Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Radu Ciprian Racovita | -- | 3349 | 2023-05-23 12:09:29 | | | |
| 2 | Rita Xu | -4 word(s) | 3345 | 2023-05-24 03:36:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ciuca, M.D.; Racovita, R.C. Health Benefits of Curcumin. Encyclopedia. Available online: https://encyclopedia.pub/entry/44710 (accessed on 01 March 2026).
Ciuca MD, Racovita RC. Health Benefits of Curcumin. Encyclopedia. Available at: https://encyclopedia.pub/entry/44710. Accessed March 01, 2026.
Ciuca, Maria D., Radu C. Racovita. "Health Benefits of Curcumin" Encyclopedia, https://encyclopedia.pub/entry/44710 (accessed March 01, 2026).
Ciuca, M.D., & Racovita, R.C. (2023, May 23). Health Benefits of Curcumin. In Encyclopedia. https://encyclopedia.pub/entry/44710
Ciuca, Maria D. and Radu C. Racovita. "Health Benefits of Curcumin." Encyclopedia. Web. 23 May, 2023.
Copy Citation
Curcumin is the principal curcuminoid found in the rhizomes of turmeric. Due to its therapeutic action against cancer, depression, diabetes, some bacteria, and oxidative stress, it has been used widely in medicine since ancient times.
curcumin
extraction methods
microemulsion
health benefits
1. Introduction
Turmeric (also known as Arab globe Curcuma, Haridra in Sanskrit, Chinese yellow ginger Jianghuang, Japanese Kyoo, or Ukon [1]) is a rhizomatous perennial plant [2] of the Zingiberaceae family [3] widely cultivated annually in tropical and subtropical regions of the world [4][5][6], the largest global producer of turmeric being India [1][4]. Curcuma longa is the most commonly recognized species within the genus Curcuma. However, other species exist within this genus, namely Curcuma amada, Curcuma zedoaria, Curcuma aromatica, and Curcuma rakatakanta [7]. At maturity, turmeric can reach a height of one meter. Its leaves are long, rectangular, and placed in two rows from which leaf sheaths and false stems, petioles, and leaf blades are formed. With a length between 2.5–7 cm and a diameter of approximately 2.5 cm, the rhizomes are roughly segmented and have a specific fragrance. Depending on the shape, two types of rhizomes are distinguished: the primary rhizomes that have a pear shape and are also known as bulbs, and the secondary rhizomes that have a cylindrical shape. The flowers are 10–15 cm long, have a matte yellow color, and are arranged in spike-like structures [8]. Since ancient times, this yellow spice has been used in the Asian medicine of Ayurveda (which emerged about 5000 years ago), Siddha, Atharveda (2000 years ago), Unani medicine, and Chinese medicine [9][10]. During the 14th century, the Western world was introduced to the golden spice by early European explorers of the Asian continent [11]. Further, it was also used in religious cult rituals [3][12]. It is also a natural spice used as a colorant in curry, mustard [13], salads, pasta, yoghurt, cheese, and baked goods [14]. It is referred to as Natural Yellow 3 as an ecological dye and has been given the E designation E100 when used as a food coloring ingredient [15]. Turmeric is made up of 69.4% carbohydrates, 13.1% moisture, 6.3% protein, 5.1% fat, and 3.5% minerals. The essential oil, with a proportion of 5.8%, contains, among other compounds, curcumin (C21H20O6) [12], a significant curcuminoid found in the rhizomes of turmeric that gives it its yellow color [16][17]. The other curcuminoids are demethoxycurcumin (C20H18O5) and bisdemethoxycurcumin (C19H16O4), which are present in lower concentrations (Figure 1) [18]. Depending on the geographical cultivation area, curcuminoids amount to around 30–150 mg/g of the turmeric rhizome [19]. Curcumin is biosynthesized from two molecules of feruloyl-CoA and one molecule of malonyl-CoA via two enzymatic conversions catalyzed by diketide-CoA synthase (DCS) and curcumin synthase. (CURS). DCS and CURS both belong to the family of type III polyketide synthases [20].
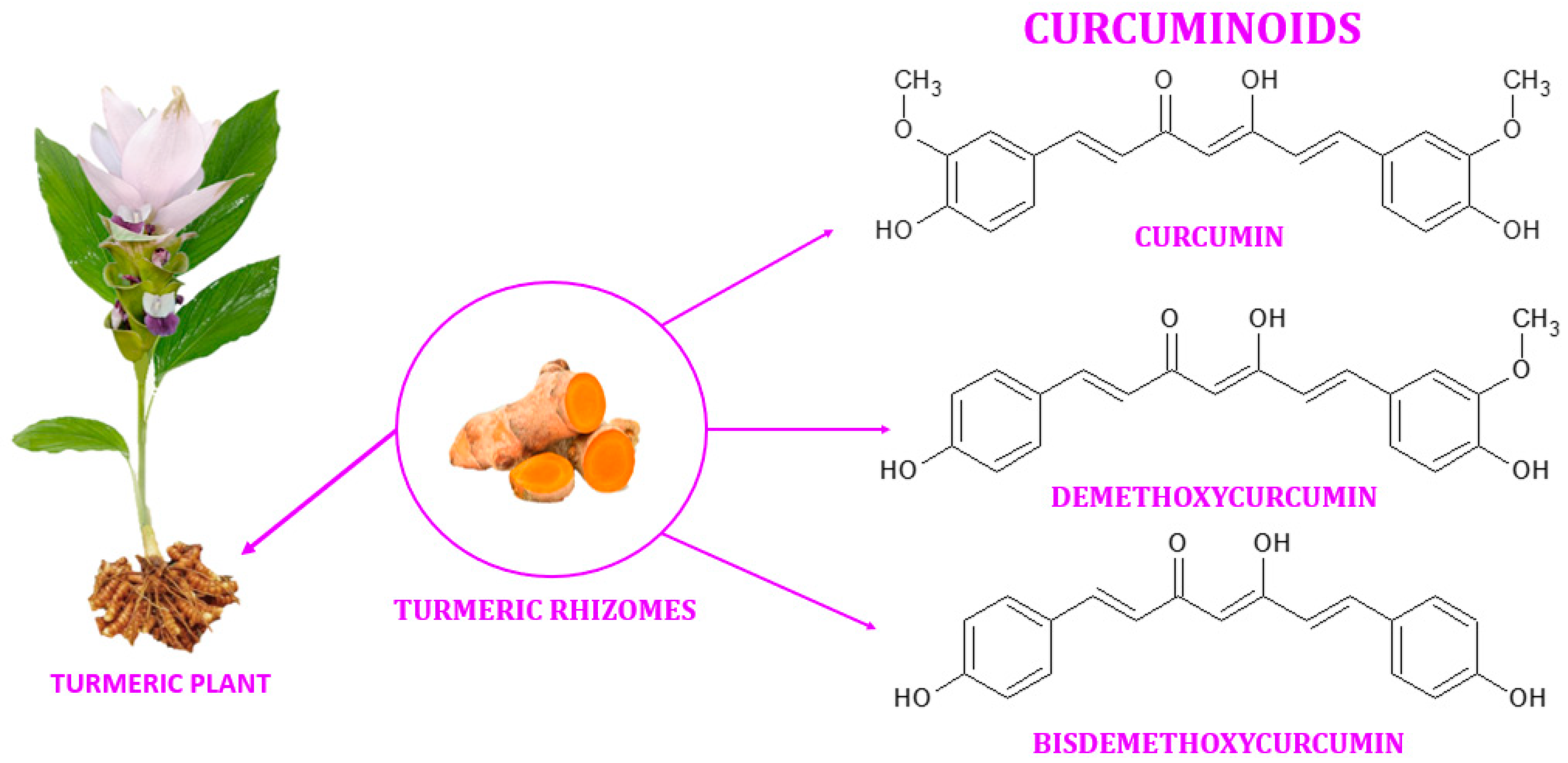
Figure 1. Curcuminoids found in the rhizomes of turmeric.
Curcumin derivatives have antioxidant, anti-diabetic, anti-cancer, anti-allergic, anticoagulant, anti-fungal, and anti-infertility properties [2][5][21][22][23]. However, the bioavailability of this bioactive compound is limited by its low solubility in water [24]. To increase its bioavailability, numerous researchers have tried multiple times and by different methods the encapsulation in micro and nano emulsions [25].
2. Chemistry of Curcumin
Curcumin, also known as diferuloylmethane [26], is a polyphenol from the group of diarylheptanoids with the IUPAC name (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, a molecular weight of 368.39 g/mol, and the chemical formula C21H20O6 [16][27]. This compound has a symmetrical molecule that exhibits in its structure two phenyl rings substituted with a methoxy group in the ortho position and a hydroxy group in the para position. The two aromatic rings are interconnected by a seven-carbon chain consisting of an alpha-beta unsaturated diketone moiety [22], which makes it both a polyphenol and a polyketide at the same time [28][29][30][31]. The diketo group exhibits keto-enol tautomerism: in the solid state, it was shown to exist 100% in the enol form [32] and also predominate as an enol in alkaline aqueous solutions, while the keto form is predominant in acidic and neutral solutions, the enol representing only about 30% of all curcumin in the latter (Figure 2) [10][33][34].
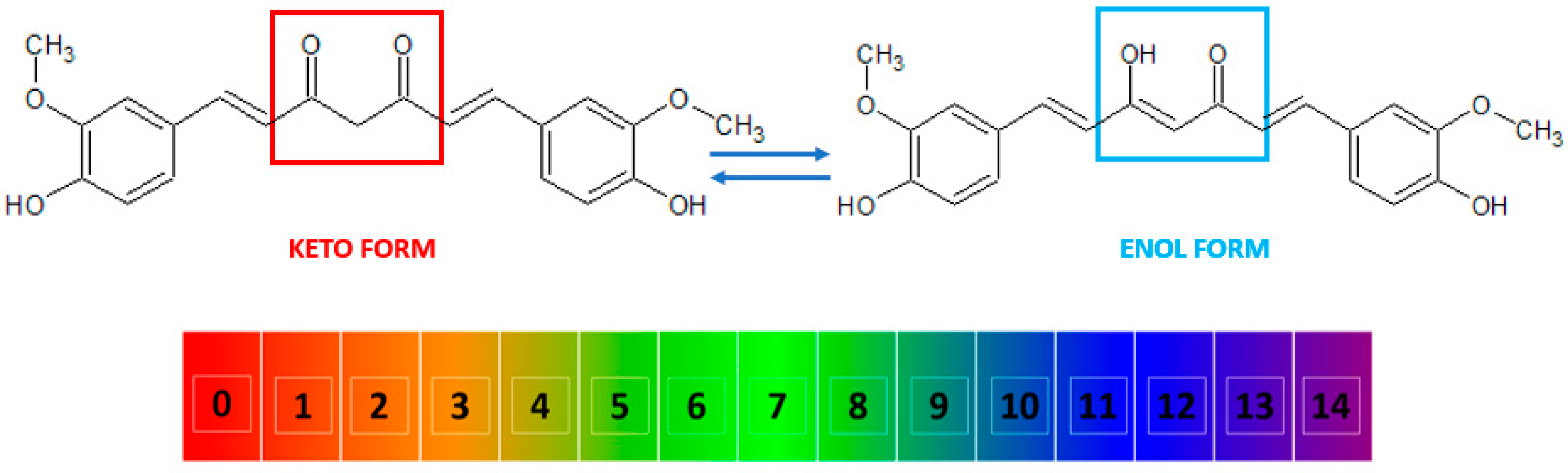
Figure 2. The keto and enol tautomers of curcumin and their reversible inter-conversion in aqueous medium as a function of pH. The numbers indicate the pH scale from 0 to 14. At low pH, the keto form (marked in red) is predominant, while at high pH, the enol form (marked in blue) predominates.
Curcumin is characterized by the presence of three ionizable protons in aqueous solution, namely the enolic proton with a pKa value of approximately 8.5 and two phenolic protons with pKa values ranging from 10 to 10.5 [11]. The essential chemical reactions associated with the biological activity of curcumin are electron-donating reactions that lead to the oxidation of the compound, reversible and irreversible nucleophilic addition reactions, hydrolysis, and enzymatic reactions [12]. The degradation products of curcumin include ferulic acid, feruloyl methane, vanillin, and dicyclopentadiene [35]. Curcumin has a yellow color, a melting point of 183 °C [10], and a partition coefficient of logP ≈ 3, which is indicative that curcumin is a rather hydrophobic compound [4][12]. However, it is soluble in polar solvents such as dimethyl sulfoxide (DMSO), methanol, ethanol, and acetone [12].
3. Health Benefits of Curcumin
After ingestion, curcumin is metabolized mainly in the liver, large intestine, and intestinal microbiota [36][37][38]. The metabolism process is carried out by enzymes and occurs in two phases: the first phase involves the reduction of the four double bonds of the heptadiene-3,5-dione [39][40] structure in heterocytes and hepatocytes by a reductase to dihydrocurcumin, tetrahydrocurcumin, hexahydrocurcumin, and octahydrocurcumin [36][37][38]. In the second phase, unmetabolized curcumin and the resulting metabolites from the first phase are transformed into glucuronidic and sulphate-O-conjugated metabolites because glucoronidases and sulphatransferases can conjugate the glucuronic acid and sulphate molecules to hydroxyl groups [38][39]. Numerous clinical studies have suggested curcumin has antioxidant, antidiabetic, antibacterial, antidepressant, and anticancer properties and beneficial effects on diabetes mellitus and other disorders [41].
3.1. Antioxidant Properties
Reactive oxygen species (ROS) are oxygen-derived molecules with short lifetimes and high reactivity due to the presence of unpaired valence electrons [42]. Both endogenous (such as mitochondria, peroxisomes, endoplasmic reticulum, etc.) and exogenous (such as pollution, alcohol, tobacco smoke, heavy metals, medications, etc.) sources contribute to the production of free radicals [43]. The primary source of ROS production is the mitochondria, where adenosine triphosphate (ATP) production by oxidative phosphorylation takes place with the reduction of molecular oxygen to H2O in the electron transport chain [44]. Although they are by-products, they play an essential role in cell signaling and homeostasis by regulating cell proliferation, differentiation, and survival [45].
The concept of oxidative stress was defined as “an imbalance between oxidants and antioxidants in favour of the oxidants, leading to disruption of redox signalling and control and/or molecular damage” in “Oxidative stress: a concept in redox biology and medicine” [46]. The best-known ROS are peroxides, superoxides, hydroxyl radicals, ozone, and nascent oxygen molecules. In high concentrations, these species have the ability to oxidize nucleic acids, lipids, and proteins. Antioxidants scavenge ROS by donating their electrons to the undervalent ROS and neutralizing it.
Curcumin can reduce mitochondrial oxidative stress by increasing the effects of superoxide dismutase, glutathione, and catalase. The three redox sites of curcumin can undergo oxidation and hydrogen abstraction, resulting in the formation of phenoxy radicals and stabilization against the enol structure [47].
Curcumin at 645 mg/24 h for 67 days boosted total antioxidant capacity and lowered malondialdehyde levels, according to a meta-analysis of 308 individuals, 60% of whom were women [48]. In an in vivo investigation, the cardioprotective and hepatoprotective properties of curcumin against hepatotoxicity and cardiotoxicity generated by doxorubicin (DOX), a medication often used in tumour disorders, were studied. To elicit unfavourable effects, mice were given a single dose of DOX (20 mg/kg) intraperitoneally. Curcumin (100 mg/kg oral) was administered to them 10 days before and 5 days after DOX treatment. Due to its anti-inflammatory and antioxidant properties, curcumin reduces lipid peroxidation, inhibits immunoexpression of nuclear factor-kB (NF-kB), inhibits tumour necrosis factor-alpha (TNF-α) and inducible nitric oxide synthase (iNOS), and lowers circular inflammatory interferon-gamma (IFN-γ) levels. As a result, it was revealed that curcumin may be administered as an adjuvant since it can serve as a protective antioxidant-based method against DOX-associated cardiotoxicity and hepatotoxicity [49].
3.2. Antibacterial Properties
Due to their both beneficial and harmful impacts, the existence of microbes is well known in the human population. When the symbiotic relationship between microbes exceeds a certain threshold, they can produce pathogenic illnesses and diseases that can be fatal to the human body. Antimicrobial agents, particularly antibiotics, are used to combat bacterial infections [50]. Bacteria have evolved and developed resistance to antibiotics throughout time as a result of their overuse or inadequate usage [51]. Antibacterial antibiotic resistance is one of the leading causes of treatment failure. Traditional medicines have been shown to have a considerable impact on pathogen therapy by regulating a variety of physiological systems [52].
The antibacterial activities of curcumin were initially documented by Schraufstatter and colleagues in 1949 [53]. Despite its poor solubility in water, limited bioavailability, and pharmacokinetic profile, modern investigations have shown high antibacterial activity for curcumin [54]. The antibacterial mechanism of action of curcumin involves damage to the cell membrane, interference with cellular processes by targeting DNA and proteins, and inhibition of bacterial quorum sensing [55].
Maleki Dizaj et al. [56] studied the antibacterial effects of curcumin nanocrystals against Posphyromones gingivalis (P. gingivalis) isolated from the gingival crevicular fluid of Iranian patients with implant failure. The disc diffusion method was utilized to test bacterial sensitivity to curcumin nanoparticles, and the broth microdilution method was employed to estimate the nanoparticles’ minimal inhibitory concentrations (MICs) against P. gingivalis. The bacteria were sensitive at 50, 25, 12.5, and 6 µg/mL concentrations. At 50 µg/mL, curcumin nanocrystals demonstrated the most significant inhibitory zone. According to the MIC test, curcumin nanoparticles inhibited P. gingivalis growth at 6.25 µg/mL. Curcumin nanoparticles had bactericidal activity against P. gingivalis at 12.5 µg/mL in the MBC test (minimum bactericidal concentration) [56]. In another study, Snetkov et al. evaluated the antibacterial properties of polymer nanofibers based on hyaluronic acid and curcumin against multidrug resistant ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebeciella pneumoniae, Acinetobacter baumannii, Pseudomona aeruginosa, and Enterobacter species). The HA-curcumin stable complex showed high antibacterial activity against Gram-positive and Gram-negative bacteria. The minimum inhibitory concentrations for Gram-positive bacteria reached 90 µg/mL, while those for Gram-negative bacteria ranged from 90–960 µg/mL [57].
3.3. Antidepressant Properties
Depression is a psychiatric condition that affects about 300 million people annually, resulting in over 800,000 deaths. This illness costs Europe EUR 92 billion annually, with a substantial proportion of the financial burden imposed by the productivity loss of affected persons [58]. Fatigue, anhedonia, sleep difficulties, and self-destructive behaviour are the most common symptoms [59]. During the SARS-CoV-2 epidemic, prevention efforts led to social isolation, economic instability, and worries about losing loved ones, resulting in both physical and mental health deterioration [60][61][62][63]. Some prevalent antidepressant medicines have been linked to a number of negative side effects, prompting their early withdrawal [64]. These effects include constipation, dry mouth, sleep difficulties, cardiotoxicity, neurotoxicity, orthostatic hypotension, and sexual dysfunction [65].
Disruptions in the intestinal microbiota and disruptions of the gut-brain axis are significant factors in the development of depression. Depression episodes may be induced by a reduction in neurotrophic factors derived from the brain and a change in the efficiency of neurotransmitters due to changes in the permeability of the intestinal barrier caused by changes in the variety of the intestinal microbiota [59]. Since the intestine and liver are the primary sites of curcumin metabolism, it may be acting on the gut microbiota to reduce intestinal inflammation and act as a neuroprotective agent because neuroinflammation is likely a factor in many psychiatric disorders [66].
Inhibition of the monoamine oxidase (MAO) enzyme, modulation of the levels of different neurotransmitters (norepinephrine, dopamine, and serotonin), promotion of hippocampal neurogenesis, and its use as an anti-inflammatory agent are just a few of the details about the phenomenon of curcumin that support its use in treating major depression [67]. A 12-week research study was carried out by Kanchanatawan et al. to assess the benefits of adjunctive curcumin for treating major depressive disorder (MDD). The 65 MDD patients in the double-blind, placebo-controlled experiment were randomized to receive either adjunctive curcumin or a placebo. They found that adding curcumin to the medication considerably improved the Montgomery–Asberg depression rating scale (MADRS) score; this impact was significant 12 weeks after commencing treatment, and the benefit was maintained four weeks after curcumin withdrawal. With substantial differences between curcumin and placebo at weeks 12 and 16, curcumin was considerably more effective than placebo at alleviating depressive symptoms in severe depression. A greater effectiveness of curcumin was reported in males compared to females [68]. In mice, Qi et al. studied the antidepressant effects of curcumin administered nasally. They created a thermosensitive hydrogel that contained curcumin. After administering it, they noticed a rise in the levels of norepinephrine, dopamine, and 5-hydroxytryptamine, as well as its metabolites, in the striatum and hippocampus, proving that curcumin has antidepressant effects [69].
3.4. Diabetes Mellitus
Diabetes mellitus is a metabolic condition defined by persistent hyperglycemia in which insulin hormone activity on cell receptors is absent or inefficient, resulting in a substantial rise in blood glucose [70]. The types of diabetes mellitus according to the classification by the American Diabetes Association are: (I) type 1 diabetes, with autoimmune β-cell destruction and absolute insulin shortage; (II) type 2 diabetes (90–95% of diabetes cases), which is characterized by insulin resistance, followed by insulin hypersecretion in β cells of Langerhans, preventing the body from using its own insulin [71][72]; (III) gestational diabetes mellitus, which affects 7% of pregnancies in the second or third trimester; (IV) other kinds of diabetes, such as monogenic diabetes syndromes, exocrine pancreas illnesses, and drug- or chemical-induced diabetes, which account for 5% of diabetes patients [72]. Obesity and overweight are two main risk factors for diabetes caused by lifestyle choices [73]. Hyperglycemia, glucotoxicity, and oxidative stress are all linked to diabetes. Together, these things make advanced glycation endproducts (AGEs) and lipid peroxidation products, which increase ROS production in the cell [74]. Many organs, such as the eyes, nerves, heart, kidneys, and blood vessels, are susceptible to long-term damage, dysfunction, and failure due to diabetes [75].
According to studies conducted on diabetic patients, curcuminoids enhance insulin resistance, decrease levels of leptin, resistin, interleukin (IL)-6 IL-1β, and tumor necrosis factor-α, increase the release of adiponectin, and decrease glucose and insulin levels. Consequently, these compounds may influence glucose homeostasis and the consequences of diabetes [76]. A study conducted on male Long-Evans Tokushima Fatty Otsuka rats and Long-Evans Tokushima Otsuka rats (LETO controls) revealed that combining exercise with a curcumin-based diet (5 g/kg) improves glucose homeostasis and lipid profiles, promotes weight loss, and reduces levels of inflammatory response indicators IL 6, TNF, IL10, and ER stress marker levels. Using a Morris water maze test, they also assessed cognitive performance and discovered that curcumin boosts memory retention and escape latency [77]. In another study, the effect of curcumin on alpha-amylase was investigated in rats administered with 10, 20, 40, and 80 mg/kg of curcumin for 30 days. Blood glucose levels were monitored once every three days, while insulin levels were assessed on the first, middle, and final days of the trial. According to the data, curcumin inhibits alpha-amylase with an IC50 equal to 51.32 µM and an inhibition constant Ki of 20.17 µM, as well as decreasing glucose and insulin levels [78]. AMPK (5′-adenosine monophosphate-activated protein kinase) may alter metabolic phenotypes from fat synthesis to fat oxidation, reducing hepatic glucose production and increasing muscle glucose absorption. Lu et al. revealed that curcumin controls hepatic oxidative stress (thiobarbituric acid reactive substances, superoxide dismutases, glutathione, and catalase) and activates AMPK in gestational diabetic mice [79].
3.5. Anticancer Properties
Cancer is the leading cause of mortality globally, with approximately 10 million deaths recorded in 2020 and an estimated 28.4 million new cases expected by 2040 [80]. The lack of physical activity, obesity, tobacco use, alcohol use [81][82], exposure to ultraviolet radiation [83], and exposure to an environment polluted with nitrogen dioxide [84], sulfur dioxide, carbon monoxide [85][86], suspended particles [87], and persistent organic pollutants (POP) such as polycyclic aromatic hydrocarbons [88][89], all contribute to the development of various types of cancer, the most prevalent of which are breast cancer [90], colorectal cancer [91][92], lung cancer [85], prostate cancer, and gastric cancer [93].
In order to eradicate the disease or slow down its rate of spreading, several therapies can be used, including surgery, radiotherapy, chemotherapy, and immunotherapy [15][80][94]. Although there are a variety of treatments, they have side effects and limited effectiveness [80]. There are numerous studies highlighting the anticancer effects of curcumin, which come from the fact that curcumin regulates cell signalling pathways such as STAT3, NF-kB, activated Egr-1, AP-1, P53 [95], Wnt/β-catenin, PI3K/Akt, JAK/STAT, and MAPK [96].
Li et al. [97] explored the effect of curcumin against colorectal cancer in an in vitro investigation. The results revealed that curcumin was not hazardous to epithelial cells of the colonic mucosa, that it reduced colon cancer cell growth, and that it promoted apoptosis via a P53-mediated mechanism, upregulation of pro-apoptotic proteins such as Bax, and cell cycle arrest during S phase by inhibiting cell-cycle-related protein production, phosphorylation of Rb signalling pathway proteins, and E2F family transcription factors [97]. The anticancer effects of curcumin on multicellular breast cancer spheroids were studied by Kamalabadi et al. Breast cancer stem cells (BCSCs) were evaluated in vitro using spheroids. Three-dimensional (3D) culture methods were chosen because they better maintained the biological properties of the original malignancies than typical two-dimensional (2D) monolayer cultures. Curcumin induces death in breast cancer cells (MCF-3) in 3D culture. This bioactive substance drastically reduced MCF-3 cell survival in 2D and 3D systems in a dose- and time-dependent manner; nevertheless, curcumin’s impact has been limited due to its low bioavailability [98]. In a recent study, Bolat et al. developed curcumin-loaded emulsions that increase the bioactive compound’s solubility by up to 10,000 times and extend the therapeutic effect against hepatocellular carcinoma. These loaded emulsions were evaluated in vitro on the androgen-dependent prostate cancer cell line LNCaP, and a substantial drop in androgen receptor gene expression levels was observed after treatment [99].
3.6. Side Effects and Potential Toxicity
The United States Food and Drug Administration (FDA) has acknowledged the general safety of curcumin, with its LD50 value determined in rats, i.e., the lethal dosage expected to cause the death of 50% of target species subjects [100], being well above 2 g/kg body weight [101]. The Joint Food and Agriculture Organization/World Health Organization (FAO/WHO) expert committee on food additives has established an acceptable daily intake (ADI) range of 0.1–3 mg/kg body weight for this substance [102][103].
Chanaken et al. conducted an in vivo study wherein they observed that the administration of curcumin at high doses and for prolonged periods (100 mg/kg/90 days) resulted in an imbalance in rats. This imbalance was characterized by an overproduction of reactive oxygen species (ROS), an increased production of the pro-oxidant cytokine IL6, and a decrease in antioxidant enzymes. These changes ultimately led to oxidative stress-mediated liver injury and inflammatory disorders [104].
In another study, fifteen people diagnosed with advanced colorectal cancer ingested curcumin-compatible capsules, with dosages ranging from 0.45 to 3.6 g per day, for a maximum duration of four months. Gastrointestinal adverse effects were reported by the patients. Two patients who were administered 0.45 g and 3.6 g of curcumin per day, respectively, experienced diarrhea (grades 1–2). A single patient who ingested a daily dose of 0.9 g of curcumin reported experiencing nausea. Elevated serum alkaline phosphatase levels were detected in four patients during the blood test [105].
Another case report was about a male patient, aged 74 years and afflicted with various medical conditions, who was admitted to the emergency department due to the presence of a sizable hematoma in his left thigh. The subject initiated a daily intake of 1 g of curcumin, in addition to the prescribed medications for his illnesses, one week prior. The researchers suggested that an elevated dosage of curcumin could potentially be linked to heightened antiaggregant and anticoagulant effects [106].
It has been found that curcumin is a potent chelator of iron [107]. According to in vivo research, adding curcumin to mice’s meals caused them to develop the symptoms of iron deficiency anaemia, including a drop in serum iron levels, a reduction in transferrin saturation, and the development of hypochromic red blood cells. Additionally, bone marrow and spleen iron levels may be decreased by curcumin [108].
According to the literature, a few other adverse effects of curcumin have been reported, indicating that the incidence of headache, skin rash, and yellow stool is dose-dependent and was observed in 7 of the 24 participants [102][109]. Additional adverse effects comprise gastrointestinal irritation, abdominal discomfort, hypersensitive dermatological responses [110], and triggered uterine contractions in pregnancy [111].
References
- Jyotirmayee, B.; Mahalik, G. A review on selected pharmacological activities of Curcuma longa L. Int. J. Food Prop. 2022, 25, 1377–1398.
- Akter, J.; Islam, M.Z.; Takara, K.; Hossain, M.A.; Gima, S.; Hou, D.X. Plant growth inhibitors in turmeric (Curcuma longa) and their effects on Bidens pilosa. Weed Biol. Manage. 2018, 18, 136–145.
- Tavares, K.; Kirk, E.; Motomura-Wages, S.; Calpito, J.; Bingham, J.-P.; Ahmad, A.A.; Flanagan, K.; Uyeda, J.; Kantar, M.B.; Radovich, T.J.K. Genotypic and Environmental Influence on Fresh Rhizome Yield of Turmeric (Curcuma longa L.). Agronomy 2022, 12, 2703.
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112.
- Sontsa-Donhoung, A.M.; Bahdjolbe, M.; Hawaou; Nwaga, D. Selecting Endophytes for Rhizome Production, Curcumin Content, Biocontrol Potential, and Antioxidant Activities of Turmeric (Curcuma longa). Biomed. Res. Int. 2022, 2022, 8321734.
- Park, J.; Do, S.; Lee, M.; Ha, S.; Lee, K.-G. Preparation of turmeric powder with various extraction and drying methods. Chem. Biol. Technol. Agric. 2022, 9, 39.
- Jiang, T.; Ghosh, R.; Charcosset, C. Extraction, purification and applications of curcumin from plant materials-A comprehensive review. Trends Food Sci. Technol. 2021, 112, 419–430.
- Kumar, A.; Singh, A.K.; Kaushik, M.S.; Mishra, S.K.; Raj, P.; Singh, P.K.; Pandey, K.D. Interaction of turmeric (Curcuma longa L.) with beneficial microbes: A review. 3 Biotech 2017, 7, 357.
- Srivastava, B.B.L.; Ripanda, A.S.; Mwanga, H.M. Ethnomedicinal, Phytochemistry and Antiviral Potential of Turmeric (Curcuma longa). Compounds 2022, 2, 200–221.
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocinska, K.; Zielinska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021.
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell Mol. Life Sci. 2008, 65, 1631–1652.
- Rathore, M.M.S.; Sharma, P.; Devi, S.; Nagar, J.C.; Khalid, M. Curcumin: A Review for Health Benefits. Int. J. Res. Rev. 2020, 7, 273–290.
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637.
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. 2018, 32, 985–995.
- Urosevic, M.; Nikolic, L.; Gajic, I.; Nikolic, V.; Dinic, A.; Miljkovic, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135.
- El-Menyawy, E.M.; Raslan, M.; Zedan, I.T. Physical characteristics of naturally isolated high-purity curcumin and its application in photosensor appliances. J. Mol. Struct. 2023, 1274, 134445.
- Wahyudi, L.D.; Yu, S.H.; Cho, M.K. The effect of curcumin on the cadmium-induced mitochondrial apoptosis pathway by metallothionein 2A regulation. Life Sci. 2022, 310, 121076.
- Prakhongsil, S.S.P.; Pewlong, W.; Picha, R.; Thamrongsiripak, N. Turmeric Sprout Inhibition and Rhizomes Quality and Post-Harvest Treatment with Gamma Irradiation. Sci. Technol. Asia 2022, 27, 234–241.
- Le-Tan, H.; Fauster, T.; Vladic, J.; Gerhardt, T.; Haas, K.; Jaeger, H. Application of Emerging Cell Disintegration Techniques for the Accelerated Recovery of Curcuminoids from Curcuma longa. Appl. Sci. 2021, 11, 8238.
- Sureshbabu, A.; Smirnova, E.; Karthikeyan, A.; Moniruzzaman, M.; Kalaiselvi, S.; Nam, K.; Goff, G.L.; Min, T. The impact of curcumin on livestock and poultry animal’s performance and management of insect pests. Front. Vet. Sci. 2023, 10, 1048067.
- Lima, M.S.D.; Resende, O.; PlÁCido, G.R.; Silva, J.A.G.E.; CÉLia, J.A.; Caliari, M.; Oliveira, D.E.C.D.; Correia, J.S.; Silva, M.A.P.D. Effects of drying temperature on the bioactive and technological properties of turmeric (Curcuma longa L.) flour. Food Sci. Technol. 2022, 42, e76122.
- Salem, M.A.; El-Shiekh, R.A.; Fernie, A.R.; Alseekh, S.; Zayed, A. Metabolomics-based profiling for quality assessment and revealing the impact of drying of Turmeric (Curcuma longa L.). Sci. Rep. 2022, 12, 10288.
- Orellana-Paucar, A.M.; Machado-Orellana, M.G. Pharmacological Profile, Bioactivities, and Safety of Turmeric Oil. Molecules 2022, 27, 5055.
- Sands, D.C.; Carsky, M.; Donovan, E.; Virgilio, L.L.; Ewart, K.V. Interaction of curcumin with a winter flounder alpha-helical antifreeze protein. Biochem. Biophys. Res. Commun. 2022, 630, 183–189.
- Verma, K.; Tarafdar, A.; Kumar, D.; Kumar, Y.; Rana, J.S.; Badgujar, P.C. Formulation and characterization of nano-curcumin fortified milk cream powder through microfluidization and spray drying. Food Res. Int. 2022, 160, 111705.
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930.
- Agrawal, N.; Jaiswal, M. Bioavailability enhancement of curcumin via esterification processes: A review. Eur. J. Med. Chem. Rep. 2022, 6, 100081.
- Hen-Avivi, S.; Savin, O.; Racovita, R.C.; Lee, W.S.; Adamski, N.M.; Malitsky, S.; Almekias-Siegl, E.; Levy, M.; Vautrin, S.; Berges, H.; et al. A Metabolic Gene Cluster in the Wheat W1 and the Barley Cer-cqu Loci Determines beta-Diketone Biosynthesis and Glaucousness. Plant Cell 2016, 28, 1440–1460.
- Racovita, R.C.; Jetter, R. Identification of Polyketides in the Cuticular Waxes of Triticum aestivum cv. Bethlehem. Lipids 2016, 51, 1407–1420.
- Racovita, R.C.; Hen-Avivi, S.; Fernandez-Moreno, J.P.; Granell, A.; Aharoni, A.; Jetter, R. Composition of cuticular waxes coating flag leaf blades and peduncles of Triticum aestivum cv. Bethlehem. Phytochemistry 2016, 130, 182–192.
- Racovita, R.C.; Jetter, R. Identification of In-Chain-Functionalized Compounds and Methyl-Branched Alkanes in Cuticular Waxes of Triticum aestivum cv. Bethlehem. PLoS ONE 2016, 11, e0165827.
- Mague, J.T.; Alworth, W.L.; Payton, F.L. Curcumin and derivatives. Acta Cryst. C 2004, 60, o608–o610.
- Al-Noor, T.; Ali, A.; Al-Sarray, A.; Al-Obaidi, O.; Obeidat, A.; Habash, R. A Short Review: Chemistry of Curcumin and Its Metal Complex Derivatives. J. Univ. Anbar Pure Sci. 2022, 16, 20–26.
- Mondal, S.; Ghosh, S.; Moulik, S.P. Stability of curcumin in different solvent and solution media: UV-visible and steady-state fluorescence spectral study. J. Photochem. Photobiol. B 2016, 158, 212–218.
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714.
- Kasprzak-Drozd, K.; Oniszczuk, T.; Gancarz, M.; Kondracka, A.; Rusinek, R.; Oniszczuk, A. Curcumin and Weight Loss: Does It Work? Int. J. Mol. Sci. 2022, 23, 639.
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147.
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499.
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620.
- Metzler, M.; Pfeiffer, E.; Schulz, S.I.; Dempe, J.S. Curcumin uptake and metabolism. BioFactors 2013, 39, 14–20.
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762.
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladacenco, O.; Roza, E.; Costachescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938.
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26.
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552.
- Waheed, T.O.; Hahn, O.; Sridharan, K.; Morke, C.; Kamp, G.; Peters, K. Oxidative Stress Response in Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2022, 23, 3435.
- Azzi, A. Oxidative Stress: What Is It? Can It Be Measured? Where Is It Located? Can It Be Good or Bad? Can It Be Prevented? Can It Be Cured? Antioxidants 2022, 11, 1431.
- Sathyabhama, M.; Dharshini, L.C.P.; Karthikeyan, A.; Kalaiselvi, S.; Min, T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules 2022, 12, 1405.
- Jakubczyk, K.; Druzga, A.; Katarzyna, J.; Skonieczna-Zydecka, K. Antioxidant Potential of Curcumin-A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092.
- Fouad, G.I.; Ahmed, K.A. Curcumin Ameliorates Doxorubicin-Induced Cardiotoxicity and Hepatotoxicity Via Suppressing Oxidative Stress and Modulating iNOS, NF-kappaB, and TNF-alpha in Rats. Cardiovasc. Toxicol. 2022, 22, 152–166.
- Hettiarachchi, S.S.; Perera, Y.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, S.; Rajapakse, R.M.G. Comparison of Antibacterial Activity of Nanocurcumin with Bulk Curcumin. ACS Omega 2022, 7, 46494–46500.
- El-Kattan, N.; Emam, A.N.; Mansour, A.S.; Ibrahim, M.A.; El-Razik, A.B.A.; Allam, K.A.M.; Riad, N.Y.; Ibrahim, S.A. Curcumin assisted green synthesis of silver and zinc oxide nanostructures and their antibacterial activity against some clinical pathogenic multi-drug resistant bacteria. RSC Adv. 2022, 12, 18022–18038.
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics 2022, 11, 322.
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants 2022, 11, 459.
- Adamczak, A.; Ozarowski, M.; Karpinski, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153.
- Trigo-Gutierrez, J.K.; Vega-Chacon, Y.; Soares, A.B.; Mima, E.G.O. Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 7130.
- Dizaj, S.M.; Shokrgozar, H.; Yazdani, J.; Memar, M.Y.; Sharifi, S.; Ghavimi, M.A. Antibacterial Effects of Curcumin Nanocrystals against Porphyromonas gingivalis Isolated from Patients with Implant Failure. Clin. Pr. 2022, 12, 809–817.
- Snetkov, P.; Rogacheva, E.; Kremleva, A.; Morozkina, S.; Uspenskaya, M.; Kraeva, L. In-Vitro Antibacterial Activity of Curcumin-Loaded Nanofibers Based on Hyaluronic Acid against Multidrug-Resistant ESKAPE Pathogens. Pharmaceutics 2022, 14, 1186.
- Polak, M.; Nowicki, G.J.; Naylor, K.; Piekarski, R.; Slusarska, B. The Prevalence of Depression Symptoms and Their Socioeconomic and Health Predictors in a Local Community with a High Deprivation: A Cross-Sectional Studies. Int. J. Environ. Res. Public Health 2022, 19, 1797.
- Wu, S.X.; Li, J.; Zhou, D.D.; Xiong, R.G.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Li, H.B. Possible Effects and Mechanisms of Dietary Natural Products and Nutrients on Depression and Anxiety: A Narrative Review. Antioxidants 2022, 11, 2132.
- Balakrishnan, V.; Ng, K.S.; Kaur, W.; Govaichelvan, K.; Lee, Z.L. COVID-19 depression and its risk factors in Asia Pacific—A systematic review and meta-analysis. J. Affect. Disord. 2022, 298, 47–56.
- Stephenson, E.; O’Neill, B.; Kalia, S.; Ji, C.; Crampton, N.; Butt, D.A.; Tu, K. Effects of COVID-19 pandemic on anxiety and depression in primary care: A retrospective cohort study. J. Affect. Disord. 2022, 303, 216–222.
- Zalewska, A.; Galczyk, M.; Van Damme-Ostapowicz, K. Level of Depression during the COVID-19 Pandemic in Poland-A Cross-Sectional Study. Healthcare 2022, 10, 1123.
- Aveiro-Robalo, T.R.; Garlisi-Torales, L.D.; Chuman-Sanchez, M.; Pereira-Victorio, C.J.; Huaman-Garcia, M.; Failoc-Rojas, V.E.; Valladares-Garrido, M.J. Prevalence and Associated Factors of Depression, Anxiety, and Stress in University Students in Paraguay during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 2930.
- Riveros, M.E.; Avila, A.; Schruers, K.; Ezquer, F. Antioxidant Biomolecules and Their Potential for the Treatment of Difficult-to-Treat Depression and Conventional Treatment-Resistant Depression. Antioxidants 2022, 11, 540.
- Zhang, Y.; Li, L.; Zhang, J. Curcumin in antidepressant treatments: An overview of potential mechanisms, pre-clinical/clinical trials and ongoing challenges. Basic Clin. Pharmacol. Toxicol. 2020, 127, 243–253.
- Lamanna-Rama, N.; Romero-Miguel, D.; Desco, M.; Soto-Montenegro, M.L. An Update on the Exploratory Use of Curcumin in Neuropsychiatric Disorders. Antioxidants 2022, 11, 353.
- Kulkarni, S.; Dhir, A.; Akula, K.K. Potentials of curcumin as an antidepressant. Sci. World J. 2009, 9, 1233–1241.
- Kanchanatawan, B.; Tangwongchai, S.; Sughondhabhirom, A.; Suppapitiporn, S.; Hemrunrojn, S.; Carvalho, A.F.; Maes, M. Add-on Treatment with Curcumin Has Antidepressive Effects in Thai Patients with Major Depression: Results of a Randomized Double-Blind Placebo-Controlled Study. Neurotox. Res. 2018, 33, 621–633.
- Qi, X.J.; Liu, X.Y.; Tang, L.M.; Li, P.F.; Qiu, F.; Yang, A.H. Anti-depressant effect of curcumin-loaded guanidine-chitosan thermo-sensitive hydrogel by nasal delivery. Pharm. Dev. Technol. 2020, 25, 316–325.
- Sena-Junior, A.S.; Aidar, F.J.; Oliveira, E.S.A.M.; Estevam, C.D.S.; de Oliveira Carvalho, C.R.; Lima, F.B.; Dos Santos, J.L.; Marcal, A.C. Whether or Not the Effects of Curcuma longa Supplementation Are Associated with Physical Exercises in T1DM and T2DM: A Systematic Review. Nutrients 2020, 13, 124.
- Hussain, Y.; Khan, H.; Alotaibi, G.; Khan, F.; Alam, W.; Aschner, M.; Jeandet, P.; Saso, L. How Curcumin Targets Inflammatory Mediators in Diabetes: Therapeutic Insights and Possible Solutions. Molecules 2022, 27, 58.
- Rivera-Mancía, S.; Trujillo, J.; Chaverri, J.P. Utility of curcumin for the treatment of diabetes mellitus: Evidence from preclinical and clinical studies. J. Nutr. Intermed. Metab. 2018, 14, 29–41.
- Altobelli, E.; Angeletti, P.M.; Marziliano, C.; Mastrodomenico, M.; Giuliani, A.R.; Petrocelli, R. Potential Therapeutic Effects of Curcumin on Glycemic and Lipid Profile in Uncomplicated Type 2 Diabetes-A Meta-Analysis of Randomized Controlled Trial. Nutrients 2021, 13, 404.
- Ghareghomi, S.; Rahban, M.; Moosavi-Movahedi, Z.; Habibi-Rezaei, M.; Saso, L.; Moosavi-Movahedi, A.A. The Potential Role of Curcumin in Modulating the Master Antioxidant Pathway in Diabetic Hypoxia-Induced Complications. Molecules 2021, 26, 7658.
- Mahmoudi, A.; Atkin, S.L.; Nikiforov, N.G.; Sahebkar, A. Therapeutic Role of Curcumin in Diabetes: An Analysis Based on Bioinformatic Findings. Nutrients 2022, 14, 3244.
- Marton, L.T.; Pescinini, E.S.L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; Dos Santos Bueno, P.C. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front. Endocrinol. 2021, 12, 669448.
- Cho, J.A.; Park, S.H.; Cho, J.; Kim, J.O.; Yoon, J.H.; Park, E. Exercise and Curcumin in Combination Improves Cognitive Function and Attenuates ER Stress in Diabetic Rats. Nutrients 2020, 12, 1309.
- Najafian, M. The Effects of Curcumin on Alpha Amylase in Diabetics Rats. Zahedan J. Res. Med. Sci. 2015, in press.
- Lu, X.; Wu, F.; Jiang, M.; Sun, X.; Tian, G. Curcumin ameliorates gestational diabetes in mice partly through activating AMPK. Pharm. Biol. 2019, 57, 250–254.
- Yang, Z.J.; Huang, S.Y.; Zhou, D.D.; Xiong, R.G.; Zhao, C.N.; Fang, A.P.; Zhang, Y.J.; Li, H.B.; Zhu, H.L. Effects and Mechanisms of Curcumin for the Prevention and Management of Cancers: An Updated Review. Antioxidants 2022, 11, 1481.
- Wang, Y.C.; Lin, C.H.; Huang, S.P.; Chen, M.; Lee, T.S. Risk Factors for Female Breast Cancer: A Population Cohort Study. Cancers 2022, 14, 788.
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114.
- Pouresmaeili, F.; Hosseini, S.J.; Farzaneh, F.; Karimpour, A.; Azargashb, E.; Yaghoobi, M.; Kamarehei, M. Evaluation of environmental risk factors for prostate cancer in a population of Iranian patients. Asian Pac. J. Cancer Prev. 2014, 15, 10603–10605.
- Amadou, A.; Praud, D.; Coudon, T.; Deygas, F.; Grassot, L.; Dubuis, M.; Faure, E.; Couvidat, F.; Caudeville, J.; Bessagnet, B.; et al. Long-term exposure to nitrogen dioxide air pollution and breast cancer risk: A nested case-control within the French E3N cohort study. Environ. Pollut. 2023, 317, 120719.
- Okunromade, O.; Yin, J.; Ray, C.; Adhikari, A. Air Quality and Cancer Prevalence Trends across the Sub-Saharan African Regions during 2005–2020. Int. J. Environ. Res. Public Health 2022, 19, 1342.
- Gawelko, J.; Cierpial-Wolan, M.; Bwanakare, S.; Czarnota, M. Association between Air Pollution and Squamous Cell Lung Cancer in South-Eastern Poland. Int. J. Environ. Res. Public Health 2022, 19, 1598.
- Youogo, L.M.K.; Parent, M.E.; Hystad, P.; Villeneuve, P.J. Ambient air pollution and prostate cancer risk in a population-based Canadian case-control study. Env. Epidemiol. 2022, 6, e219.
- Racovita, R.C.; Secuianu, C.; Ciuca, M.D.; Israel-Roming, F. Effects of Smoking Temperature, Smoking Time, and Type of Wood Sawdust on Polycyclic Aromatic Hydrocarbon Accumulation Levels in Directly Smoked Pork Sausages. J. Agric. Food. Chem. 2020, 68, 9530–9536.
- Racovita, R.C.; Secuianu, C.; Israel-Roming, F. Quantification and risk assessment of carcinogenic polycyclic aromatic hydrocarbons in retail smoked fish and smoked cheeses. Food Control 2021, 121, 107586.
- Barcelos, K.A.; Mendonca, C.R.; Noll, M.; Botelho, A.F.; Francischini, C.R.D.; Silva, M.A.M. Antitumor Properties of Curcumin in Breast Cancer Based on Preclinical Studies: A Systematic Review. Cancers 2022, 14, 2165.
- Ojo, O.A.; Adeyemo, T.R.; Rotimi, D.; Batiha, G.E.; Mostafa-Hedeab, G.; Iyobhebhe, M.E.; Elebiyo, T.C.; Atunwa, B.; Ojo, A.B.; Lima, C.M.G.; et al. Anticancer Properties of Curcumin Against Colorectal Cancer: A Review. Front. Oncol. 2022, 12, 881641.
- Wang, S.; Gao, X.; Li, J.; Wei, S.; Shao, Y.; Yin, Y.; Zhang, D.; Tang, M. The anticancer effects of curcumin and clinical research progress on its effects on esophageal cancer. Front. Pharmacol. 2022, 13, 1058070.
- Picetti, R.; Deeney, M.; Pastorino, S.; Miller, M.R.; Shah, A.; Leon, D.A.; Dangour, A.D.; Green, R. Nitrate and nitrite contamination in drinking water and cancer risk: A systematic review with meta-analysis. Environ. Res. 2022, 210, 112988.
- Shahriari, M.; Kesharwani, P.; Johnston, T.P.; Sahebkar, A. Anticancer potential of curcumin-cyclodextrin complexes and their pharmacokinetic properties. Int. J. Pharm. 2023, 631, 122474.
- Hosseini, S.S.; Reihani, R.Z.; Doustvandi, M.A.; Amini, M.; Zargari, F.; Baradaran, B.; Yari, A.; Hashemi, M.; Tohidast, M.; Mokhtarzadeh, A. Synergistic anticancer effects of curcumin and crocin on human colorectal cancer cells. Mol. Biol. Rep. 2022, 49, 8741–8752.
- Wang, M.; Jiang, S.; Zhou, L.; Yu, F.; Ding, H.; Li, P.; Zhou, M.; Wang, K. Potential Mechanisms of Action of Curcumin for Cancer Prevention: Focus on Cellular Signaling Pathways and miRNAs. Int. J. Biol. Sci. 2019, 15, 1200–1214.
- Li, P.; Pu, S.; Lin, C.; He, L.; Zhao, H.; Yang, C.; Guo, Z.; Xu, S.; Zhou, Z. Curcumin selectively induces colon cancer cell apoptosis and S cell cycle arrest by regulates Rb/E2F/p53 pathway. J. Mol. Struct. 2022, 1263, 133180.
- Farahani, M.K.; Atashi, A.; Asadi, A. Evaluation of Anticancer Effects of Curcumin on Multicellular Breast Cancer Spheroids. Turk. J. Oncol. 2022, 37, 285–290.
- Bolat, Z.B.; Islek, Z.; Sahin, F.; Ucisik, M.H. Delivery of curcumin within emulsome nanoparticles enhances the anti-cancer activity in androgen-dependent prostate cancer cell. Mol. Biol. Rep. 2023, 50, 2531–2543.
- Gad, S.C. LD50/LC50 (Lethal Dosage 50/Lethal Concentration 50). In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 58–60.
- Kohli, K.; Ali, J.; Ansari, M.; Raheman, Z. Curcumin: A natural antiinflammatory agent. Indian J. Pharmacol. 2005, 37, 141–147.
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92.
- Naz, R.K.; Lough, M.L.; Barthelmess, E.K. Curcumin: A Novel Non-Steroidal Contraceptive with Antimicrobial Properties. Front. Biosci. 2016, 8, 113–128.
- Jantawong, C.; Priprem, A.; Intuyod, K.; Pairojkul, C.; Pinlaor, P.; Waraasawapati, S.; Mongkon, I.; Chamgramol, Y.; Pinlaor, S. Curcumin-loaded nanocomplexes: Acute and chronic toxicity studies in mice and hamsters. Toxicol. Rep. 2021, 8, 1346–1357.
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer. Res. 2004, 10, 6847–6854.
- Gronich, N. Spontaneous bleeding and curcumin: Case report. J. Clin. Images Med. Case Rep. 2022, 3, 2141.
- Burgos-Moron, E.; Calderon-Montano, J.M.; Salvador, J.; Robles, A.; Lopez-Lazaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775.
- Jiao, Y.; Wilkinson, J.t.; Di, X.; Wang, W.; Hatcher, H.; Kock, N.D.; D’Agostino, R., Jr.; Knovich, M.A.; Torti, F.M.; Torti, S.V. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood 2009, 113, 462–469.
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J. Nutr. Biochem. 2014, 25, 144–150.
- Hassan, S.A.; Bhateja, S.; Arora, G.; Prathyusha, F. Use of Curcumin in Oral Health-A Review. Indian J. Integr. Med. 2020, 2, 20–23.
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
807
Revisions:
2 times
(View History)
Update Date:
24 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





