L.; Lamberti, F.; Castro, A.; Pandozi, C.; Palamara, A.; et al. Relation of C-reactive protein to long-term risk of recurrence of atrial fibrillation after electrical cardioversion. Am. J. Cardiol. 2007, 99, 1421–1424.
- Lombardi, F.; Tundo, F.; Belletti, S.; Mantero, A.; Melzi D’eril, G.V. C-reactive protein but not atrial dysfunction predicts recurrences of atrial fibrillation after cardioversion in patients with preserved left ventricular function. J. Cardiovasc. Med. 2008, 9, 581–588.
- Barassi, A.; Pezzilli, R.; Morselli-Labate, A.M.; Lombardi, F.; Belletti, S.; Dogliotti, G.; Corsi, M.M.; Merlini, G.; Melzi d’Eril, G.V. Serum amyloid a and C-reactive protein independently predict the recurrences of atrial fibrillation after cardioversion in patients with preserved left ventricular function. Can. J. Cardiol. 2012, 28, 537–541.
- Korantzopoulos, P.; Kalantzi, K.; Siogas, K.; Goudevenos, J.A. Long-term prognostic value of baseline C-reactive protein in predicting recurrence of atrial fibrillation after electrical cardioversion. Pacing Clin. Electrophysiol. 2008, 31, 1272–1276.
- Watanabe, E.; Arakawa, T.; Uchiyama, T.; Kodama, I.; Hishida, H. High-sensitivity C-reactive protein is predictive of successful cardioversion for atrial fibrillation and maintenance of sinus rhythm after conversion. Int. J. Cardiol. 2006, 108, 346–353.
- Henningsen, K.M.; Therkelsen, S.K.; Bruunsgaard, H.; Krabbe, K.S.; Pedersen, B.K.; Svendsen, J.H. Prognostic impact of hs-CRP and IL-6 in patients with persistent atrial fibrillation treated with electrical cardioversion. Scand. J. Clin. Lab. Investig. 2009, 69, 425–432.
- Liu, T.; Li, L.; Korantzopoulos, P.; Goudevenos, J.A.; Li, G. Meta-analysis of association between C-reactive protein and immediate success of electrical cardioversion in persistent atrial fibrillation. Am. J. Cardiol. 2008, 101, 1749–1752.
- Yo, C.H.; Lee, S.H.; Chang, S.S.; Lee, M.C.; Lee, C.C. Value of high-sensitivity C-reactive protein assays in predicting atrial fibrillation recurrence: A systematic review and meta-analysis. BMJ Open 2014, 4, e004418.
- Celebi, O.O.; Celebi, S.; Canbay, A.; Ergun, G.; Aydogdu, S.; Diker, E. The effect of sinus rhythm restoration on high-sensitivity C-reactive protein levels and their association with long-term atrial fibrillation recurrence after electrical cardioversion. Cardiology 2011, 118, 168–174.
- Krishnan, A.; Chilton, E.; Raman, J.; Saxena, P.; McFarlane, C.; Trollope, A.F.; Kinobe, R.; Chilton, L. Are Interactions between Epicardial Adipose Tissue, Cardiac Fibroblasts and Cardiac Myocytes Instrumental in Atrial Fibrosis and Atrial Fibrillation? Cells 2021, 10, 2501.
- Berezin, A.E.; Berezin, A.A.; Lichtenauer, M. Myokines and Heart Failure: Challenging Role in Adverse Cardiac Remodeling, Myopathy, and Clinical Outcomes. Dis. Markers 2021, 2021, 6644631.
- Suffee, N.; Moore-Morris, T.; Jagla, B.; Mougenot, N.; Dilanian, G.; Berthet, M.; Proukhnitzky, J.; Le Prince, P.; Tregouet, D.A.; Pucéat, M.; et al. Reactivation of the Epicardium at the Origin of Myocardial Fibro-Fatty Infiltration During the Atrial Cardiomyopathy. Circ. Res. 2020, 126, 1330–1342.
- Fujita, K.; Maeda, N.; Sonoda, M.; Ohashi, K.; Hibuse, T.; Nishizawa, H.; Nishida, M.; Hiuge, A.; Kurata, A.; Kihara, S.; et al. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 863–870.
- Kim, Y.; Lim, J.H.; Kim, E.N.; Hong, Y.A.; Park, H.J.; Chung, S.; Choi, B.S.; Kim, Y.S.; Park, J.Y.; Kim, H.W.; et al. Adiponectin receptor agonist ameliorates cardiac lipotoxicity via enhancing ceramide metabolism in type 2 diabetic mice. Cell Death Dis. 2022, 13, 282.
- Singh, R.; Kaundal, R.K.; Zhao, B.; Bouchareb, R.; Lebeche, D. Resistin induces cardiac fibroblast-myofibroblast differentiation through JAK/STAT3 and JNK/c-Jun signaling. Pharmacol. Res. 2021, 167, 105414.
- Martínez-Martínez, E.; Jurado-López, R.; Valero-Muñoz, M.; Bartolomé, M.V.; Ballesteros, S.; Luaces, M.; Briones, A.M.; López-Andrés, N.; Miana, M.; Cachofeiro, V. Leptin induces cardiac fibrosis through galectin-3, mTOR and oxidative stress: Potential role in obesity. J. Hypertens. 2014, 32, 1104–1114; discussion 1114.
- Rienstra, M.; Sun, J.X.; Lubitz, S.A.; Frankel, D.S.; Vasan, R.S.; Levy, D.; Magnani, J.W.; Sullivan, L.M.; Meigs, J.B.; Ellinor, P.T.; et al. Plasma resistin, adiponectin, and risk of incident atrial fibrillation: The Framingham Offspring Study. Am. Heart J. 2012, 163, 119–124.e1.
- Peller, M.; Kapłon-Cieślicka, A.; Rosiak, M.; Tymińska, A.; Ozierański, K.; Eyileten, C.; Kondracka, A.; Mirowska-Guzel, D.; Opolski, G.; Postuła, M.; et al. Are adipokines associated with atrial fibrillation in type 2 diabetes? Endokrynol. Pol. 2020, 71, 34–41.
- Velliou, M.; Sanidas, E.; Papadopoulos, D.; Iliopoulos, D.; Mantzourani, M.; Toutouzas, K.; Barbetseas, J. Adipokines and atrial fibrillation: The important role of apelin. Hell. J. Cardiol. 2021, 62, 89–91.
- Agbaedeng, T.A.; Zacharia, A.L.; Iroga, P.E.; Rathnasekara, V.M.; Munawar, D.A.; Bursill, C.; Noubiap, J.J. Associations between adipokines and atrial fibrillation: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 853–862.
- Antushevich, H.; Wójcik, M. Review: Apelin in disease. Clin. Chim. Acta 2018, 483, 241–248.
- Luo, H.; Wei, W.; Ye, Z.; Zheng, J.; Xu, R.H. Liquid Biopsy of Methylation Biomarkers in Cell-Free DNA. Trends Mol. Med. 2021, 27, 482–500.
- Grosse, G.M.; Blume, N.; Abu-Fares, O.; Götz, F.; Ernst, J.; Leotescu, A.; Gabriel, M.M.; van Gemmeren, T.; Worthmann, H.; Lichtinghagen, R.; et al. Endogenous Deoxyribonuclease Activity and Cell-Free Deoxyribonucleic Acid in Acute Ischemic Stroke: A Cohort Study. Stroke 2022, 53, 1235–1244.
- Berezina, T.A.; Kopytsya, M.P.; Petyunina, O.V.; Berezin, A.A.; Obradovic, Z.; Schmidbauer, L.; Lichtenauer, M.; Berezin, A.E. Lower Circulating Cell-Free Mitochondrial DNA Is Associated with Heart Failure in Type 2 Diabetes Mellitus Patients. Cardiogenetics 2023, 13, 15–30.
- Li, X.; Hu, R.; Luo, T.; Peng, C.; Gong, L.; Hu, J.; Yang, S.; Li, Q. Serum cell-free DNA and progression of diabetic kidney disease: A prospective study. BMJ Open Diabetes Res. Care 2020, 8, e001078.
- Gianni, C.; Palleschi, M.; Merloni, F.; Di Menna, G.; Sirico, M.; Sarti, S.; Virga, A.; Ulivi, P.; Cecconetto, L.; Mariotti, M.; et al. Cell-Free DNA Fragmentomics: A Promising Biomarker for Diagnosis, Prognosis and Prediction of Response in Breast Cancer. Int. J. Mol. Sci. 2022, 23, 14197.
- Hashimoto, T.; Ueki, S.; Kamide, Y.; Miyabe, Y.; Fukuchi, M.; Yokoyama, Y.; Furukawa, T.; Azuma, N.; Oka, N.; Takeuchi, H.; et al. Increased Circulating Cell-Free DNA in Eosinophilic Granulomatosis with Polyangiitis: Implications for Eosinophil Extracellular Traps and Immunothrombosis. Front. Immunol. 2022, 12, 801897.
- Yamazoe, M.; Sasano, T.; Ihara, K.; Takahashi, K.; Nakamura, W.; Takahashi, N.; Komuro, H.; Hamada, S.; Furukawa, T. Sparsely methylated mitochondrial cell free DNA released from cardiomyocytes contributes to systemic inflammatory response accompanied by atrial fibrillation. Sci. Rep. 2021, 11, 5837.
- Wiersma, M.; van Marion, D.M.S.; Bouman, E.J.; Li, J.; Zhang, D.; Ramos, K.S.; Lanters, E.A.H.; de Groot, N.M.S.; Brundel, B.J.J.M. Cell-Free Circulating Mitochondrial DNA: A Potential Blood-Based Marker for Atrial Fibrillation. Cells 2020, 9, 1159.
- Soltész, B.; Urbancsek, R.; Pös, O.; Hajas, O.; Forgács, I.N.; Szilágyi, E.; Nagy-Baló, E.; Szemes, T.; Csanádi, Z.; Nagy, B. Quantification of peripheral whole blood, cell-free plasma and exosome encapsulated mitochondrial DNA copy numbers in patients with atrial fibrillation. J. Biotechnol. 2019, 299, 66–71.
- Geurts, S.; Mens, M.M.J.; Bos, M.M.; Ikram, M.A.; Ghanbari, M.; Kavousi, M. Circulatory MicroRNAs in Plasma and Atrial Fibrillation in the General Population: The Rotterdam Study. Genes 2021, 13, 11.
- Garcia-Elias, A.; Tajes, M.; Yañez-Bisbe, L.; Enjuanes, C.; Comín-Colet, J.; Serra, S.A.; Fernández-Fernández, J.M.; Aguilar-Agon, K.W.; Reilly, S.; Martí-Almor, J.; et al. Atrial Fibrillation in Heart Failure Is Associated with High Levels of Circulating microRNA-199a-5p and 22-5p and a Defective Regulation of Intracellular Calcium and Cell-to-Cell Communication. Int. J. Mol. Sci. 2021, 22, 10377.
- Chen, H.; Zhang, F.; Zhang, Y.L.; Yang, X.C. Relationship between circulating miRNA-21, atrial fibrosis, and atrial fibrillation in patients with atrial enlargement. Ann. Palliat. Med. 2021, 10, 12742–12749.
- Benito, B.; García-Elías, A.; Ois, Á.; Tajes, M.; Vallès, E.; Ble, M.; Yáñez Bisbe, L.; Giralt-Steinhauer, E.; Rodríguez-Campello, A.; Cladellas Capdevila, M.; et al. Plasma levels of miRNA-1-3p are associated with subclinical atrial fibrillation in patients with cryptogenic stroke. Rev. Esp. Cardiol. (Engl. Ed). 2022, 75, 717–726, (In English and Spanish).
- Park, H.; Park, H.; Park, J. Circulating microRNA-423 attenuates the phosphorylation of calcium handling proteins in atrial fibrillation. Mol. Med. Rep. 2022, 25, 186.
- Arroyo, A.B.; de Los Reyes-García, A.M.; Rivera-Caravaca, J.M.; Valledor, P.; García-Barberá, N.; Roldán, V.; Vicente, V.; Martínez, C.; González-Conejero, R. MiR-146a Regulates Neutrophil Extracellular Trap Formation That Predicts Adverse Cardiovascular Events in Patients with Atrial Fibrillation. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 892–902.
- Da Silva, A.M.G.; de Araújo, J.N.G.; de Oliveira, K.M.; Novaes, A.E.M.; Lopes, M.B.; de Sousa, J.C.V.; Filho, A.A.A.; Luchessi, A.D.; de Rezende, A.A.; Hirata, M.H.; et al. Circulating miRNAs in acute new-onset atrial fibrillation and their target mRNA network. J. Cardiovasc. Electrophysiol. 2018, 29, 1159–1166.
- Zhou, Q.; Maleck, C.; von Ungern-Sternberg, S.N.I.; Neupane, B.; Heinzmann, D.; Marquardt, J.; Duckheim, M.; Scheckenbach, C.; Stimpfle, F.; Gawaz, M.; et al. Circulating MicroRNA-21 Correlates with Left Atrial Low-Voltage Areas and Is Associated with Procedure Outcome in Patients Undergoing Atrial Fibrillation Ablation. Circ. Arrhythm. Electrophysiol. 2018, 11, e006242.
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257.
- Büttner, P.; Bahls, M.; Böger, R.H.; Hindricks, G.; Thiele, H.; Schwedhelm, E.; Kornej, J. Arginine derivatives in atrial fibrillation progression phenotypes. J. Mol. Med. 2020, 98, 999–1008.
- Ramuschkat, M.; Appelbaum, S.; Atzler, D.; Zeller, T.; Bauer, C.; Ojeda, F.M.; Sinning, C.R.; Hoffmann, B.; Lackner, K.J.; Böger, R.H.; et al. ADMA, subclinical changes and atrial fibrillation in the general population. Int. J. Cardiol. 2016, 203, 640–646.
- Horowitz, J.D.; De Caterina, R.; Heresztyn, T.; Alexander, J.H.; Andersson, U.; Lopes, R.D.; Steg, P.G.; Hylek, E.M.; Mohan, P.; Hanna, M.; et al. Asymmetric and Symmetric Dimethylarginine Predict Outcomes in Patients with Atrial Fibrillation: An ARISTOTLE Substudy. J. Am. Coll. Cardiol. 2018, 72, 721–733.
- Goette, A.; Hammwöhner, M.; Bukowska, A.; Scalera, F.; Martens-Lobenhoffer, J.; Dobrev, D.; Ravens, U.; Weinert, S.; Medunjanin, S.; Lendeckel, U.; et al. The impact of rapid atrial pacing on ADMA and endothelial NOS. Int. J. Cardiol. 2012, 154, 141–146.
- Goni, L.; Razquin, C.; Toledo, E.; Guasch-Ferré, M.; Clish, C.B.; Babio, N.; Wittenbecher, C.; Atzeni, A.; Li, J.; Liang, L.; et al. Arginine catabolism metabolites and atrial fibrillation or heart failure risk: 2 case-control studies within the Prevención con Dieta Mediterránea (PREDIMED) trial. Am. J. Clin. Nutr. 2022, 116, 653–662.
- Li, J.; Xia, W.; Feng, W.; Qu, X. Effects of rosuvastatin on serum asymmetric dimethylarginine levels and atrial structural remodeling in atrial fibrillation dogs. Pacing Clin. Electrophysiol. 2012, 35, 456–464.
- Lao, M.C.; Liu, L.J.; Luo, C.F.; Lu, G.H.; Zhai, Y.S.; Chen, X.L.; Gao, X.R. Effect of asymmetrical dimethylarginine for predicting pro-thrombotic risk in atrial fibrillation. Zhonghua Yi Xue Za Zhi 2016, 96, 2059–2063. (In Chinese)
- Chao, T.F.; Lu, T.M.; Lin, Y.J.; Tsao, H.M.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Tuan, T.C.; Hsieh, M.H.; Chen, S.A. Plasma asymmetric dimethylarginine and adverse events in patients with atrial fibrillation referred for coronary angiogram. PLoS ONE 2013, 8, e71675.
- Xia, W.; Qu, X.; Yu, Y.; Zhang, X.; Feng, W.; Song, Y. Asymmetric dimethylarginine concentration and early recurrence of atrial fibrillation after electrical cardioversion. Pacing Clin. Electrophysiol. 2008, 31, 1036–1040.
- Tveit, A.; Arnesen, H.; Smith, P.; Bratseth, V.; Seljeflot, I. L-arginine, asymmetric dimethylarginine and rhythm outcome after electrical cardioversion for atrial fibrillation. Cardiology 2010, 117, 176–180.
- Schnabel, R.B.; Maas, R.; Wang, N.; Yin, X.; Larson, M.G.; Levy, D.; Ellinor, P.T.; Lubitz, S.A.; McManus, D.D.; Magnani, J.W.; et al. Asymmetric dimethylarginine, related arginine derivatives, and incident atrial fibrillation. Am. Heart J. 2016, 176, 100–106.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
3 times
(View History)
Update Date:
25 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




