Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Curcumin is the principal curcuminoid found in the rhizomes of turmeric. Due to its therapeutic action against cancer, depression, diabetes, some bacteria, and oxidative stress, it has been used widely in medicine since ancient times.
- curcumin
- extraction methods
- microemulsion
- health benefits
1. Introduction
Turmeric (also known as Arab globe Curcuma, Haridra in Sanskrit, Chinese yellow ginger Jianghuang, Japanese Kyoo, or Ukon [1]) is a rhizomatous perennial plant [2] of the Zingiberaceae family [3] widely cultivated annually in tropical and subtropical regions of the world [4,5,6], the largest global producer of turmeric being India [1,4]. Curcuma longa is the most commonly recognized species within the genus Curcuma. However, other species exist within this genus, namely Curcuma amada, Curcuma zedoaria, Curcuma aromatica, and Curcuma rakatakanta [7]. At maturity, turmeric can reach a height of one meter. Its leaves are long, rectangular, and placed in two rows from which leaf sheaths and false stems, petioles, and leaf blades are formed. With a length between 2.5–7 cm and a diameter of approximately 2.5 cm, the rhizomes are roughly segmented and have a specific fragrance. Depending on the shape, two types of rhizomes are distinguished: the primary rhizomes that have a pear shape and are also known as bulbs, and the secondary rhizomes that have a cylindrical shape. The flowers are 10–15 cm long, have a matte yellow color, and are arranged in spike-like structures [8]. Since ancient times, this yellow spice has been used in the Asian medicine of Ayurveda (which emerged about 5000 years ago), Siddha, Atharveda (2000 years ago), Unani medicine, and Chinese medicine [9,10]. During the 14th century, the Western world was introduced to the golden spice by early European explorers of the Asian continent [11]. Further, it was also used in religious cult rituals [3,12]. It is also a natural spice used as a colorant in curry, mustard [13], salads, pasta, yoghurt, cheese, and baked goods [14]. It is referred to as Natural Yellow 3 as an ecological dye and has been given the E designation E100 when used as a food coloring ingredient [15]. Turmeric is made up of 69.4% carbohydrates, 13.1% moisture, 6.3% protein, 5.1% fat, and 3.5% minerals. The essential oil, with a proportion of 5.8%, contains, among other compounds, curcumin (C21H20O6) [12], a significant curcuminoid found in the rhizomes of turmeric that gives it its yellow color [16,17]. The other curcuminoids are demethoxycurcumin (C20H18O5) and bisdemethoxycurcumin (C19H16O4), which are present in lower concentrations (Figure 1) [18]. Depending on the geographical cultivation area, curcuminoids amount to around 30–150 mg/g of the turmeric rhizome [19]. Curcumin is biosynthesized from two molecules of feruloyl-CoA and one molecule of malonyl-CoA via two enzymatic conversions catalyzed by diketide-CoA synthase (DCS) and curcumin synthase. (CURS). DCS and CURS both belong to the family of type III polyketide synthases [20].
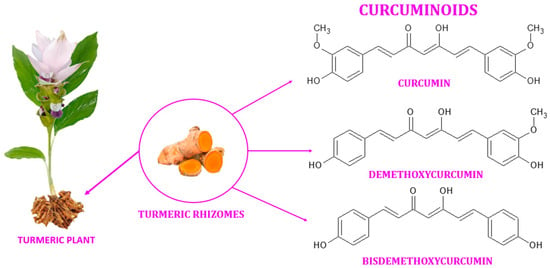
Figure 1. Curcuminoids found in the rhizomes of turmeric.
Curcumin derivatives have antioxidant, anti-diabetic, anti-cancer, anti-allergic, anticoagulant, anti-fungal, and anti-infertility properties [2,5,21,22,23]. However, the bioavailability of this bioactive compound is limited by its low solubility in water [24]. To increase its bioavailability, numerous researchers have tried multiple times and by different methods the encapsulation in micro and nano emulsions [25].
2. Chemistry of Curcumin
Curcumin, also known as diferuloylmethane [26], is a polyphenol from the group of diarylheptanoids with the IUPAC name (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, a molecular weight of 368.39 g/mol, and the chemical formula C21H20O6 [16,27]. This compound has a symmetrical molecule that exhibits in its structure two phenyl rings substituted with a methoxy group in the ortho position and a hydroxy group in the para position. The two aromatic rings are interconnected by a seven-carbon chain consisting of an alpha-beta unsaturated diketone moiety [22], which makes it both a polyphenol and a polyketide at the same time [28,29,30,31]. The diketo group exhibits keto-enol tautomerism: in the solid state, it was shown to exist 100% in the enol form [32] and also predominate as an enol in alkaline aqueous solutions, while the keto form is predominant in acidic and neutral solutions, the enol representing only about 30% of all curcumin in the latter (Figure 2) [10,33,34].
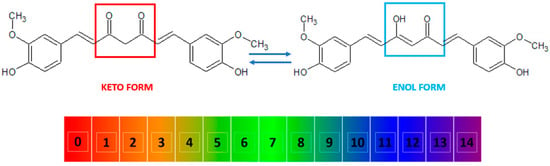
Figure 2. The keto and enol tautomers of curcumin and their reversible inter-conversion in aqueous medium as a function of pH. The numbers indicate the pH scale from 0 to 14. At low pH, the keto form (marked in red) is predominant, while at high pH, the enol form (marked in blue) predominates.
Curcumin is characterized by the presence of three ionizable protons in aqueous solution, namely the enolic proton with a pKa value of approximately 8.5 and two phenolic protons with pKa values ranging from 10 to 10.5 [11]. The essential chemical reactions associated with the biological activity of curcumin are electron-donating reactions that lead to the oxidation of the compound, reversible and irreversible nucleophilic addition reactions, hydrolysis, and enzymatic reactions [12]. The degradation products of curcumin include ferulic acid, feruloyl methane, vanillin, and dicyclopentadiene [35]. Curcumin has a yellow color, a melting point of 183 °C [10], and a partition coefficient of logP ≈ 3, which is indicative that curcumin is a rather hydrophobic compound [4,12]. However, it is soluble in polar solvents such as dimethyl sulfoxide (DMSO), methanol, ethanol, and acetone [12].
3. Health Benefits of Curcumin
After ingestion, curcumin is metabolized mainly in the liver, large intestine, and intestinal microbiota [105,106,107]. The metabolism process is carried out by enzymes and occurs in two phases: the first phase involves the reduction of the four double bonds of the heptadiene-3,5-dione [108,109] structure in heterocytes and hepatocytes by a reductase to dihydrocurcumin, tetrahydrocurcumin, hexahydrocurcumin, and octahydrocurcumin [105,106,107]. In the second phase, unmetabolized curcumin and the resulting metabolites from the first phase are transformed into glucuronidic and sulphate-O-conjugated metabolites because glucoronidases and sulphatransferases can conjugate the glucuronic acid and sulphate molecules to hydroxyl groups [107,108]. Numerous clinical studies have suggested curcumin has antioxidant, antidiabetic, antibacterial, antidepressant, and anticancer properties and beneficial effects on diabetes mellitus and other disorders [110].
3.1. Antioxidant Properties
Reactive oxygen species (ROS) are oxygen-derived molecules with short lifetimes and high reactivity due to the presence of unpaired valence electrons [111]. Both endogenous (such as mitochondria, peroxisomes, endoplasmic reticulum, etc.) and exogenous (such as pollution, alcohol, tobacco smoke, heavy metals, medications, etc.) sources contribute to the production of free radicals [112]. The primary source of ROS production is the mitochondria, where adenosine triphosphate (ATP) production by oxidative phosphorylation takes place with the reduction of molecular oxygen to H2O in the electron transport chain [113]. Although they are by-products, they play an essential role in cell signaling and homeostasis by regulating cell proliferation, differentiation, and survival [114].
The concept of oxidative stress was defined as “an imbalance between oxidants and antioxidants in favour of the oxidants, leading to disruption of redox signalling and control and/or molecular damage” in “Oxidative stress: a concept in redox biology and medicine” [115]. The best-known ROS are peroxides, superoxides, hydroxyl radicals, ozone, and nascent oxygen molecules. In high concentrations, these species have the ability to oxidize nucleic acids, lipids, and proteins. Antioxidants scavenge ROS by donating their electrons to the undervalent ROS and neutralizing it.
Curcumin can reduce mitochondrial oxidative stress by increasing the effects of superoxide dismutase, glutathione, and catalase. The three redox sites of curcumin can undergo oxidation and hydrogen abstraction, resulting in the formation of phenoxy radicals and stabilization against the enol structure [116].
Curcumin at 645 mg/24 h for 67 days boosted total antioxidant capacity and lowered malondialdehyde levels, according to a meta-analysis of 308 individuals, 60% of whom were women [117]. In an in vivo investigation, the cardioprotective and hepatoprotective properties of curcumin against hepatotoxicity and cardiotoxicity generated by doxorubicin (DOX), a medication often used in tumour disorders, were studied. To elicit unfavourable effects, mice were given a single dose of DOX (20 mg/kg) intraperitoneally. Curcumin (100 mg/kg oral) was administered to them 10 days before and 5 days after DOX treatment. Due to its anti-inflammatory and antioxidant properties, curcumin reduces lipid peroxidation, inhibits immunoexpression of nuclear factor-kB (NF-kB), inhibits tumour necrosis factor-alpha (TNF-α) and inducible nitric oxide synthase (iNOS), and lowers circular inflammatory interferon-gamma (IFN-γ) levels. As a result, it was revealed that curcumin may be administered as an adjuvant since it can serve as a protective antioxidant-based method against DOX-associated cardiotoxicity and hepatotoxicity [118].
3.2. Antibacterial Properties
Due to their both beneficial and harmful impacts, the existence of microbes is well known in the human population. When the symbiotic relationship between microbes exceeds a certain threshold, they can produce pathogenic illnesses and diseases that can be fatal to the human body. Antimicrobial agents, particularly antibiotics, are used to combat bacterial infections [119]. Bacteria have evolved and developed resistance to antibiotics throughout time as a result of their overuse or inadequate usage [120]. Antibacterial antibiotic resistance is one of the leading causes of treatment failure. Traditional medicines have been shown to have a considerable impact on pathogen therapy by regulating a variety of physiological systems [121].
The antibacterial activities of curcumin were initially documented by Schraufstatter and colleagues in 1949 [122]. Despite its poor solubility in water, limited bioavailability, and pharmacokinetic profile, modern investigations have shown high antibacterial activity for curcumin [123]. The antibacterial mechanism of action of curcumin involves damage to the cell membrane, interference with cellular processes by targeting DNA and proteins, and inhibition of bacterial quorum sensing [124].
Maleki Dizaj et al. [125] studied the antibacterial effects of curcumin nanocrystals against Posphyromones gingivalis (P. gingivalis) isolated from the gingival crevicular fluid of Iranian patients with implant failure. The disc diffusion method was utilized to test bacterial sensitivity to curcumin nanoparticles, and the broth microdilution method was employed to estimate the nanoparticles’ minimal inhibitory concentrations (MICs) against P. gingivalis. The bacteria were sensitive at 50, 25, 12.5, and 6 µg/mL concentrations. At 50 µg/mL, curcumin nanocrystals demonstrated the most significant inhibitory zone. According to the MIC test, curcumin nanoparticles inhibited P. gingivalis growth at 6.25 µg/mL. Curcumin nanoparticles had bactericidal activity against P. gingivalis at 12.5 µg/mL in the MBC test (minimum bactericidal concentration) [125]. In another study, Snetkov et al. evaluated the antibacterial properties of polymer nanofibers based on hyaluronic acid and curcumin against multidrug resistant ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebeciella pneumoniae, Acinetobacter baumannii, Pseudomona aeruginosa, and Enterobacter species). The HA-curcumin stable complex showed high antibacterial activity against Gram-positive and Gram-negative bacteria. The minimum inhibitory concentrations for Gram-positive bacteria reached 90 µg/mL, while those for Gram-negative bacteria ranged from 90–960 µg/mL [126].
3.3. Antidepressant Properties
Depression is a psychiatric condition that affects about 300 million people annually, resulting in over 800,000 deaths. This illness costs Europe EUR 92 billion annually, with a substantial proportion of the financial burden imposed by the productivity loss of affected persons [127]. Fatigue, anhedonia, sleep difficulties, and self-destructive behaviour are the most common symptoms [128]. During the SARS-CoV-2 epidemic, prevention efforts led to social isolation, economic instability, and worries about losing loved ones, resulting in both physical and mental health deterioration [129,130,131,132]. Some prevalent antidepressant medicines have been linked to a number of negative side effects, prompting their early withdrawal [133]. These effects include constipation, dry mouth, sleep difficulties, cardiotoxicity, neurotoxicity, orthostatic hypotension, and sexual dysfunction [134].
Disruptions in the intestinal microbiota and disruptions of the gut-brain axis are significant factors in the development of depression. Depression episodes may be induced by a reduction in neurotrophic factors derived from the brain and a change in the efficiency of neurotransmitters due to changes in the permeability of the intestinal barrier caused by changes in the variety of the intestinal microbiota [128]. Since the intestine and liver are the primary sites of curcumin metabolism, it may be acting on the gut microbiota to reduce intestinal inflammation and act as a neuroprotective agent because neuroinflammation is likely a factor in many psychiatric disorders [135].
Inhibition of the monoamine oxidase (MAO) enzyme, modulation of the levels of different neurotransmitters (norepinephrine, dopamine, and serotonin), promotion of hippocampal neurogenesis, and its use as an anti-inflammatory agent are just a few of the details about the phenomenon of curcumin that support its use in treating major depression [136]. A 12-week research study was carried out by Kanchanatawan et al. to assess the benefits of adjunctive curcumin for treating major depressive disorder (MDD). The 65 MDD patients in the double-blind, placebo-controlled experiment were randomized to receive either adjunctive curcumin or a placebo. They found that adding curcumin to the medication considerably improved the Montgomery–Asberg depression rating scale (MADRS) score; this impact was significant 12 weeks after commencing treatment, and the benefit was maintained four weeks after curcumin withdrawal. With substantial differences between curcumin and placebo at weeks 12 and 16, curcumin was considerably more effective than placebo at alleviating depressive symptoms in severe depression. A greater effectiveness of curcumin was reported in males compared to females [137]. In mice, Qi et al. studied the antidepressant effects of curcumin administered nasally. They created a thermosensitive hydrogel that contained curcumin. After administering it, they noticed a rise in the levels of norepinephrine, dopamine, and 5-hydroxytryptamine, as well as its metabolites, in the striatum and hippocampus, proving that curcumin has antidepressant effects [138].
3.4. Diabetes Mellitus
Diabetes mellitus is a metabolic condition defined by persistent hyperglycemia in which insulin hormone activity on cell receptors is absent or inefficient, resulting in a substantial rise in blood glucose [139]. The types of diabetes mellitus according to the classification by the American Diabetes Association are: (I) type 1 diabetes, with autoimmune β-cell destruction and absolute insulin shortage; (II) type 2 diabetes (90–95% of diabetes cases), which is characterized by insulin resistance, followed by insulin hypersecretion in β cells of Langerhans, preventing the body from using its own insulin [140,141]; (III) gestational diabetes mellitus, which affects 7% of pregnancies in the second or third trimester; (IV) other kinds of diabetes, such as monogenic diabetes syndromes, exocrine pancreas illnesses, and drug- or chemical-induced diabetes, which account for 5% of diabetes patients [141]. Obesity and overweight are two main risk factors for diabetes caused by lifestyle choices [142]. Hyperglycemia, glucotoxicity, and oxidative stress are all linked to diabetes. Together, these things make advanced glycation endproducts (AGEs) and lipid peroxidation products, which increase ROS production in the cell [143]. Many organs, such as the eyes, nerves, heart, kidneys, and blood vessels, are susceptible to long-term damage, dysfunction, and failure due to diabetes [144].
According to studies conducted on diabetic patients, curcuminoids enhance insulin resistance, decrease levels of leptin, resistin, interleukin (IL)-6 IL-1β, and tumor necrosis factor-α, increase the release of adiponectin, and decrease glucose and insulin levels. Consequently, these compounds may influence glucose homeostasis and the consequences of diabetes [145]. A study conducted on male Long-Evans Tokushima Fatty Otsuka rats and Long-Evans Tokushima Otsuka rats (LETO controls) revealed that combining exercise with a curcumin-based diet (5 g/kg) improves glucose homeostasis and lipid profiles, promotes weight loss, and reduces levels of inflammatory response indicators IL 6, TNF, IL10, and ER stress marker levels. Using a Morris water maze test, they also assessed cognitive performance and discovered that curcumin boosts memory retention and escape latency [146]. In another study, the effect of curcumin on alpha-amylase was investigated in rats administered with 10, 20, 40, and 80 mg/kg of curcumin for 30 days. Blood glucose levels were monitored once every three days, while insulin levels were assessed on the first, middle, and final days of the trial. According to the data, curcumin inhibits alpha-amylase with an IC50 equal to 51.32 µM and an inhibition constant Ki of 20.17 µM, as well as decreasing glucose and insulin levels [147]. AMPK (5′-adenosine monophosphate-activated protein kinase) may alter metabolic phenotypes from fat synthesis to fat oxidation, reducing hepatic glucose production and increasing muscle glucose absorption. Lu et al. revealed that curcumin controls hepatic oxidative stress (thiobarbituric acid reactive substances, superoxide dismutases, glutathione, and catalase) and activates AMPK in gestational diabetic mice [148].
3.5. Anticancer Properties
Cancer is the leading cause of mortality globally, with approximately 10 million deaths recorded in 2020 and an estimated 28.4 million new cases expected by 2040 [149]. The lack of physical activity, obesity, tobacco use, alcohol use [150,151], exposure to ultraviolet radiation [152], and exposure to an environment polluted with nitrogen dioxide [153], sulfur dioxide, carbon monoxide [154,155], suspended particles [156], and persistent organic pollutants (POP) such as polycyclic aromatic hydrocarbons [157,158], all contribute to the development of various types of cancer, the most prevalent of which are breast cancer [159], colorectal cancer [36,160], lung cancer [154], prostate cancer, and gastric cancer [161].
In order to eradicate the disease or slow down its rate of spreading, several therapies can be used, including surgery, radiotherapy, chemotherapy, and immunotherapy [15,149,162]. Although there are a variety of treatments, they have side effects and limited effectiveness [149]. There are numerous studies highlighting the anticancer effects of curcumin, which come from the fact that curcumin regulates cell signalling pathways such as STAT3, NF-kB, activated Egr-1, AP-1, P53 [163], Wnt/β-catenin, PI3K/Akt, JAK/STAT, and MAPK [164].
Li et al. [165] explored the effect of curcumin against colorectal cancer in an in vitro investigation. The results revealed that curcumin was not hazardous to epithelial cells of the colonic mucosa, that it reduced colon cancer cell growth, and that it promoted apoptosis via a P53-mediated mechanism, upregulation of pro-apoptotic proteins such as Bax, and cell cycle arrest during S phase by inhibiting cell-cycle-related protein production, phosphorylation of Rb signalling pathway proteins, and E2F family transcription factors [165]. The anticancer effects of curcumin on multicellular breast cancer spheroids were studied by Kamalabadi et al. Breast cancer stem cells (BCSCs) were evaluated in vitro using spheroids. Three-dimensional (3D) culture methods were chosen because they better maintained the biological properties of the original malignancies than typical two-dimensional (2D) monolayer cultures. Curcumin induces death in breast cancer cells (MCF-3) in 3D culture, according to this study. This bioactive substance drastically reduced MCF-3 cell survival in 2D and 3D systems in a dose- and time-dependent manner; nevertheless, curcumin’s impact has been limited due to its low bioavailability [166]. In a recent study, Bolat et al. developed curcumin-loaded emulsions that increase the bioactive compound’s solubility by up to 10,000 times and extend the therapeutic effect against hepatocellular carcinoma. These loaded emulsions were evaluated in vitro on the androgen-dependent prostate cancer cell line LNCaP, and a substantial drop in androgen receptor gene expression levels was observed after treatment [167].
3.6. Side Effects and Potential Toxicity
The United States Food and Drug Administration (FDA) has acknowledged the general safety of curcumin, with its LD50 value determined in rats, i.e., the lethal dosage expected to cause the death of 50% of target species subjects [168], being well above 2 g/kg body weight [169]. The Joint Food and Agriculture Organization/World Health Organization (FAO/WHO) expert committee on food additives has established an acceptable daily intake (ADI) range of 0.1–3 mg/kg body weight for this substance [170,171].
Chanaken et al. conducted an in vivo study wherein they observed that the administration of curcumin at high doses and for prolonged periods (100 mg/kg/90 days) resulted in an imbalance in rats. This imbalance was characterized by an overproduction of reactive oxygen species (ROS), an increased production of the pro-oxidant cytokine IL6, and a decrease in antioxidant enzymes. These changes ultimately led to oxidative stress-mediated liver injury and inflammatory disorders [172].
In another study, fifteen people diagnosed with advanced colorectal cancer ingested curcumin-compatible capsules, with dosages ranging from 0.45 to 3.6 g per day, for a maximum duration of four months. Gastrointestinal adverse effects were reported by the patients. Two patients who were administered 0.45 g and 3.6 g of curcumin per day, respectively, experienced diarrhea (grades 1–2). A single patient who ingested a daily dose of 0.9 g of curcumin reported experiencing nausea. Elevated serum alkaline phosphatase levels were detected in four patients during the blood test [173].
Another case report was about a male patient, aged 74 years and afflicted with various medical conditions, who was admitted to the emergency department due to the presence of a sizable hematoma in his left thigh. The subject initiated a daily intake of 1 g of curcumin, in addition to the prescribed medications for his illnesses, one week prior. The researchers suggested that an elevated dosage of curcumin could potentially be linked to heightened antiaggregant and anticoagulant effects [174].
It has been found that curcumin is a potent chelator of iron [175]. According to in vivo research, adding curcumin to mice’s meals caused them to develop the symptoms of iron deficiency anaemia, including a drop in serum iron levels, a reduction in transferrin saturation, and the development of hypochromic red blood cells. Additionally, bone marrow and spleen iron levels may be decreased by curcumin [176].
According to the literature, a few other adverse effects of curcumin have been reported, indicating that the incidence of headache, skin rash, and yellow stool is dose-dependent and was observed in 7 of the 24 participants [170,177]. Additional adverse effects comprise gastrointestinal irritation, abdominal discomfort, hypersensitive dermatological responses [178], and triggered uterine contractions in pregnancy [179].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24108874
This entry is offline, you can click here to edit this entry!
