Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Atrial fibrillation (AF) is associated with atrial remodeling, cardiac dysfunction, and poor clinical outcomes. External direct electrical cardioversion is a well-developed urgent treatment strategy for patients presenting with recent-onset AF.
- atrial fibrillation
- electrical cardioversion
- post-procedural complications
- biomarkers
1. Introduction
Atrial fibrillation (AF) is the most common form of sustained cardiac arrhythmia in the world [1]. The prevalence of AF advances with increasing age. After the age of 80, atrial fibrillation affects 10–17% of the population [2]. The morbidity is increased and mortality rises up to 3.5-fold in men and women [3]. Along with it, AF frequently occurs in patients at higher risk of cardiovascular diseases (CVD) as well as among individuals with known CVD [4]. Unfortunately, AF and CVD exacerbate each other and mutually intervene in prognosis. Indeed, patients with any form of AF demonstrated poorer clinical outcomes if there is concomitant heart failure (HF), coronary artery disease (CAD), type 2 diabetes mellitus (T2DM), obesity, obstructive sleep apnea, chronic kidney disease (CKD), or peripheral artery disease [5,6,7]. Further, the prognosis of patients with AF is poorer than the prognosis of patients with various CVD and comorbid conditions (i.e., HF, CKD) without AF [8]. Multi-morbidity among patients with AF seems to play a pivotal role in natural evolution of primary and secondary AF through direct and indirect impact on the structural and/or electrophysiological abnormalities that occur in AF [9,10]. AF influences electrical remodeling, i.e., shortening of refractoriness due to the high atrial rate itself, resulting in adverse cardiac remodeling [11]. Yet, the persistence of AF itself modulates the risk of cerebrovascular and cardiovascular events [12,13].
The management of AF includes either rhythm restoration or rate control along with comorbidity management, prevention of stroke, and systemic thromboembolism [14]. Synchronized electrical cardioversion can terminate AF. Combined with sedation, it is a safe procedure and highly effective, restoring sinus rhythm in more than 90% [15,16,17]. It is important to detect AF recurrence after successful electrical cardioversion. In this case, early cardioversion could prolong the subsequent duration of sinus rhythm and slow disease progression compared to delayed sinus rhythm restoration [18].
Although the current clinical protocol of initial AF management seems to be very useful in practice [1], it poses challenges in predicting incidental AF and early detection of AF-related complications [19,20]. There are many factors associated with AF recurrence, such as duration of AF, higher age, sex, HF, LA volume index, chronic obstructive pulmonary disease, hypertension, obstructive sleep apnea, hyperthyroidism, smoking, and obesity [21,22].
2. Promoting Factors and Electrophysiological/Anatomical Substrates of AF
Vulnerable substrates for the occurrence, support, and recurrence of AF are electrophysiological and adverse cardiac remodeling, along with structural remodeling, mechanical dysfunction, and trigger activity, which are mediated by genetic ion channel alterations, concomitant cardiovascular (CV) diseases (acute and chronic coronary syndromes, multifocal atherosclerosis, primary and secondary cardiomyopathy, etc.), CV risk factors (hypertension, smoking, obesity, diabetes mellitus, resistance to insulin, and dyslipidemia), and comorbidities (chronic obstructive pulmonary disease, bronchial asthma, chronic kidney disease) (Figure 1). In addition, concomitant hemodynamic factors as a result of numerous diseases (heart failure, atrial cardiomyopathy, pulmonary hypertension, inherited and acquired heart diseases, myocarditis) and conditions (chemotherapy, cardiac toxicity) play a crucial role in secondary structural remodeling of the heart [23,24,25]. These factors contribute to AF occurrence by maintaining afterdepolarization-induced triggered ectopic activity, focal enhanced automaticity, altered function of ion channels, micro-reentrant circus rotor, less dynamic head–tail interactions during re-entry in cardiac tissue, altered ion accumulation on the dynamics of re-entry and electrical heterogeneity [26]. Indeed, head–tail interactions have previously been known to have a causative impact on the dynamics of the reentrant action potential, which plays a pivotal role in inducing AF [26]. To note, intracellular ions, mainly Ca2+ and Na+, accumulated during reentrant arrhythmia through the rapid repetitive cellular excitation may lead to spontaneous termination of re-entry or break-up of the re-entry loop into multiple pathways resulting in AF. Along with it, the initiation and persistence of AF are controlled by both parasympathetic and sympathetic stimulation, as well as hormonal influences, which also seem to play a role in AF recurrence [27]. However, the continuous interaction between electrophysiological, structural, and anatomical remodeling leads to intercellular uncoupling and a pro-fibrotic response, which is crucial for trigger activity, the presence of AF, and the transformation of cardiac dysfunction into HF [28].
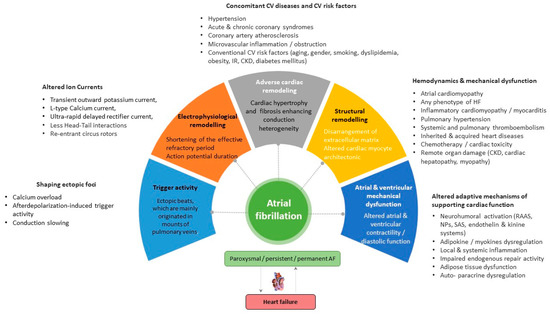
Figure 1. Promoting factors and plausible pathogenetic mechanisms of AF. Abbreviations: AF, atrial fibrillation; CV, cardiovascular; CKD, chronic kidney disease; SAS, sympathoadrenal system; IR, insulin resistance; HF, heart failure; RAAS, renin-angiotensin-aldosterone system.
3. Electrical Cardioversion of AF: Safety and Outcomes
It seems that standard external direct current electrical cardioversion is a well-developed urgent treatment strategy for patients presenting recent-onset AF [1,60]. Numerous retrospective one-center studies and multicenter trials yielded 86–88% efficacy of the approach in restoring sinus rhythm along with 6–10% relapse of AF in a short-term perspective (7–28 days) [61,62,63]. Overall, electrical cardioversion in AF patients who required emergency department transportation was associated with infrequent hospital admission and few mild-to-moderate complications [61]. However, the duration of AF in the majority of studies was less than 48 h in 99% of the patients. Burton JH et al. (2004) [61] observed in a retrospective multicenter study that electrical cardioversion had an 86% success rate, and only 10% of the patients returned to the emergency department within 7 days. Fried AM et al. (2021) [16] reported that the efficacy of this procedure, defined as restoration of sinus rhythm, reached 88% in routine clinical practice, whereas major complications (post-cardioversion stroke, thromboembolic events, jaw thrust maneuver for hypoxia, and overnight observation for hypotension) and predefined minor adverse events (frequently related to general anesthesia, skin burns) were detected in 0.3% and 14%, respectively. In addition, electrical cardioversion was about 2.5 times more effective than conventional pharmacological treatment in restoring sinus rhythm [62,63]. Although there are numerous potential complications of electrical cardioversion (i.e., ventricular fibrillation, thromboembolism due to inadequate anticoagulant therapy, nonsustained ventricular tachycardia, various forms of atrial arrhythmias, bradycardia, transient left bundle branch block, myocardial necrosis, asymptomatic myocardial dysfunction, acute HF, transient hypotension, pulmonary edema, and stroke), they occur less frequently than recurrent AF. Further, 6.4% of patients revisited the emergency department within 30 days, and 4.8% returned with AF or atrial flutter. It is noteworthy that the return visit rate for patients with relapsed AF varies between 3% and 17% [64].
Overall, 30-day all-cause mortality among AF patients undergoing direct-current electrical cardioversion was 0.8% [65]. Data received from the FIRE (Atrial Fibrillation/flutter Italian Registry) registry showed that predictors of unsuccessful electrical cardioversion were onset of AF > 48 h, concomitant HF, increasing age, syncope, transient ischemic attack (TIA)/stroke as well as previous admission to a non-cardiology department [66]. The investigators also found several predictors of in-hospital mortality in this patient population, including age, HF, diabetes mellitus, previous admission to a non-cardiology department, and TIA/stroke [66]. Thus, patients at low risk for thromboembolic complications, including stroke and heart failure, seem to benefit more from electric cardioversion than other individuals with recent-onset AF [67].
Another reason for physicians to use this approach may be cost savings and a short period of emergency department admission [67,68]. Houghton AR et al. (2000) [69] and Boriani G. et al. (2007) [70] did not identify concise hemodynamic predictors of successful external electrical cardioversion or relapses after electrical cardioversion among patients with persistent AF or atrial flutter. However, only two predictors (duration of arrhythmia ≥1 year and previous cardioversion) were found to be powerful for this matter [69,70], whereas, in previous investigations, relapse of AF was associated with reduced left ventricular ejection fraction [71]. Along with it, standard external biphasic direct current electrical cardioversion has better efficacy than monophasic electrical cardioversion (360-J) for restoration of sinus rhythm in AF patients, although dual external monophasic 360-J cardioversion may increase the success rate as a rescue technique after failing standard external direct current cardioversion [72,73]. In this concept, the prediction of plausible cardiovascular events, including relapsed AF, with a biomarker strategy seems promising in patients with recent-onset AF.
4. Predictors for AF Recurrence Following Electrical Cardioversion
Biomarkers reflecting the complex pathophysiological mechanisms underlying AF seem to be an effective tool to predict rhythm status after cardioversion as well as other AF-related complications, which can intervene in mortality, hospital admission, cardiovascular (CV), and non-CV outcomes (Table A1).
4.1. Natriuretic Peptides
Natriuretic peptides (brain natriuretic peptide [BNP], N-terminal pro-B-type natriuretic peptide (NT-proBNP), mid-regional pro-A type natriuretic peptide (MR-proANP)) serve as circulating cardiac biomarkers of biomechanical stress, adverse cardiac remodeling and fluid overload with established diagnostic and predictive values for acute and chronic HF involving any phenotypes [74,75]. Along with it, elevated levels of NPs were strongly associated with all-cause and CV mortality and urgent hospitalization among patients with AF, T2DM, CKD, hypertension, and cardiac hypertrophy [76,77]. Moreover, NT-proBNP and BNP were found to be predictors for AF [78,79,80]. However, it has been suggested that restoration of sinus rhythm through effective electric cardioversion may associate with a reduction in NP concentrations and thereby predict the recurrence of new episodes of arrhythmia. Xu X et al. (2017) [81] observed in a meta-analysis that low levels of BNP and NT-proBNP were associated with the maintenance of sinus rhythm and that the baseline concentrations of both biomarkers may be a predictor of AF recurrence after successful electrical cardioversion. Ari H. et al. (2008) [82] reported that a significant decrease in BNP levels 30 min after electric cardioversion corresponded to six-month maintenance of sinus rhythm in follow-up.
In the GAPP-AF (The gene expression patterns for the prediction of atrial fibrillation) study, Meyre PB (2022) [83] investigated 21 conventional and new circulating biomarkers reflecting inflammation, myocardial injury, cardiac biomechanical stress, and renal dysfunction before and 30 days after electrical cardioversion and evaluated plausible associations of changes in circulating biomarker levels with rhythm status at 30-day follow-up. The patients included in the study had no acute HF, severe valvular disease, or life-limiting active or chronic serious concomitant diseases. The authors found that low levels of NT-proBNP were independently associated with sinus rhythm restoration after electric cardioversion. On the other hand, initial levels of BNP and NT-proBNP in patients with persistent AF without established CVD did not predict long-term sinus rhythm maintenance, although conversion to sinus rhythm related to a significant decrease in circulating BNP but not NT-proBNP level [84]. In contrast, NT-proBNP levels were found to be a predictor of AF recurrence 30 days after successful electric cardioversion among patients with persistent AF and CV risk factors, including hypertension and dyslipidemia [85]. In another study, pre-procedural NT-proBNP levels, but not post-procedural levels of the peptide, independently predicted the relapse of AF after successful electrical cardioversion [86]. These controversial issues perhaps may relate to the presence of concomitant HF. Indeed, in the CAPRAF (Candesartan in the Prevention of Relapsing Atrial Fibrillation) trial, plasma NT-proBNP concentrations measured before electrical cardioversion did not predict cardioversion success nor the relapse of AF in patients without HF [87]. Mabuchi N et al. (2000) [88] noticed that low atrial natriuretic peptide (ANP) and high BNP levels before electric cardioversion were independent predictors of recurrent AF in mild chronic HF patients. Moreover, the authors established that ANP to BNP ratio <0.44 was a significant risk factor for AF recurrence [88]. The BNP level of 700 fmol/mL or higher on day 7 after cardioversion was most predictive for AF recurrence (sensitivity, 78%; specificity, 71%), whereas ANP did not predict the relapse of AF [89]. Buccelletti F. et al. (2011) [90] measured the levels of NT-proBNP in 200 patients admitted to the emergency department due to new-onset AF (<2 weeks) regardless of HF presence. The authors found that NT-proBNP levels of either ≤450 pg/mL or >1800 pg/mL seem to show positive and negative predictive values for cardioversion in rate-control and rhythm-control strategies, respectively. In the range of 450 to 1800 pg/mL, NT-proBNP did not exhibit serious clinical utility [90]. However, it remained unclear whether continuous monitoring of the dynamic changes of NPs after sinus rhythm restoration predicts recurrent AF [91]. Overall, the restoration of sinus rhythm after electric cardioversion in AF patients is associated with a decrease in circulating levels of NPs and low levels of NT-proBNP predicts a sustainable maintains of sinus rhythm in follow-up.
4.2. Biomarkers of Fibrosis
Cardiac fibrosis was found to be closely associated with AF. Circulating biomarkers of fibrosis have already been proposed as a promising tool in its evaluation, but which biomarkers are most appropriate for AF remains unclear [92]. There are a large number of circulating biomarkers, which characterize the accumulation of extracellular matrix components and fibrosis, such as soluble suppressor tumorigenicity-2 (sST2), galectin-3 (Gal-3), procollagen type III N terminal peptide (PIIINP), type I collagen carboxyl telopeptide (ICTP), and fibroblast growth factor 23 (FGF-23) [93].
4.3. Biomarkers of Inflammation
4.3.1. GDF15
GDF15 is a member of the TGF-beta superfamily whose expression is increased in response to biomechanical myocardial stress, inflammation, or ischemia/hypoxia [121]. GDF15 is involved in the regulation of energy homeostasis, thermogenesis, and eating behavior [122]. Yet, GDF15 also exerts anti-inflammatory and anti-proliferative properties, although the underlying molecular mechanisms are still unclear [123]. Elevated GDF15 levels were found in patients with any phenotypes of chronic HF, stroke, AF, and T2DM [124,125,126,127,128]. In the general population, GDF-15 did not show a positive association with the prevalence of AF and the risk of AF occurrence [129]. The suitability of GDF15 for predicting bleeding and/or atrial thrombosis during anticoagulant therapy remains questionable [130]. Clinical evidence for the discriminative value of GDF15 for AF relapse or sinus rhythm maintenance is extremely limited. There is one small study that prospectively included 82 patients with persistent AF [101]. Although log10 serum GDF-15 levels correlated positively with the CHA2DS2-VASc score, there was no close association between GDF-15 levels and sinus rhythm maintenance in patients after successful electric cardioversion [101]. Thus, a discriminative potency of GDF15 for the prediction of clinical efficacy of electrical cardioversion among patients with nonvalvular/valvular AF is not completely understood and requires scrutiny in large clinical studies.
4.3.2. hs-CRP
High-sensitivity C-reactive protein (hs-CRP) is a classic biomarker of inflammation and is a component of the inflammatory profile observed in AF patients. Elevated hs-CRP levels were found in patients with all forms of nonvalvular/valvular AF, regardless of etiology and concomitant comorbidities [131,132]. hs-CRP predicted new-onset AF both in the general population as well as in patients with established cardiovascular or metabolic diseases, such as HF, acute myocardial infarction, T2DM, and metabolic syndrome [133,134,135]. Among patients with AF complicated by systemic thromboembolism, the levels of hs-CRP correlated positively with the CHA2DS2-VASc score [136].
Loricchio ML et al. (2007) [137] investigated plausible predictors for a 1-year risk of AF recurrence after electrical cardioversion. In a Cox regression analysis, the authors found that age, gender, hypertension, T2DM, LVEF, left atrial diameter, use of various antiarrhythmic and antihypertensive (including angiotensin-converting enzyme inhibitors or angiotensin II antagonists) drugs, and statins were not associated with relapsing AF. On the contrary, a low quartile of hs-CRP levels was found to be a strong predictor for this outcome [137]. Lombardi F. et al. (2008) [138] did not find any changes in hs-CRP levels after cardioversion in patients with persistent AF and preserved LVEF, regardless of the post-procedural underlying rhythm. However, NT-proBNP levels decreased significantly in patients who maintained sinus rhythm but not in those who had AF. Yet, baseline hs-CRP levels, but not echocardiographic features of atrial dysfunction and initial NT-proBNP levels, predicted recurrences of AF after cardioversion in patients without pre-existing left ventricular dysfunction [138]. Barassi A et al. (2012) [139] and Korantzopoulos P et al. (2008) [140] confirmed that in patients with persistent AF and preserved LVEF, elevated hs-CRP levels independently predicted subacute AF recurrence rate, whereas NT-proBNP concentrations were not associated with arrhythmic outcome but corresponded to the alterations of cardiac hemodynamics secondary to the presence of AF.
Overall, there is ample strong evidence that elevated preprocedural hs-CRP levels may provide independent predictive information for both successful electrical cardioversion of AF and maintenance of sinus rhythm after conversion [141,142]. The meta-analysis by Liu et al. [143], which included six prospective observational studies (n = 366 patients), showed that peripheral blood CRP levels were higher in patients with failed electric cardioversion than in those with successful restoration of sinus rhythm. In another meta-analysis by Yo CH et al. (2014) [144], a cut-off value of 1.9 mg/L hs-CRP predicted long-term AF recurrence (77% sensitivity, 65% specificity), and more than 3 mg/L predicted short-term AF relapse (73% sensitivity, 71% specificity). Thus, the measurement of CRP levels before the procedure may provide additional prognostic information about the success of sinus rhythm maintenance.
In addition, there are data illustrating that hs-CRP levels measured shortly after electrical cardioversion may be a powerful biomarker for assessing the risk of relapsing AF in the long-term. In particular, Celebi OO et al. (2011) [145] reported that hs-CRP levels measured before and 2 days after electrical cardioversion predicted the 1-year risk of AF relapse. Whether postprocedural hs-CRP provides more information to predict the event than preprocedural hs-CRP is still unclear. However, elevated levels of hs-CRP predicted new-onset AF in the general population and among patients with known cardiovascular diseases, while their role as a marker of sustainable sinus rhythm control places under question.
4.4. Myokines and Adipocytokines
Several interdependent canonic signaling pathways, such as the renin-angiotensin-aldosterone system; TGF-beta pathway, inflammatory chemokines, and cytokines lead to cardiac fibrosis through modulation of oxidative stress and inflammation. However, the direct mechanical stretch may act as a modulator of extracellular matrix remodeling by attenuating the expression of matrix metalloproteinases and their inhibitors. Recently, another signaling pathway has been identified that induces atrial fibrosis via the secretion of adipokines from epicardial, perivascular, and adipose tissue white adipocytes. In addition, recent studies have shown that myokines derived from cardiac and skeletal muscle myocytes may act as adaptive regulators of extracellular matrix remodeling and can attenuate fibrosis [146,147]. Depending on their origin, adipokines and myokines may modulate myofibroblast capabilities, regulate myocyte energy homeostasis and protect against inflammation and fibrosis [148,149]. However, some pro-fibrotic adipokines and myokines can switch a generation of reactive oxygen species to pro-inflammatory and pro-fibrotic stimuli, stimulate myofibroblast differentiation through JAK/STAT3 and JNK/c-Jun signaling, interfere with myocyte electrophysiology, and promote fibrosis in the myocardium [150,151,152]. Numerous previous studies have shown that resistin, apelin, and adiponectin are adipokines associated with several known risk factors for AF and risk of AF [153,154,155,156]. A recent meta-analysis of 34 studies (total number of patients = 31,479) showed that some adipokines, mainly adiponectin, apelin, and resistin, were associated with the risk of AF in the pooled univariate data, whereas the associations were not apparent after multivariate adjustment [157]. However, there is limited evidence of the relation between adipokine and myokine signatures and the risk of AF-related outcomes after electric cardioversion.
4.5. Biomarkers of Oxidative Stress and Endothelial Dysfunction
4.5.1. Cell-Free Circulating DNA
Cell-free circulating DNA (cfcDNA) circulates in two main pools: circular and single-stranded molecules belonging to mitochondrial-derived and nuclear-derived subpopulations, reflecting patterns of DNA methylation and a variety of neutrophil extracellular traps (NETosis) [196,197]. The cfcDNA are determined in subdetectable concentrations under certain physiological conditions, such as physical exercise, whereas increased circulating levels of these fragments are strongly associated with cardiovascular, autoimmune, rheumatic diseases, infections, and malignancy [198,199,200,201,202]. The main causes of cfcDNA production are mitochondrial dysfunction and inflammation, which are powerful drivers of numerous diseases and conditions, including AF [203].
Wiersma M. et al. (2020) [204] reported that levels of cell-free circulating mitochondrial DNA (cfc-mtDNA) were significantly increased in patients with paroxysmal AF undergoing AF treatment, especially in men and in patients with AF recurrence after electrical cardioversion or pulmonary vein isolation. In contrast, cfc-mtDNA levels gradually decreased in patients with persistent AF and long-standing persistent AF. Nevertheless, the authors suggested that cfc-mtDNA levels might be associated with the stage of AF and the risk of AF recurrence after treatment, especially in men. Gender differences in descriptive values of cfc-mtDNA for AF recurrence remain poorly understood but could be related to different comorbidities in both subpopulations. However, another study found no significant changes in mtDNA copy number in the peripheral blood of AF patients of different sex and age [205]. Perhaps, cfcDNA may be included in the multiple biomarker models with the aim of improving their predictive potency in AF patients with low levels of NT-proBNP or in AF patients with malignancy who are treated with chemotherapy.
4.5.2. mRNA
MicroRNAs (miRNAs) participate in atrial remodeling and cardiac fibrosis, contributing to the development of AF [206]. Garcia-Elias A et al. (2021) [207] established that circulating levels of miR-199a-5p and miR-22-5p, which regulate fibrogenic response in the myocardium, were higher in HFrEF patients with AF than in those without AF [207]. MiR-21, which corresponds to atrial fibrosis, is associated with the risk of persistent AF in patients with left atrial enlargement [208]. Interestingly, increased circulating levels of miR-1-3p, which is a myosine gene regulator involved in hypertrophy, myocardial infarction, and cardiac arrhythmogenesis, predicted a high risk of subclinical AF [209]. MiR423, which downregulates fibrosis-related genes such as collagen I, collagen III, fibronectin, and TGF-beta, may be a pivotal factor in stratifying patients at risk of AF occurrence and persistence [210]. Moreover, differences in miRNA expression in the atrial myocardium of men and women may mediate a sex-specific association between circulating miRNAs in plasma and AF at the population level [206]. In addition, there is evidence that epigenetic regulation of NETosis may participate in the development of AF susceptibility. As a matter of fact, miR-146a and miR21 may provide prognostic information in patients with AF [211,212] due to its direct effects on NETosis. In a study by da Silva AMG (2018) [213], miR-21, miR-133b, and miR-499, which are directly involved in the downregulation of apoptosis and fibrosis, were found to be directly involved in AF. However, it remains to be determined whether a signature of mi-Rs can be used to predict poor response to AF treatment, including electrical cardioversion. At the same time, Zhou Q et al. (2018) [213] reported that among 123 miRs affecting cardiac fibrosis, hypertrophy, and inflammation by relation with the SMAD7 and FASLG genes, only miR-21 demonstrated a positive correlation with left atrial low-voltage areas in patients with persistent AF and was associated with post-ablation outcome. Overall, the signature of miRs appears to be a more promising tool for higher AF risk than for outcomes after treatment, although this conjecture needs to be further investigated in the future.
4.5.3. Asymmetric Dimethylarginine
Asymmetric dimethylarginine (ADMA) is a well-known biomarker of endothelial dysfunction that indirectly reflects vascular NO production and exhibits certain predictive information for mortality and morbidity of cardiovascular diseases, including AF [214,215]. In the population-based Gutenberg Health Study (n = 5000), ADMA levels were correlated with left ventricular hypertrophy and AF prevalence [216]. An ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) substudy showed that elevated ADMA levels exhibited a weak association with thromboembolic events in AF patients treated with anticoagulants (warfarin or apixaban) for a median of 1.9 years [217]. The investigators found that tertile groups of ADMA levels were sufficiently associated with death, stroke, and systemic embolism and that incorporating ADMA into CHA2DS2-VASc or HAS-BLED predictive models significantly improved C-indices for those clinical outcomes [217].
There is strong evidence that acute and persistent episodes of AF seem to show elevated ADMA levels accompanied by increased biomarkers of ischemic myocardial injury like cardiac troponins [218]. In the animal AF model, ADMA concentrations in peripheral blood returned to normal within 24 h after successful electrical cardioversion [218]. Along with it, increased circulating levels of ADMA in AF may be reduced by a Mediterranean diet and statin treatment [219,220]. Thus, being closely associated with thrombus formation and CHADS2/CHA2DS2-VASc score, ADMA is a biomarker for predicting pro-thrombotic risk in AF [221,222].
There are controversial data for ADMA’s predictive ability regarding AF recurrence after electrical cardioversion. Xia W et al. (208) [223] reported that elevated ADMA levels were strongly associated with an increased risk of AF relapse within 1 month after electrical cardioversion. On the contrary, Tveit A et al. (2010) [224] found that the levels of ADMA and the L-arginine/ADMA ratio did not exert predictive ability for sinus rhythm maintenance after electrical cardioversion, while the L-arginine/ADMA ratio remained elevated in patients with sinus rhythm for 6 months compared with patients with AF recurrence. The discriminative potency of ADMA may be strongly related to comorbidities. Indeed, serum ADMA levels were not associated with incident AF in the general population after adjusting for other cardiovascular risk factors [224]. Overall, the utility of ADMA refines clinical risk stratification in AF regardless of the treatment strategy.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11051452
This entry is offline, you can click here to edit this entry!
