
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexander Jordan | -- | 2247 | 2023-05-21 17:33:16 | | | |
| 2 | Lindsay Dong | -1 word(s) | 2246 | 2023-05-22 02:57:59 | | | | |
| 3 | Lindsay Dong | + 4 word(s) | 2250 | 2023-05-22 02:58:32 | | |
Video Upload Options
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease with distinct phenotypes, each having distinct treatment needs. Eosinophilic airway inflammation is present in a subset of COPD patients in whom it can act as a driver of exacerbations. Blood eosinophil counts are a reliable way to identify patients with an eosinophilic phenotype, and these measurements have proven to be successful in guiding the use of corticosteroids in moderate and severe COPD exacerbations. Antibiotic use in COPD patients induces a risk of Clostridium difficile infection, diarrhea, and antibiotic resistance. Procalcitonin could possibly guide antibiotic treatment in patients admitted with acute exacerbations of COPD (AECOPD). Current studies in COPD patients were successful in reducing exposure to antibiotics with no changes in mortality or length of stay. Daily monitoring of blood eosinophils is a safe and effective way to reduce oral corticosteroid exposure and side effects for acute exacerbations.
1. Introduction
2. Blood Eosinophils as a Treatment Response Marker
3. Eosinophil-Guided OCS for Acute Exacerbations
4. Eosinophil-Guided ICS for Stable COPD
5. Future Perspectives of Eosinophil-Guided Treatment
6. Procalcitonin as a Biomarker in Respiratory Infections
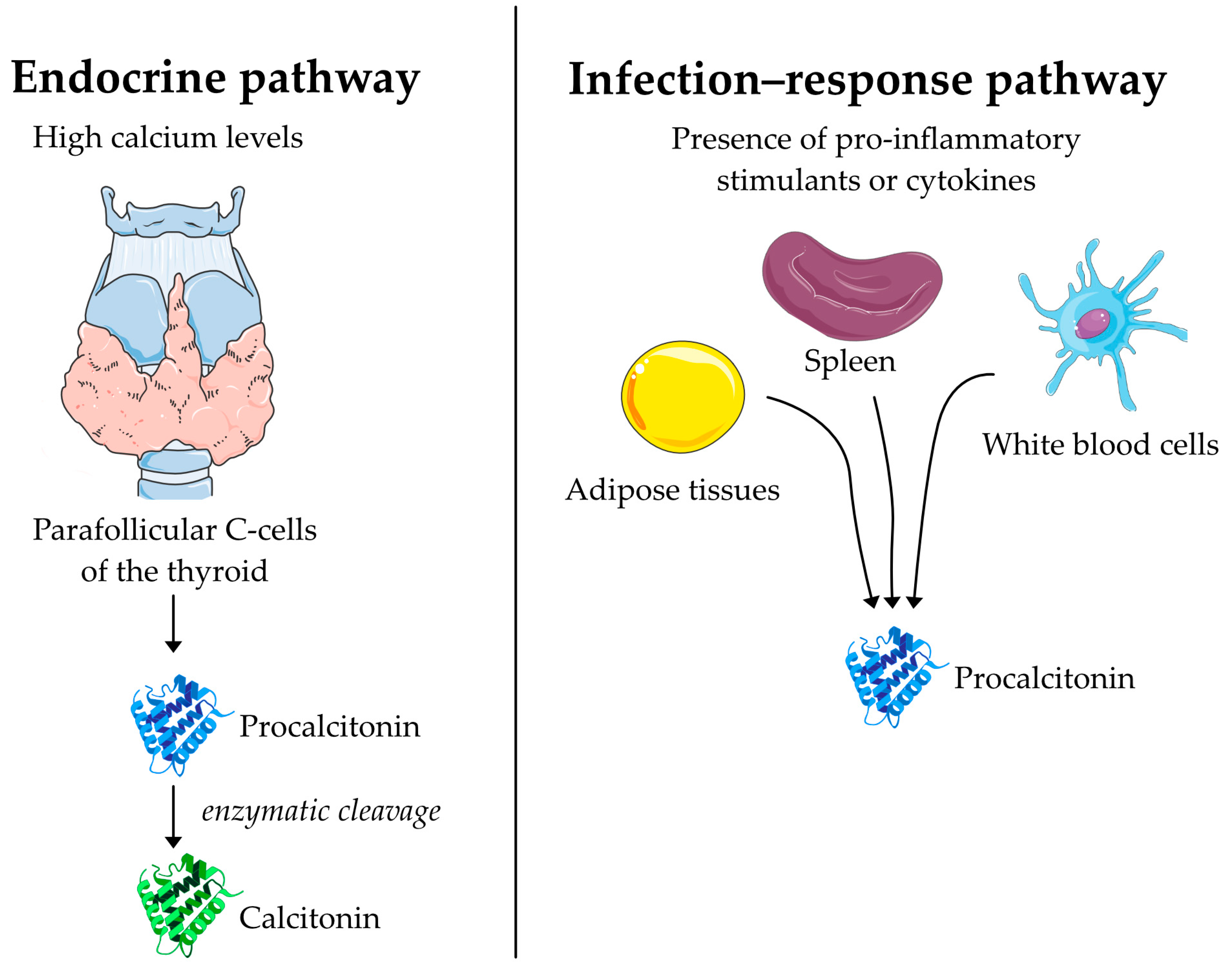
7. Procalcitonin Guidance to Reduce Antibiotic Exposure in Acute Exacerbations of COPD
8. Conclusions
References
- Christenson, S.A.; Smith, B.M.; Bafadhel, M.; Putcha, N. Chronic obstructive pulmonary disease. Lancet 2022, 399, 2227–2242.
- Diseases, G.B.D.; Injuries, C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222.
- Agusti, A.; Calverley, P.M.; Celli, B.; Coxson, H.O.; Edwards, L.D.; Lomas, D.A.; MacNee, W.; Miller, B.E.; Rennard, S.; Silverman, E.K.; et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir. Res. 2010, 11, 122.
- Brightling, C.; Greening, N. Airway inflammation in COPD: Progress to precision medicine. Eur. Respir. J. 2019, 54, 1900651.
- Woodruff, P.G.; Barr, R.G.; Bleecker, E.; Christenson, S.A.; Couper, D.; Curtis, J.L.; Gouskova, N.A.; Hansel, N.N.; Hoffman, E.A.; Kanner, R.E.; et al. Clinical Significance of Symptoms in Smokers with Preserved Pulmonary Function. N. Engl. J. Med. 2016, 374, 1811–1821.
- Doiron, D.; de Hoogh, K.; Probst-Hensch, N.; Fortier, I.; Cai, Y.; De Matteis, S.; Hansell, A.L. Air pollution, lung function and COPD: Results from the population-based UK Biobank study. Eur. Respir. J. 2019, 54, 1802140.
- Silverman, E.K. Genetics of COPD. Annu. Rev. Physiol. 2020, 82, 413–431.
- Beech, A.; Jackson, N.; Singh, D. Identification of COPD Inflammatory Endotypes Using Repeated Sputum Eosinophil Counts. Biomedicines 2022, 10, 2611.
- Schumann, D.M.; Tamm, M.; Kostikas, K.; Stolz, D. Stability of the Blood Eosinophilic Phenotype in Stable and Exacerbated COPD. Chest 2019, 156, 456–465.
- Benson, V.S.; Hartl, S.; Barnes, N.; Galwey, N.; Van Dyke, M.K.; Kwon, N. Blood eosinophil counts in the general population and airways disease: A comprehensive review and meta-analysis. Eur. Respir. J. 2022, 59, 2004590.
- Kolsum, U.; Donaldson, G.C.; Singh, R.; Barker, B.L.; Gupta, V.; George, L.; Webb, A.J.; Thurston, S.; Brookes, A.J.; McHugh, T.D.; et al. Blood and sputum eosinophils in COPD; relationship with bacterial load. Respir. Res. 2017, 18, 88.
- Wang, Z.; Locantore, N.; Haldar, K.; Ramsheh, M.Y.; Beech, A.S.; Ma, W.; Brown, J.R.; Tal-Singer, R.; Barer, M.R.; Bafadhel, M.; et al. Inflammatory Endotype-associated Airway Microbiome in Chronic Obstructive Pulmonary Disease Clinical Stability and Exacerbations: A Multicohort Longitudinal Analysis. Am. J. Respir. Crit. Care Med. 2021, 203, 1488–1502.
- Konig, I.R.; Fuchs, O.; Hansen, G.; von Mutius, E.; Kopp, M.V. What is precision medicine? Eur. Respir. J. 2017, 50, 1700391.
- Meteran, H.; Sivapalan, P.; Staehr Jensen, J.U. Treatment Response Biomarkers in Asthma and COPD. Diagnostics 2021, 11, 1668.
- Paudel, R.; Dogra, P.; Montgomery-Yates, A.A.; Coz Yataco, A. Procalcitonin: A promising tool or just another overhyped test? Int. J. Med. Sci. 2020, 17, 332–337.
- Mathioudakis, A.G.; Chatzimavridou-Grigoriadou, V.; Corlateanu, A.; Vestbo, J. Procalcitonin to guide antibiotic administration in COPD exacerbations: A meta-analysis. Eur. Respir. Rev. 2017, 26, 160073.
- Halici, A.; Hur, I.; Abatay, K.; Cetin, E.; Halici, F.; Ozkan, S. The role of presepsin in the diagnosis of chronic obstructive pulmonary disease acute exacerbation with pneumonia. Biomark. Med. 2020, 14, 31–41.
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27.
- Barnes, P.J. Inflammatory endotypes in COPD. Allergy 2019, 74, 1249–1256.
- Culpitt, S.V.; Maziak, W.; Loukidis, S.; Nightingale, J.A.; Matthews, J.L.; Barnes, P.J. Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 160, 1635–1639.
- O’Sullivan, J.A.; Bochner, B.S. Eosinophils and eosinophil-associated diseases: An update. J. Allergy Clin. Immunol. 2018, 141, 505–517.
- Fahy, J.V. Type 2 inflammation in asthma--present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65.
- Schleimer, R.P.; Bochner, B.S. The effects of glucocorticoids on human eosinophils. J. Allergy Clin. Immunol. 1994, 94, 1202–1213.
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671.
- Negewo, N.A.; McDonald, V.M.; Baines, K.J.; Wark, P.A.; Simpson, J.L.; Jones, P.W.; Gibson, P.G. Peripheral blood eosinophils: A surrogate marker for airway eosinophilia in stable COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1495–1504.
- DiSantostefano, R.L.; Hinds, D.; Le, H.V.; Barnes, N.C. Relationship between blood eosinophils and clinical characteristics in a cross-sectional study of a US population-based COPD cohort. Respir. Med. 2016, 112, 88–96.
- Martinez-Gestoso, S.; Garcia-Sanz, M.T.; Calvo-Alvarez, U.; Doval-Oubina, L.; Camba-Matos, S.; Salgado, F.J.; Munoz, X.; Perez-Lopez-Corona, P.; Gonzalez-Barcala, F.J. Variability of blood eosinophil count and prognosis of COPD exacerbations. Ann. Med. 2021, 53, 1152–1158.
- Bafadhel, M.; Peterson, S.; De Blas, M.A.; Calverley, P.M.; Rennard, S.I.; Richter, K.; Fageras, M. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: A post-hoc analysis of three randomised trials. Lancet Respir. Med. 2018, 6, 117–126.
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632.
- Vedel-Krogh, S.; Nielsen, S.F.; Lange, P.; Vestbo, J.; Nordestgaard, B.G. Blood Eosinophils and Exacerbations in Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am. J. Respir. Crit. Care Med. 2016, 193, 965–974.
- Zeiger, R.S.; Tran, T.N.; Butler, R.K.; Schatz, M.; Li, Q.; Khatry, D.B.; Martin, U.; Kawatkar, A.A.; Chen, W. Relationship of Blood Eosinophil Count to Exacerbations in Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. Pract. 2018, 6, 944–954.
- Duman, D.; Aksoy, E.; Agca, M.C.; Kocak, N.D.; Ozmen, I.; Akturk, U.A.; Gungor, S.; Tepetam, F.M.; Eroglu, S.A.; Oztas, S.; et al. The utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophilia. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 2469–2478.
- Brightling, C.E.; McKenna, S.; Hargadon, B.; Birring, S.; Green, R.; Siva, R.; Berry, M.; Parker, D.; Monteiro, W.; Pavord, I.D.; et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax 2005, 60, 193–198.
- Pascoe, S.; Barnes, N.; Brusselle, G.; Compton, C.; Criner, G.J.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Hartley, B.; Lange, P.; et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: Analysis of the IMPACT trial. Lancet Respir. Med. 2019, 7, 745–756.
- Suissa, S.; Dell’Aniello, S.; Ernst, P. Comparative effectiveness of LABA-ICS versus LAMA as initial treatment in COPD targeted by blood eosinophils: A population-based cohort study. Lancet Respir. Med. 2018, 6, 855–862.
- de Groot, J.C.; Storm, H.; Amelink, M.; de Nijs, S.B.; Eichhorn, E.; Reitsma, B.H.; Bel, E.H.; Ten Brinke, A. Clinical profile of patients with adult-onset eosinophilic asthma. ERJ Open Res. 2016, 2, 00100–2015.
- Hastie, A.T.; Martinez, F.J.; Curtis, J.L.; Doerschuk, C.M.; Hansel, N.N.; Christenson, S.; Putcha, N.; Ortega, V.E.; Li, X.; Barr, R.G.; et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: An analysis of the SPIROMICS cohort. Lancet Respir. Med. 2017, 5, 956–967.
- Pizzichini, E.; Pizzichini, M.M.; Leigh, R.; Djukanovic, R.; Sterk, P.J. Safety of sputum induction. Eur. Respir. J. Suppl. 2002, 37, 9s–18s.
- Walters, J.A.; Tan, D.J.; White, C.J.; Gibson, P.G.; Wood-Baker, R.; Walters, E.H. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2014, 9, CD001288.
- Volmer, T.; Effenberger, T.; Trautner, C.; Buhl, R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: A focused review of the impact data in the literature. Eur. Respir. J. 2018, 52, 1800703.
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Pancholi, M.; Venge, P.; Lomas, D.A.; Barer, M.R.; Johnston, S.L.; Pavord, I.D.; et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: A randomized placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2012, 186, 48–55.
- Ramakrishnan, S.; Jeffers, H.; Langford-Wiley, B.; Davies, J.; Mahdi, M.; Court, C.; Binnian, I.; Bright, S.; Cartwright, S.; Fox, R.; et al. Point of care blood eosinophil guided oral prednisolone for COPD exacerbations: A multi-centre double blind randomised controlled trial (The STARR2 trial). Eur. Respir. J. 2022, 60, 4728.
- Eosinophil-Driven Corticotherapy for Patients Hospitalized for COPD Exacerbation (eo-Drive). Available online: https://clinicaltrials.gov/ct2/show/NCT04234360?recrs=abdf&type=Intr&cond=Copd&draw=3&rank=54 (accessed on 15 January 2023).
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease; 2023 Report. Available online: https://goldcopd.org/2023-gold-report-2/ (accessed on 7 March 2023).
- Wedzicha, J.A.; Banerji, D.; Chapman, K.R.; Vestbo, J.; Roche, N.; Ayers, R.T.; Thach, C.; Fogel, R.; Patalano, F.; Vogelmeier, C.F.; et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N. Engl. J. Med. 2016, 374, 2222–2234.
- Calverley, P.M.; Anderson, J.A.; Celli, B.; Ferguson, G.T.; Jenkins, C.; Jones, P.W.; Yates, J.C.; Vestbo, J.; TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N. Engl. J. Med. 2007, 356, 775–789.
- Eklof, J.; Ingebrigtsen, T.S.; Sorensen, R.; Saeed, M.I.; Alispahic, I.A.; Sivapalan, P.; Boel, J.B.; Bangsborg, J.; Ostergaard, C.; Dessau, R.B.; et al. Use of inhaled corticosteroids and risk of acquiring Pseudomonas aeruginosa in patients with chronic obstructive pulmonary disease. Thorax 2022, 77, 573–580.
- Mohsin, R.U.; Heerfordt, C.K.; Eklof, J.; Sivapalan, P.; Saeed, M.I.; Ingebrigtsen, T.S.; Nielsen, S.D.; Harboe, Z.B.; Iversen, K.K.; Bangsborg, J.; et al. Use of Inhaled Corticosteroids and Risk of Acquiring Haemophilus influenzae in Patients with Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2022, 11, 3539.
- Ronn, C.; Sivapalan, P.; Eklof, J.; Kamstrup, P.; Biering-Sorensen, T.; Bonnesen, B.; Harboe, Z.B.; Browatzki, A.; Kjaergaard, J.L.; Meyer, C.N.; et al. Hospitalization for chronic obstructive pulmonary disease and pneumonia: Association with the dose of inhaled corticosteroids. A nation-wide cohort study of 52 100 outpatients. Clin. Microbiol. Infect. 2022, 29, 523–529.
- Suissa, S.; Kezouh, A.; Ernst, P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am. J. Med. 2010, 123, 1001–1006.
- Ernst, P.; Baltzan, M.; Deschenes, J.; Suissa, S. Low-dose inhaled and nasal corticosteroid use and the risk of cataracts. Eur. Respir. J. 2006, 27, 1168–1174.
- Jordan, A.; Sivapalan, P.; Eklof, J.; Vestergaard, J.B.; Meteran, H.; Saeed, M.I.; Biering-Sorensen, T.; Lokke, A.; Seersholm, N.; Jensen, J.U.S. The Association between Use of ICS and Psychiatric Symptoms in Patients with COPD-A Nationwide Cohort Study of 49,500 Patients. Biomedicines 2021, 9, 1492.
- van der Meulen, M.; Amaya, J.M.; Dekkers, O.M.; Meijer, O.C. Association between use of systemic and inhaled glucocorticoids and changes in brain volume and white matter microstructure: A cross-sectional study using data from the UK Biobank. BMJ Open 2022, 12, e062446.
- Eosinophil-guided Reduction of Inhaled Corticosteroids (COPERNICOS). ClinicalTrials.gov. 2020. Available online: https://ClinicalTrials.gov/show/NCT04481555 (accessed on 7 March 2023).
- Allison, J.; Hall, L.; MacIntyre, I.; Craig, R.K. The construction and partial characterization of plasmids containing complementary DNA sequences to human calcitonin precursor polyprotein. Biochem. J. 1981, 199, 725–731.
- Le Moullec, J.M.; Jullienne, A.; Chenais, J.; Lasmoles, F.; Guliana, J.M.; Milhaud, G.; Moukhtar, M.S. The complete sequence of human preprocalcitonin. FEBS Lett. 1984, 167, 93–97.
- Kiriakopoulos, A.; Giannakis, P.; Menenakos, E. Calcitonin: Current concepts and differential diagnosis. Ther. Adv. Endocrinol. Metab. 2022, 13, 20420188221099344.
- Vijayan, A.L.; Ravindran, S.; Saikant, R.; Lakshmi, S.; Kartik, R. Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J. Intensive Care 2017, 5, 51.
- Assicot, M.; Gendrel, D.; Carsin, H.; Raymond, J.; Guilbaud, J.; Bohuon, C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993, 341, 515–518.
- Müller, B.; White, J.C.; Nylén, E.S.; Snider, R.H.; Becker, K.L.; Habener, J.F. Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J. Clin. Endocrinol. Metab. 2001, 86, 396–404.
- Lopez-Campos, J.L.; Hartl, S.; Pozo-Rodriguez, F.; Roberts, C.M.; On behalf of the European COPD Audit Team. Antibiotic Prescription for COPD Exacerbations Admitted to Hospital: European COPD Audit. PLoS ONE 2015, 10, e0124374.
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912.
- Huttner, A.; Harbarth, S.; Carlet, J.; Cosgrove, S.; Goossens, H.; Holmes, A.; Jarlier, V.; Voss, A.; Pittet, D. Antimicrobial resistance: A global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob. Resist. Infect. Control 2013, 2, 31.
- Schuetz, P.; Christ-Crain, M.; Thomann, R.; Falconnier, C.; Wolbers, M.; Widmer, I.; Neidert, S.; Fricker, T.; Blum, C.; Schild, U.; et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA 2009, 302, 1059–1066.
- Huang, D.T.; Yealy, D.M.; Filbin, M.R.; Brown, A.M.; Chang, C.H.; Doi, Y.; Donnino, M.W.; Fine, J.; Fine, M.J.; Fischer, M.A.; et al. Procalcitonin-Guided Use of Antibiotics for Lower Respiratory Tract Infection. N. Engl. J. Med. 2018, 379, 236–249.
- Christ-Crain, M.; Jaccard-Stolz, D.; Bingisser, R.; Gencay, M.M.; Huber, P.R.; Tamm, M.; Muller, B. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: Cluster-randomised, single-blinded intervention trial. Lancet 2004, 363, 600–607.
- Corti, C.; Fally, M.; Fabricius-Bjerre, A.; Mortensen, K.; Jensen, B.N.; Andreassen, H.F.; Porsbjerg, C.; Knudsen, J.D.; Jensen, J.U. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1381–1389.
- Stolz, D.; Christ-Crain, M.; Bingisser, R.; Leuppi, J.; Miedinger, D.; Muller, C.; Huber, P.; Muller, B.; Tamm, M. Antibiotic treatment of exacerbations of COPD: A randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007, 131, 9–19.
- Verduri, A.; Luppi, F.; D’Amico, R.; Balduzzi, S.; Vicini, R.; Liverani, A.; Ruggieri, V.; Plebani, M.; Barbaro, M.P.; Spanevello, A.; et al. Antibiotic treatment of severe exacerbations of chronic obstructive pulmonary disease with procalcitonin: A randomized noninferiority trial. PLoS ONE 2015, 10, e0118241.




