Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrea Duranti | -- | 2897 | 2023-05-22 17:34:45 | | | |
| 2 | Beatrix Zheng | + 6 word(s) | 2903 | 2023-05-23 11:13:44 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 2903 | 2023-05-23 11:14:23 | | | | |
| 4 | Beatrix Zheng | -2 word(s) | 2901 | 2023-05-24 08:26:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Centofanti, F.; Buono, A.; Verboni, M.; Tomino, C.; Lucarini, S.; Duranti, A.; Pandolfi, P.P.; Novelli, G. Synthetic Methodologies of Indole-3-Carbinol. Encyclopedia. Available online: https://encyclopedia.pub/entry/44675 (accessed on 07 February 2026).
Centofanti F, Buono A, Verboni M, Tomino C, Lucarini S, Duranti A, et al. Synthetic Methodologies of Indole-3-Carbinol. Encyclopedia. Available at: https://encyclopedia.pub/entry/44675. Accessed February 07, 2026.
Centofanti, Federica, Alessandro Buono, Michele Verboni, Carlo Tomino, Simone Lucarini, Andrea Duranti, Pier Paolo Pandolfi, Giuseppe Novelli. "Synthetic Methodologies of Indole-3-Carbinol" Encyclopedia, https://encyclopedia.pub/entry/44675 (accessed February 07, 2026).
Centofanti, F., Buono, A., Verboni, M., Tomino, C., Lucarini, S., Duranti, A., Pandolfi, P.P., & Novelli, G. (2023, May 22). Synthetic Methodologies of Indole-3-Carbinol. In Encyclopedia. https://encyclopedia.pub/entry/44675
Centofanti, Federica, et al. "Synthetic Methodologies of Indole-3-Carbinol." Encyclopedia. Web. 22 May, 2023.
Copy Citation
Indole-3-Carbinol (I3C) is an important phytochemical contained in cruciferous vegetables and is able to exert various activities among which are cardioprotective, antioxidant, anti-inflammatory, antiangiogenesis, and antimicrobial activities, the promotion of tumor cell apoptosis and, an important inhibition of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS)-CoV-2 viral egression, including the Omicron variant.
Indole-3-carbinol
phytochemical
natural products
1. I3C Biosynthesis
With regard to the environment of plants, the role of glucosinolates is to protect them. Upon damage to plant tissues, they undergo enzymatic hydrolysis and are transformed into bioactive molecules toxic to herbivores and pathogens. When plant tissues are damaged, the β-thioglucosidase [1] enzyme myrosinase [2] can hydrolyze the thioglucosidic bond in the structure of the glucosinolates [3]. In particular, Indole-3-Carbinol (I3C) is obtained from the degradation of the glucosinate glucobrassicin. This process is carried out through the formation of unstable intermediates thiohydroximate-O-sulfonate and 3-indolylmethyl isothiocyanate, which is finally converted into the desired compound I3C (Figure 1).
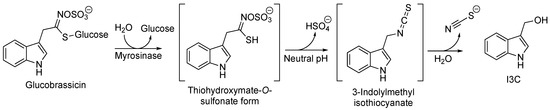
Figure 1. Biosynthesis of I3C starting from glucobrassicin.
2. I3C Synthetic Methodologies
Among the numerous synthetic methodologies followed to obtain I3C, those with the best characteristics to be reproduced on a very large scale (the first illustrated) and the one with very high yield were selected.
A classical method to obtain I3C requires highly basic conditions such as the reduction of the corresponding aldehyde with sodium boron hydride (NaBH4) [4][5], as follows in Scheme 1.
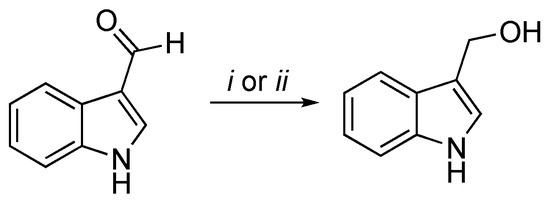
Scheme 1. Reagents and conditions: (i) NaBH4, MeOH, 0 °C, 30′; (ii) NaBH4, EtOH/THF, rt, 3 h.
The second scheme describes the method to obtain I3C from indole proposed by Downey et al. [6] (Scheme 2). Indoles undergo Friedel–Crafts addition to aldehydes in the presence of trimethylsilyl trifluoromethanesulfonate (TMSOTf) and a trialkylamine to form 3-(1-syloxyalkyl)indoles. The reaction is quenched by the addition of pyridine followed by deprotection under basic conditions with tetrabutylammonium fluoride (TBAF) and provides the free desired product. This method prevents the formation of bisindolyl methanes, a thermodynamically favored process typically observed when indoles react with aldehydes under acidic conditions.
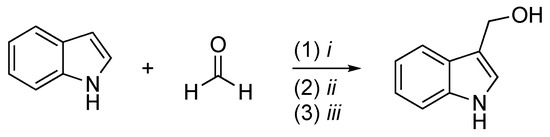
Scheme 2. Reagents and conditions: (i) TMSOTf, i-Pr2NEt, Et2O, N2, −78 °C, 1 h; (ii) pyridine; (iii) TBAF, THF, rt, 5′.
As mentioned before, I3C is a well-researched and interesting compound due to its extensive biological properties and may activate multiple antiproliferative cascades [7].
For an extended period, I3C has been widely explored for potential roles in different cellular mechanisms, including the suppression of cell cycle progression, blocking of cancer cell migration, the promotion of apoptosis, and the inhibition of tumor growth [8][9][10]. Although it interacts with different pathways, it has been proposed that I3C and its synthetic derivatives may influence human cells by directly inhibiting them with specific enzymatic target proteins. Specifically, they are potent natural inhibitors of homologous to E6AP carboxyl-terminus (HECT) family members of E3 Ubiquitin-Ligase, emphasizing the potential importance of I3C in developing highly potent and stable anti-cancer molecules, and not only [11]. I3C and its derivatives are also able to inhibit neuronal precursor cell-expressed developmentally downregulated 4 (NEDD4) and WW domain-containing ubiquitin E3 ligase 1 (WWP1) ubiquitination enzymatic activity through the interaction with their catalytic subunit [11][12][13][14].
Since I3C affects many cellular mechanisms, it is not surprising to find proposed applications in the treatment or prevention of different cancer types (e.g., breast, ovarian, prostate, lung, liver, and colon) and in other diseases [15][16][17][18][19][20][21][22][23]. To date, it has been tested in many clinical trials for the prevention and treatment of obesity, chronic inflammation, lupus erythematosus (SLE), and breast, colon, and prostate cancer [24]. Details about all of the applications of the potential of I3C and its derivatives in different pathologies are reported below.
3. I3C Antitumor Activity: In Vitro, In Vivo, and Clinical Studies
Cancer is one of the leading causes of disease and death worldwide and is defined as uncontrollable and abnormal cell growth [25]. In breast cancer, gland cells grow abnormally and uncontrolled, resulting in the development of a malignant and metastatic phenotype [26]. It is divided into four molecular subtypes based on the expression of the estrogen receptor (ER), progesterone receptor (PgR), and the epidermal growth factor receptor 2 (HER2): two are characterized by the presence of hormone receptors, which provide hormonal therapeutic treatment; then, there is the HER2 subtype, which constitutes about 20% of all cases and is a biologically treatable tumor with some monoclonal antibodies specific to the substance used; finally, there is the triple-negative tumor (it has no receptors for either hormones or HER2), which is sometimes more aggressive and is subject to studies aimed at specific therapies [27].
Although conventional treatments are clinically effective against breast cancer, they present severe side effects associated with the development of resistance and high toxicity in healthy cells leading to a poor prognosis [25]. Therefore, the discovery of new treatments and in particular, the use of natural compounds is one of the primary goals in the field of breast cancer biology, due to therapeutic potential with minimum side effects compared to the traditional methods such as chemotherapy and radiotherapy [28].
In this context, different studies have been conducted on different phytochemical compounds that have brought to light significant antitumor effects, preventing malignant neoformations, inhibiting the metastatic process, and also optimizing first-line anticancer therapies [26]. For many years, several studies have highlighted the potential benefits of the phytochemical compound I3C (and its derivatives; in particular, DIM) as promising antitumoral options with negligible toxicity [8][29][30][31], due to the fact that many of these antiproliferative responses are selectively controlled by I3C-activated pathways. Now, the researchers will illustrate in molecular detail how I3C and its derivatives exert their antitumor activity in the human cancer cell.
Regardless of the type of tumor, I3C triggers DNA repair, cell-cycle arrest, apoptosis, disruption of cell migration, and modulates hormone receptor signaling [32][33][34]. The first identified I3C target protein was serine protease elastase [35]. In human breast cancer, it has been observed that inhibition of elastase activity by I3C prevents CD40 cleavage, resulting in the disruption of the NFkB-dependent cell cycle and proliferation [36][37].
Moreover, I3C and its derivatives also play a fundamental role in DNA damage repair processes by influencing the expression of tumor suppressor genes such as BRCA1 and BRCA2 and DNA repair proteins (i.e., RAD51, which regulates responses in the case of DNA damage) [38][39]. In all estrogen-dependent tumors (including breast cancer), I3C inhibits cyclin-dependent kinase (CDK) expression, resulting in the stopping of the cell cycle in the G1 phase. In particular, I3C has also been reported to induce apoptosis in the PC-3 human prostate cancer cell line by inhibiting the activation of serine/threonine kinase Akt [40][41]. In addition, I3C has the ability to counteract the metastatic process, tumor angiogenesis, and migration of cancer cells, inhibiting CDK6 with the consequent blockage of cell growth [42]. These observations suggest that I3C may mediate its anti-proliferative effects by directly interacting with other classes of target proteins with enzymatic activities.
More recently, through the identification of genes involved in tumorigenesis and cancer susceptibility, it was found that members of the HECT family of E3 ubiquitin ligases were often over-expressed in human cancers. They display oncogenic properties through the ubiquitin-dependent regulation of several protein substrates such as phosphatase and tensin (PTEN) homolog, a negative regulator of phosphatidylinositol-3,4,5-trisphosphate and the Akt/PKB signaling pathway [14]. Specifically, two members of a subgroup of HECT-E3 ligases, known as C2-WW-HECT (NEDD4-like), have been identified to be most involved in cancer: NEDD4 and WWP1. They are characterized by WW domains that act on protein–protein interactions by recognizing Pro-rich and Ser/Thr-Pro phosphorylated patterns. These domains provide scaffolding to recruit protein substrates and regulators [11][12][13].
Quirit et al. have demonstrated through in vitro assay that I3C directly inhibits NEDD4 ubiquitination activity. Moreover, protein thermal shift assays, in combination with the in silico binding simulations and crystallographic structure of NEDD4, showed the binding involvement to the purified catalytic HECT domain of NEDD4 [11]. Likewise, due to its structural similarity to NEDD4, WWP1 was also demonstrated to be inhibited by I3C. It is interesting to note that the MST (microscale thermophoresis) binding assay found that I3C binds to the WWP1 HECT domain with a dissociation constant much higher than that related to NEDD4 [12].
In addition to directly influencing specific molecules, I3C (and DIM) also seem to be able to regulate gene expression by modulating DNA methyltransferases, histone deacetylases, miRNAs, lncRNAs, and some transcription factors such as the aryl hydrocarbon receptor (AhR), ER, and NF-kB [43][44][45]. In fact, the researchers know, for example, that DNA methylation involves the silencing of tumor suppressor genes, an important mechanism for developing target molecules for chemoprevention, and able to modulate the methylation of these genes. In this regard, it has been seen that the inhibition of DNMT (DNA methyl transferase) by DIM has shown a lower expression of oncogenes and an increase in tumor suppressor genes [46]. This mechanism has emerged from some experiments on mouse models and on human prostate cancer cells, on healthy prostate cells (PrEC), as well as on androgen-dependent (LnCAP) or androgen-independent (PC3) prostate cancer [47].
These results suggest that I3C could provide a starting point to develop highly potent anticancer compounds based on target-protein interactions to have a more potent antiproliferative response in human breast cancer cells (and also for other types of cancer) compared to other inhibitors [37].
In recent years, the demand for natural products with prophylactic action against a series of diseases and their adverse reactions is increasing. According to some studies, I3C exerts a potential prophylactic action either directly or through its metabolites [48].
Recently, Lee et al. identified, in either Myc-driven or PTEN heterozygous mice, the reactivation of PTEN due to a pharmacological inactivation of WWP1 by I3C, leading to the potent suppression of tumorigenesis driven by the PI3K-Akt pathway [12]. Moreover, PTEN deletion by CRISPR-Cas9 in Hi-Myc tumor organoids conferred partial resistance to I3C, supporting that I3C exerts its function in a PTEN-dependent manner. In addition, an I3C pharmacokinetic analysis in male C57BL/6 mice after intraperitoneal administration at 20 mg/kg every day or every other day was carried out. These results support again that I3C targets WWP1 E3 ligase, triggering reactivation of the PTEN tumor suppressive function, and promoting its plasma membrane recruitment, and suppression of MYC-driven tumorigenesis in vivo. These findings highlight the use of I3C as a potential therapeutic strategy to reactivate PTEN (through WWP1 inhibition) and to treat all of those patients with multiple tumors or other diseases associated with PTEN germline mutations and for cancer prevention, through the targeting of WWP1-PTEN axis pharmacologically [12].
There are also several studies carried out on mouse models to test the effectiveness of I3C and its derivatives. Studies on immunodeficient mice, such as the naked mouse or the non-obese diabetic (NOD) mouse, in which human cancerous cell lines (xenotransplantations) are implanted and follow the dietary administration of I3C (or DIM), have observed the inhibition of tumor cell proliferation over time [49][50][51]. Again, following the implantation of human cancer cell lines in SCID (severe combined immunodeficiency) mice (NOD. CB17-Prkdcscid/SzJ), the efficacy of I3C in comparison to that of DIM from food in inhibiting tumor growth has been analyzed: both tumor size and doubling time of human T-ALL CCRF-CEM cell xenografts in these mice were significantly affected by 100 ppm of dietary DIM, while they were sensitive to 500 and 2000 ppm of I3C from the diet [49]. The chronic administration of I3C in rodents has led to the development of hepatocarcinogenesis [52][53], while in the infantile model, it led to a high decrease of liver tumors induced by diethylnitrosamine [54]. Some studies on colon cancer have brought to light activity of chemoprevention of I3C and DIM according to an AhR-dependent modality, also susceptible to the microbial production of indole AhR ligands starting from the metabolism of dietary tryptophan [55][56]. For the analysis of breast cancer in rats induced by DMBA (7,12-dimethylbenzanthracene) or MNU (N-methylnitrosourea), a direct-acting carcinogen, I3C, unlike DIM, has been shown to be effective in chemoprevention [57]. All of this evidence justifies the ability to attribute I3C chemopreventive activity to DIM in each study performed. Protection against TBD-dependent in female, but not male, offspring originating from mothers fed I3C during gestation required the expression of ERβ [58]. These results would be in line with the chemoprevention of I3C and ER-dependent DIM in breast cancer [8][59][60][61][62][63][64][65][66].
Preclinical studies of I3C as chemopreventive agents have yielded excellent results [9][67][68]. For example, in addition to liver, breast, and colon cancer, dietary I3C leads to a decrease in lung carcinogenesis caused by the specific nitrosamine contained in tobacco, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, PAH (pulmonary arterial hypertension), and BAP (broader autism phenotype) [69].
Since numerous in vitro and in vivo studies have shown its powerful chemopreventive action in different cancers, I3C has also been the subject of some promising clinical studies concerning cancer therapy. In a double-blind placebo-controlled study, the ability of I3C to prevent the onset of breast cancer was evaluated [70]. The study involved 60 women aged 22 to 74 years who received doses of 50 mg or 400 mg of I3C and in no case did adverse effects occur except in patients with a history of elevated aminotransferase levels. The study showed that I3C exerts a preventive effect on breast cancer and all forms of estrogen-dependent cancer [70]. It is important to consider that breast cancer in women has been placed in the foreground following the proven activity of I3C (and DIM) to act on CYP1-dependent estrogen metabolism. With regard to the metabolites of estradiol (E2), 2-hydroxyestrogen has a lower pharmacological activity, in contrast to 16-α-hydroxyestrogen, which maintains estrogenic activity. The ratio (2-hydroxyestrogen)/(16-α-hydroxyestrogen) to date is considered a biomarker for the identification of the risk of an estrogen-dependent tumor and for cervical intraepithelial neoplasia (CIN), such that clinical studies carried out with I3C (and DIM) have determined an increase in the ratio between the two estrogens, and therefore, the prevalence of the non-estrogenic metabolite, and, in the specific case of CIN, an improvement in the pathology.
I3C (and DIM) also affect the action of flavin-containing monooxygenases, i.e., a superfamily of enzymes contained in the endoplasmic reticulum of tissues such as the liver, lung, intestine, and kidney, which catalyze redox reactions in which NADPH is identified as a reducing agent and, at the same time, an O2 atom is added in a substrate while the other is used for the formation of H2O [71]. Humans express five FMOs (FMO 1–5) but in mammals, excluding primates, FMO1 is the main form present in the liver capable of metabolizing a wide range of xenobiotics [72]. Specifically, dietary I3C (and DIM) inhibits FMO1 only in rats and FMO3 in humans [73][74][75][76][77]. The ratio of FMO/CYP-mediated metabolism of DMA was reduced in a dose-dependent manner following the intake of dietary I3C (and DIMs). When considering (S)-nicotine, the rate of metabolism operated by CYP did not change with diet, while N-oxygenation was significantly inhibited, and no forms of N-oxide were detected in liver microsomes of rats fed 2500 ppm I3C (or 1000 ppm DIM). Tamoxifen, an estrogen receptor antagonist ER used in chemoprevention or treatment of ER-dependent breast cancer, allows limited use from ovarian toxicity due to the hydroxylation reaction at position 4 [78]. Again, the N-oxidized form is the eliminable form and the inhibition of N-oxide metabolism is likely to increase the toxicity of both (S)-nicotine and tamoxifen [79].
However, the actual clinical and preclinical I3C concentrations as well as the consumption of large quantities are still unclear; a recent paper published by Centofanti et al. evaluated potential adverse in vivo toxicity effects through two different routes of administration [intraperitoneally (i.p.) and intragastrically (i.g.)] in both male and female mice, because in females, the effect of I3C may be subject to hormonal changes. This analysis showed a different tolerability dose in males and females to be considered for any future clinical trials in which I3C will be used. Overall, below 550 mg/kg for i.g. and 250 mg/kg for i.p. values, I3C induces neither death nor abnormal toxic symptoms, as well as no histopathological lesions [80].
It is always important to remember that the doses used in preclinical and clinical studies are significantly higher than the doses that can be derived from food sources; for this reason, supplementation is essential to achieve the same health effects attributed to I3C (and DIM).
Finally, it is also important to highlight some controversies that emerged from different studies on the cellular effect of high I3C concentration. Rather than I3C, this effect should be attributed to its DIM derivative(s), because I3C rarely, if ever, is detected in the blood after oral ingestion [81]. Moreover, it has been proven that in humans, a significant portion of DIM is actually present in the form of its metabolites, having an unknown pharmacological activity [82].
Although I3C and DIM have been subjected to clinical trials in humans, essentially to investigate their efficacy against breast and prostate cancer [44][81][83][84][85][86][87][88], different epidemiological studies have been carried out on the population that have brought to light a negative relationship between the intake of cruciferous vegetables and some cancers [68][89][90][91][92].
Notably, except for one case, these clinical studies consider the therapeutic potential of DIM in cancer and not the chemopreventive activity, which in fact requires double-blind, placebo-controlled studies in disease-free subjects to confirm its potency and efficacy as a supplement.
References
- Kliebenstein, D.J.; Kroymann, J.; Mitchell-Olds, T. The glucosinolate–myrosinase system in an ecological and evolutionary context. Curr. Opin. Plant Biol. 2005, 8, 264–271.
- Zhao, Y.; Wang, J.; Liu, Y.; Miao, H.; Cai, C.; Shao, Z.; Guo, R.; Sun, B.; Jia, C.; Zhang, L.; et al. Classic myrosinase-dependent degradation of indole glucosinolate attenuates fumonisin B1-induced programmed cell death in Arabidopsis. Plant J. 2015, 81, 920–933.
- Ağagündüz, D.; Şahin, T.Ö.; Yılmaz, B.; Ekenci, K.D.; Duyar, Ö.Ş.; Capasso, R. Cruciferous vegetables and their bioactive metabolites: From prevention to novel therapies of colorectal cancer. Evid. Based Complement. Alternat. Med. 2022, 11, 1534083.
- Chen, X.; Fan, H.; Zhang, S.; Yu, C.; Wang, W. Facile installation of 2-reverse prenyl functionality into indoles by a tandem n-alkylation-aza-cope rearrangement reaction and its application in synthesis. Chem. Eur. J. 2016, 22, 716–723.
- Zhang, C.; Xu, D.; Wang, J.; Kang, C. Efficient synthesis and biological activity of novel indole derivatives as VEGFR-2 tyrosine kinase inhibitors. Russ. J. Gen. Chem. 2017, 87, 3006–3016.
- Downey, C.W.; Poff, C.D.; Nizinski, A.N. Friedel-Crafts hydroxyalkylation of indoles mediated by trimethylsilyl trifluoromethanesulfonate. J. Org. Chem. 2015, 80, 10364–10369.
- Verhoeven, D.T.; Goldbohm, R.A.; van Poppel, G.; Verhagen, H.; van den Brandt, P.A. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol. Biomarkers Prev. 1996, 5, 733–748.
- Firestone, G.L.; Bjeldanes, L.F. Indole-3-carbinol and 3-3’-diindolylmethane antiproliferative signaling pathways control cell-cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 transcription factor interactions. J. Nutr. 2003, 133, 2448S–2455S.
- Weng, J.-R.; Tsai, C.-H.; Kulp, S.K.; Chen, C.-S. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008, 262, 153–163.
- Katz, E.; Nisani, S.; Chamovitz, D.A. Indole-3-carbinol: A plant hormone combatting cancer. F1000Research 2018, 7, 689.
- Quirit, J.G.; Lavrenov, S.N.; Poindexter, K.; Xu, J.; Kyauk, C.; Durkin, K.A.; Aronchik, I.; Tomasiak, T.; Solomatin, Y.A.; Preobrazhenskaya, M.N.; et al. Indole-3-carbinol (I3C) analogues are potent small molecule inhibitors of NEDD4-1 ubiquitin ligase activity that disrupt proliferation of human melanoma cells. Biochem. Pharmacol. 2017, 127, 13–27.
- Lee, Y.-R.; Chen, M.; Lee, J.D.; Zhang, J.; Lin, S.-Y.; Fu, T.-M.; Chen, H.; Ishikawa, T.; Chiang, S.-Y.; Katon, J.; et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science 2019, 364, eaau0159.
- Kishikawa, T.; Higuchi, H.; Wang, L.; Panch, N.; Maymi, V.; Best, S.; Lee, S.; Notoya, G.; Toker, A.; Matesic, L.E.; et al. WWP1 inactivation enhances efficacy of PI3K inhibitors while suppressing their toxicities in breast cancer models. J. Clin. Investig. 2021, 131, e140436.
- Song, M.S.; Pandolfi, P.P. The HECT family of E3 ubiquitin ligases and PTEN. Semin. Cancer Biol. 2022, 85, 43–51.
- Del Priore, G.; Gudipudi, D.K.; Montemarano, N.; Restivo, A.M.; Malanowska-Stega, J.; Arslan, A.A. Oral diindolylmethane (DIM): Pilot evaluation of a nonsurgical treatment for cervical dysplasia. Gynecol. Oncol. 2010, 116, 464–467.
- Rajoria, S.; Suriano, R.; Parmar, P.S.; Wilson, Y.L.; Megwalu, U.; Moscatello, A.; Bradlow, H.L.; Sepkovic, D.W.; Geliebter, J.; Schantz, S.P.; et al. 3,3’-diindolylmethane modulates estrogen metabolism in patients with thyroid proliferative disease: A pilot study. Thyroid 2011, 21, 299–304.
- Sepkovic, D.W.; Raucci, L.; Stein, J.; Carlisle, A.D.; Auborn, K.; Ksieski, H.B.; Nyirenda, T.; Bradlow, H.L. 3,3’-Diindolylmethane increases serum interferon-γ levels in the K14-HPV16 transgenic mouse model for cervical cancer. In Vivo 2012, 26, 207–211.
- Fan, S.; Meng, Q.; Xu, J.; Jiao, Y.; Zhao, L.; Zhang, X.; Sarkar, F.H.; Brown, M.L.; Dritschilo, A.; Rosen, E.M. DIM (3,3’-diindolylmethane) confers protection against ionizing radiation by a unique mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18650–18655.
- Perez-Chacon, G.; de Los Rios, C.; Zapata, J.M. Indole-3-carbinol induces cMYC and IAP-family downmodulation and promotes apoptosis of Epstein-Barr virus (EBV)-positive but not of EBV-negative Burkitt’s lymphoma cell lines. Pharmacol. Res. 2014, 89, 46–56.
- Ashrafian, L.; Sukhikh, G.; Kiselev, V.; Paltsev, M.; Drukh, V.; Kuznetsov, I.; Muyzhnek, E.; Apolikhina, I.; Andrianova, E. Double-blind randomized placebo-controlled multicenter clinical trial (phase IIa) on diindolylmethane’s efficacy and safety in the treatment of CIN: Implications for cervical cancer prevention. EPMA J. 2015, 6, 25.
- Morales-Prieto, D.M.; Herrmann, J.; Osterwald, H.; Kochhar, P.S.; Schleussner, E.; Markert, U.R.; Oettel, M. Comparison of dienogest effects upon 3,3’-diindolylmethane supplementation in models of endometriosis and clinical cases. Reprod. Biol. 2018, 18, 252–258.
- Rzemieniec, J.; Wnuk, A.; Lasoń, W.; Bilecki, W.; Kajta, M. The neuroprotective action of 3,3’-diindolylmethane against ischemia involves an inhibition of apoptosis and autophagy that depends on HDAC and AhR/CYP1A1 but not ERα/CYP19A1 signaling. Apoptosis 2019, 24, 435–452.
- Esteve, M. Mechanisms underlying biological effects of cruciferous glucosinolate-derived isothiocyanates/indoles: A focus on metabolic syndrome. Front. Nutr. 2020, 7, 111.
- NIH ClinicalTrials.gov. Available online: https://beta.clinicaltrials.gov/search?distance=50&term=indole-3-carbinol&viewType=Table&limit=25 (accessed on 20 December 2022).
- Karimabad, M.N.; Mahmoodi, M.; Jafarzadeh, A.; Darekordi, A.; Hajizadeh, M.R.; Hassanshahi, G. Molecular targets, anti-cancer properties and potency of synthetic indole-3-carbinol derivatives. Mini Rev. Med. Chem. 2019, 19, 540–554.
- Mazurakova, A.; Koklesova, L.; Samec, M.; Kudela, E.; Kajo, K.; Skuciova, V.; Csizmár, S.H.; Mestanova, V.; Pec, M.; Adamkov, M.; et al. Anti-breast cancer effects of phytochemicals: Primary, secondary, and tertiary care. EPMA J. 2022, 13, 315–334.
- Biancolella, M.; Testa, B.; Baghernajad Salehi, L.; D’Apice, M.R.; Novelli, G. Genetics and genomics of breast cancer: Update and translational perspectives. Semin. Cancer Biol. 2021, 72, 27–35.
- Costa, K.M.N.; Araújo, C.B.B.; Barros, A.L.S.; Sato, M.R.; Oshiro-Júnior, J.A. Nanostructured lipid carrier as a strategy for the treatment of breast cancer. In Interdisciplinary Cancer Research; Springer: Cham, Switzerland, 2022; pp. 1–27.
- Firestone, G.L.; Sundar, S.N. Minireview: Modulation of hormone receptor signaling by dietary anticancer indoles. Mol. Endocrinol. 2009, 23, 1940–1947.
- Ahmad, A.; Sakr, W.A.; Rahman, K.M.W. Anticancer properties of indole compounds: Mechanism of apoptosis induction and role in chemotherapy. Curr. Drug Targets 2010, 11, 652–666.
- Ahmad, A.; Sakr, W.A.; Rahman, K.M.W. Novel targets for detection of cancer and their modulation by chemopreventive natural compounds. Front. Biosci. 2012, 4, 410–425.
- Sarkar, F.H.; Li, Y.; Wang, Z.; Kong, D. Cellular signaling perturbation by natural products. Cell. Signal. 2009, 21, 1541–1547.
- Bai, L.-Y.; Weng, J.-R.; Chiu, C.-F.; Wu, C.-Y.; Yeh, S.-P.; Sargeant, A.M.; Lin, P.-H.; Liao, Y.-M. OSU-A9, an indole-3-carbinol derivative, induces cytotoxicity in acute myeloid leukemia through reactive oxygen species-mediated apoptosis. Biochem. Pharmacol. 2013, 86, 1430–1440.
- Aronchik, I.; Kundu, A.; Quirit, J.G.; Firestone, G.L. The antiproliferative response of indole-3-carbinol in human melanoma cells is triggered by an interaction with NEDD4-1 and disruption of wild-type PTEN degradation. Mol. Cancer Res. 2014, 12, 1621–1634.
- Nguyen, H.H.; Aronchik, I.; Brar, G.A.; Nguyen, D.H.H.; Bjeldanes, L.F.; Firestone, G.L. The dietary phytochemical indole-3-carbinol is a natural elastase enzymatic inhibitor that disrupts cyclin E protein processing. Proc. Natl. Acad. Sci. USA 2008, 105, 19750–19755.
- Aronchik, I.; Bjeldanes, L.F.; Firestone, G.L. Direct inhibition of elastase activity by indole-3-carbinol triggers a CD40-TRAF regulatory cascade that disrupts NF-kappaB transcriptional activity in human breast cancer cells. Cancer Res. 2010, 70, 4961–4971.
- Aronchik, I.; Chen, T.; Durkin, K.A.; Horwitz, M.S.; Preobrazhenskaya, M.N.; Bjeldanes, L.F.; Firestone, G.L. Target protein interactions of indole-3-carbinol and the highly potent derivative 1-benzyl-I3C with the C-terminal domain of human elastase uncouples cell cycle arrest from apoptotic signaling. Mol. Carcinog. 2012, 51, 881–894.
- Scully, R.; Chen, J.; Plug, A.; Xiao, Y.; Weaver, D.; Feunteun, J.; Ashley, T.; Livingston, D.M. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell 1997, 88, 265–275.
- Reed, G.A.; Peterson, K.S.; Smith, H.J.; Gray, J.C.; Sullivan, D.K.; Mayo, M.S.; Crowell, J.A.; Hurwitz, A. A phase I study of indole-3-carbinol in women: Tolerability and effects. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1953–1960.
- McMenamin, M.E.; Soung, P.; Perera, S.; Kaplan, I.; Loda, M.; Sellers, W.R. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999, 59, 4291–4296.
- Vincent, E.E.; Elder, D.J.E.; Thomas, E.C.; Phillips, L.; Morgan, C.; Pawade, J.; Sohail, M.; May, M.T.; Hetzel, M.R.; Tavaré, J.M. Akt phosphorylation on Thr308 but not on Ser473 correlates with Akt protein kinase activity in human non-small cell lung cancer. Br. J. Cancer 2011, 104, 1755–1761.
- Cover, C.M.; Hsieh, S.J.; Tran, S.H.; Hallden, G.; Kim, G.S.; Bjeldanes, L.F.; Firestone, G.L. Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling. J. Biol. Chem. 1998, 273, 3838–3847.
- Kim, Y.S.; Milner, J.A. Targets for indole-3-carbinol in cancer prevention. J. Nutr. Biochem. 2005, 16, 65–73.
- Watson, G.W.; Beaver, L.M.; Williams, D.E.; Dashwood, R.H.; Ho, E. Phytochemicals from cruciferous vegetables, epigenetics, and prostate cancer prevention. AAPS J. 2013, 15, 951–961.
- Kaur, P.; Shorey, L.E.; Ho, E.; Dashwood, R.H.; Williams, D.E. The epigenome as a potential mediator of cancer and disease prevention in prenatal development. Nutr. Rev. 2013, 71, 441–457.
- Wu, T.-Y.; Khor, T.O.; Su, Z.-Y.; Saw, C.L.-L.; Shu, L.; Cheung, K.-L.; Huang, Y.; Yu, S.; Kong, A.-N.T. Epigenetic modifications of Nrf2 by 3,3’-diindolylmethane in vitro in TRAMP C1 cell line and in vivo TRAMP prostate tumors. AAPS J. 2013, 15, 864–874.
- Wong, C.P.; Hsu, A.; Buchanan, A.; Palomera-Sanchez, Z.; Beaver, L.M.; Houseman, E.A.; Williams, D.E.; Dashwood, R.H.; Ho, E. Effects of sulforaphane and 3,3’-diindolylmethane on genome-wide promoter methylation in normal prostate epithelial cells and prostate cancer cells. PLoS ONE 2014, 9, e86787.
- Singh, A.A.; Patil, M.P.; Kang, M.-J.; Niyonizigiye, I.; Kim, G.-D. Biomedical application of Indole-3-carbinol: A mini-review. Phytochem. Lett. 2021, 41, 49–54.
- Shorey, L.E.; Hagman, A.M.; Williams, D.E.; Ho, E.; Dashwood, R.H.; Benninghoff, A.D. 3,3’-Diindolylmethane induces G1 arrest and apoptosis in human acute T-cell lymphoblastic leukemia cells. PLoS ONE 2012, 7, e34975.
- Kiselev, V.I.; Drukh, V.M.; Muyzhnek, E.L.; Kuznetsov, I.N.; Pchelintseva, O.I.; Paltsev, M.A. Preclinical antitumor activity of the diindolylmethane formulation in xenograft mouse model of prostate cancer. Exp. Oncol. 2014, 36, 90–93.
- Wu, Y.; Li, R.W.; Huang, H.; Fletcher, A.; Yu, L.; Pham, Q.; Yu, L.; He, Q.; Wang, T.T.Y. Inhibition of Tumor Growth by Dietary Indole-3-Carbinol in a Prostate Cancer Xenograft Model May Be Associated with Disrupted Gut Microbial Interactions. Nutrients 2019, 11, 467.
- Kim, D.J.; Han, B.S.; Ahn, B.; Hasegawa, R.; Shirai, T.; Ito, N.; Tsuda, H. Enhancement by indole-3-carbinol of liver and thyroid gland neoplastic development in a rat medium-term multiorgan carcinogenesis model. Carcinogenesis 1997, 18, 377–381.
- Shimamoto, K.; Hayashi, H.; Taniai, E.; Morita, R.; Imaoka, M.; Ishii, Y.; Suzuki, K.; Shibutani, M.; Mitsumori, K. Antioxidant N-acetyl-L-cysteine (NAC) supplementation reduces reactive oxygen species (ROS)-mediated hepatocellular tumor promotion of indole-3-carbinol (I3C) in rats. J. Toxicol. Sci. 2011, 36, 775–786.
- Oganesian, A.; Hendricks, J.D.; Williams, D.E. Long term dietary indole-3-carbinol inhibits diethylnitrosamine-initiated hepatocarcinogenesis in the infant mouse model. Cancer Lett. 1997, 118, 87–94.
- Kawajiri, K.; Kobayashi, Y.; Ohtake, F.; Ikuta, T.; Matsushima, Y.; Mimura, J.; Pettersson, S.; Pollenz, R.S.; Sakaki, T.; Hirokawa, T.; et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc. Natl. Acad. Sci. USA 2009, 106, 13481–13486.
- Kim, Y.H.; Kwon, H.-S.; Kim, D.H.; Shin, E.K.; Kang, Y.-H.; Park, J.H.Y.; Shin, H.-K.; Kim, J.-K. 3,3’-diindolylmethane attenuates colonic inflammation and tumorigenesis in mice. Inflamm. Bowel Dis. 2009, 15, 1164–1173.
- Lubet, R.A.; Heckman, B.M.; De Flora, S.L.; Steele, V.E.; Crowell, J.A.; Juliana, M.M.; Grubbs, C.J. Effects of 5,6-benzoflavone, indole-3-carbinol (I3C) and diindolylmethane (DIM) on chemically-induced mammary carcinogenesis: Is DIM a substitute for I3C? Oncol. Rep. 2011, 26, 731–736.
- Benninghoff, A.D.; Williams, D.E. The role of estrogen receptor β in transplacental cancer prevention by indole-3-carbinol. Cancer Prev. Res. 2013, 6, 339–348.
- Staub, R.E.; Feng, C.; Onisko, B.; Bailey, G.S.; Firestone, G.L.; Bjeldanes, L.F. Fate of indole-3-carbinol in cultured human breast tumor cells. Chem. Res. Toxicol. 2002, 15, 101–109.
- Wang, T.T.Y.; Milner, M.J.; Milner, J.A.; Kim, Y.S. Estrogen receptor alpha as a target for indole-3-carbinol. J. Nutr. Biochem. 2006, 17, 659–664.
- Staub, R.E.; Onisko, B.; Bjeldanes, L.F. Fate of 3,3’-diindolylmethane in cultured MCF-7 human breast cancer cells. Chem. Res. Toxicol. 2006, 19, 436–442.
- Bradlow, H.L. Review. Indole-3-carbinol as a chemoprotective agent in breast and prostate cancer. In Vivo 2008, 22, 441–445.
- Saati, G.E.; Archer, M.C. Inhibition of fatty acid synthase and Sp1 expression by 3,3’-diindolylmethane in human breast cancer cells. Nutr. Cancer 2011, 63, 790–794.
- Tin, A.S.; Park, A.H.; Sundar, S.N.; Firestone, G.L. Essential role of the cancer stem/progenitor cell marker nucleostemin for indole-3-carbinol anti-proliferative responsiveness in human breast cancer cells. BMC Biol. 2014, 12, 72.
- Thomson, C.A.; Ho, E.; Strom, M.B. Chemopreventive properties of 3,3’-diindolylmethane in breast cancer: Evidence from experimental and human studies. Nutr. Rev. 2016, 74, 432–443.
- Lee, J. 3,3’-Diindolylmethane inhibits TNF-α- and TGF-β-induced epithelial- mesenchymal transition in breast cancer cells. Nutr. Cancer 2019, 71, 992–1006.
- Maruthanila, V.L.; Poornima, J.; Mirunalini, S. Attenuation of carcinogenesis and the mechanism underlying by the influence of indole-3-carbinol and its metabolite 3,3’-diindolylmethane: A therapeutic marvel. Adv. Pharmacol. Sci. 2014, 2014, 832161.
- Fujioka, N.; Fritz, V.; Upadhyaya, P.; Kassie, F.; Hecht, S.S. Research on cruciferous vegetables, indole-3-carbinol, and cancer prevention: A tribute to Lee W. Wattenberg. Mol. Nutr. Food Res. 2016, 60, 1228–1238.
- Kassie, F.; Anderson, L.B.; Scherber, R.; Yu, N.; Lahti, D.; Upadhyaya, P.; Hecht, S.S. Indole-3-carbinol inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo(a)pyrene-induced lung tumorigenesis in A/J mice and modulates carcinogen-induced alterations in protein levels. Cancer Res. 2007, 67, 6502–6511.
- Wong, G.Y.; Bradlow, L.; Sepkovic, D.; Mehl, S.; Mailman, J.; Osborne, M.P. Dose-ranging study of indole-3-carbinol for breast cancer prevention. J. Cell. Biochem. Suppl. 1997, 28–29, 111–116.
- Cashman, J.R.; Zhang, J. Human flavin-containing monooxygenases. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 65–100.
- Krueger, S.K.; Williams, D.E. Mammalian flavin-containing monooxygenases: Structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol. Ther. 2005, 106, 357–387.
- Larsen-Su, S.; Williams, D.E. Dietary indole-3-carbinol inhibits FMO activity and the expression of flavin-containing monooxygenase form 1 in rat liver and intestine. Drug Metab. Dispos. 1996, 24, 927–931.
- Cashman, J.R.; Xiong, Y.; Lin, J.; Verhagen, H.; van Poppel, G.; van Bladeren, P.J.; Larsen-Su, S.; Williams, D.E. In vitro and in vivo inhibition of human flavin-containing monooxygenase form 3 (FMO3) in the presence of dietary indoles. Biochem. Pharmacol. 1999, 58, 1047–1055.
- Katchamart, S.; Stresser, D.M.; Dehal, S.S.; Kupfer, D.; Williams, D.E. Concurrent flavin-containing monooxygenase down-regulation and cytochrome P-450 induction by dietary indoles in rat: Implications for drug-drug interaction. Drug Metab. Dispos. 2000, 28, 930–936.
- Wang, T.; Shankar, K.; Ronis, M.J.; Mehendale, H.M. Potentiation of thioacetamide liver injury in diabetic rats is due to induced CYP2E1. J. Pharmacol. Exp. Ther. 2000, 294, 473–479.
- Katchamart, S.; Williams, D.E. Indole-3-carbinol modulation of hepatic monooxygenases CYP1A1, CYP1A2 and FMO1 in guinea pig, mouse and rabbit. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2001, 129, 377–384.
- Vogel, V.G. Reducing the risk of breast cancer with tamoxifen in women at increased risk. J. Clin. Oncol. 2001, 19, 87S–92S.
- Poon, G.K.; Walter, B.; Lønning, P.E.; Horton, M.N.; McCague, R. Identification of tamoxifen metabolites in human Hep G2 cell line, human liver homogenate, and patients on long-term therapy for breast cancer. Drug Metab. Dispos. 1995, 23, 377–382.
- Centofanti, F.; Alonzi, T.; Latini, A.; Spitaleri, P.; Murdocca, M.; Chen, X.; Cui, W.; Shang, Q.; Goletti, D.; Shi, Y.; et al. Indole-3-carbinol in vitro antiviral activity against SARS-CoV-2 virus and in vivo toxicity. Cell Death Discov. 2022, 8, 491.
- Bradlow, H.L.; Zeligs, M.A. Diindolylmethane (DIM) spontaneously forms from indole-3-carbinol (I3C) during cell culture experiments. In Vivo 2010, 24, 387–391.
- Maier, M.L.V.; Siddens, L.K.; Uesugi, S.L.; Choi, J.; Leonard, S.W.; Pennington, J.M.; Tilton, S.C.; Smith, J.N.; Ho, E.; Chow, H.H.S.; et al. 3,3’-Diindolylmethane Exhibits Significant Metabolism after Oral Dosing in Humans. Drug Metab. Dispos. 2021, 49, 694–705.
- Dalessandri, K.M.; Firestone, G.L.; Fitch, M.D.; Bradlow, H.L.; Bjeldanes, L.F. Pilot study: Effect of 3,3’-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr. Cancer 2004, 50, 161–167.
- Heath, E.I.; Heilbrun, L.K.; Li, J.; Vaishampayan, U.; Harper, F.; Pemberton, P.; Sarkar, F.H. A phase I dose-escalation study of oral BR-DIM (BioResponse 3,3’- Diindolylmethane) in castrate-resistant, non-metastatic prostate cancer. Am. J. Transl. Res. 2010, 2, 402–411.
- Li, Y.; Sarkar, F.H. Role of BioResponse 3,3’-diindolylmethane in the treatment of human prostate cancer: Clinical experience. Med. Princ. Pract. 2016, 25 (Suppl. S2), 11–17.
- Hwang, C.; Sethi, S.; Heilbrun, L.K.; Gupta, N.S.; Chitale, D.A.; Sakr, W.A.; Menon, M.; Peabody, J.O.; Smith, D.W.; Sarkar, F.H.; et al. Anti-androgenic activity of absorption-enhanced 3, 3’-diindolylmethane in prostatectomy patients. Am. J. Transl. Res. 2016, 8, 166–176.
- Paltsev, M.; Kiselev, V.; Drukh, V.; Muyzhnek, E.; Kuznetsov, I.; Andrianova, E.; Baranovskiy, P. First results of the double-blind randomized placebo-controlled multicenter clinical trial of DIM-based therapy designed as personalized approach to reverse prostatic intraepithelial neoplasia (PIN). EPMA J. 2016, 7, 5.
- Thomson, C.A.; Chow, H.H.S.; Wertheim, B.C.; Roe, D.J.; Stopeck, A.; Maskarinec, G.; Altbach, M.; Chalasani, P.; Huang, C.; Strom, M.B.; et al. A randomized, placebo-controlled trial of diindolylmethane for breast cancer biomarker modulation in patients taking tamoxifen. Breast Cancer Res. Treat. 2017, 165, 97–107.
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236.
- Hayes, J.D.; Kelleher, M.O.; Eggleston, I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr. 2008, 47 (Suppl. S2), 73–88.
- Ambrosone, C.B.; Tang, L. Cruciferous vegetable intake and cancer prevention: Role of nutrigenetics. Cancer Prev. Res. 2009, 2, 298–300.
- Kim, M.K.; Park, J.H.Y. Cruciferous vegetable intake and the risk of human cancer: Epidemiological evidence. Proc. Nutr. Soc. 2009, 68, 103–110.
More
Information
Subjects:
Food Science & Technology; Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
4 times
(View History)
Update Date:
24 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




