Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease with distinct phenotypes, each having distinct treatment needs. Eosinophilic airway inflammation is present in a subset of COPD patients in whom it can act as a driver of exacerbations. Blood eosinophil counts are a reliable way to identify patients with an eosinophilic phenotype, and these measurements have proven to be successful in guiding the use of corticosteroids in moderate and severe COPD exacerbations. Antibiotic use in COPD patients induces a risk of Clostridium difficile infection, diarrhea, and antibiotic resistance. Procalcitonin could possibly guide antibiotic treatment in patients admitted with AECOPD. Current studies in COPD patients were successful in reducing exposure to antibiotics with no changes in mortality or length of stay. Daily monitoring of blood eosinophils is a safe and effective way to reduce oral corticosteroid exposure and side effects for acute exacerbations.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a common disease worldwide with a substantial impact on quality of life and mortality, making it an important contributor to the global burden of disease [
1,
2]. It is characterized by chronic airway inflammation leading to respiratory symptoms and airway obstruction [
1].
COPD is a heterogeneous disease in which the presence of emphysema, airway obstruction, excess mucus production, vascular dysfunction, and inflammation varies considerably among patients and none of these factors are particularly good predictors of symptom burden or disease development [
3,
4,
5]. The disease occurs at the intersection of airborne insults to the lung tissue such as cigarette smoke, pollutants, allergens, genetic predisposition, pathogens, and altered immune response [
4,
6,
7]. Therefore, the understanding of COPD has shifted from viewing the disease as a single entity to viewing it as a combination of distinct phenotypes that may differ in natural history and treatment needs. This understanding of COPD opens the door to individualized treatment. However, there is still a need for the better identification of treatable phenotypes.
Furthermore, patient phenotypes are not necessarily constant [
8,
9]. Even within the same phenotype, disease activity will vary based on factors such as airborne exposure to smoke, allergens, and pathogens, the control of co-morbidity, and the treatment of the lung disease [
10,
11,
12]. Thus, it is perhaps still reductive to consider the patient as belonging to a single phenotype, and the concept should be adapted to consider the time-dependent variation in the disease. This approach could lead to the earlier identification of patients that are insufficiently treated and could act as a potential method to reduce unnecessary treatment, thus reducing drug side effects. Time-updated phenotypic guidance of therapy takes this idea and applies it in practice by continually monitoring the phenotypic state of the patient and adapting treatment as needed. Biomarkers predicting treatment response have been identified in COPD with blood eosinophils as a proven tool for corticosteroid treatment, per the growing body of high-quality evidence, acting as a model for phenotypic guided therapy [
13,
14]. Furthermore, procalcitonin (PCT) has shown promising potential to guide antibiotic therapy in respiratory tract infections [
15], though evidence in COPD patients is still limited [
16]. Other biomarkers are in the process of being used for the diagnosis and treatment of COPD exacerbation [
17].
2. Blood Eosinophils as a Treatment Response Marker
The inflammatory response in COPD involves both the innate and adaptive immune system and is mediated by neutrophils, macrophages, and CD8+ T-cells (Tc1) as well as CD4+ T-cells (Th1 and Th17) [
18,
19]. The presence of pro-inflammatory factors such as cigarette smoke, bacteria, or viruses in the airways stimulates the epithelium to release cytokines, causing the recruitment and activation of immune cells. This neutrophil-dominated type of inflammation responds poorly to corticosteroids [
20].
Eosinophilic inflammation is characterized by elevated eosinophil counts in the blood and sputum and the activation of Th2 T-cells. This inflammatory phenotype is associated with atopic diseases such as allergies and asthma [
21] and tends to respond well to treatment with corticosteroids [
22,
23]. However, some COPD patients exhibit increased eosinophilic inflammation, which can be a major driver of COPD. The prevalence of eosinophilic COPD varies depending on whether or not eosinophilia is measured in sputum or blood as well as does the definition used for when eosinophils are elevated. Sputum eosinophil counts of >3% are present in 28–32% [
24,
25] of COPD patients while blood eosinophil counts of >300 cells/μL are present in 14–24% [
26,
27]. Patients with a degree of eosinophilic COPD have been shown to have more frequent exacerbations [
28,
29,
30,
31], a higher risk of readmission [
32] and better responses to treatment with inhaled corticosteroids (ICS) [
28,
33,
34,
35]. Sputum eosinophils have a closer association with clinical outcomes such as FEV1 or the exacerbation rate compared to blood eosinophils, though blood eosinophils are well-correlated with sputum eosinophils [
36,
37]. However, there are a range of challenges that come with using sputum eosinophils in clinical practice. Some patients may not be able to spontaneously produce sputum and will need induction with hypertonic saline. This process may cause bronchoconstriction in some patients and is thus unsuited to patients with severe airway obstructions such as during acute exacerbations [
38].
3. Eosinophil-Guided OCS for Acute Exacerbations
In the treatment of acute exacerbations of COPD (AECOPD), oral corticosteroid (OCS) plays an important role. In a large meta-analysis of 16 studies (
n = 1787), OCS has been shown to reduce the rate of treatment failure and the rate of relapse and to improve lung function and breathlessness [
41]. However, no effect was seen on 30-day mortality, and OCS has numerous and severe side effects including an increased risk of infections, muscle weakness, osteoporosis, metabolic dysregulation, diabetes, cataracts, glaucoma, and gastrointestinal bleeding [
42]. Therefore, some randomized trials have been conducted, examining whether or not blood eosinophils can be used to reduce OCS usage and prevent side effects. In one trial, COPD patients with moderate or severe acute exacerbations were randomized to either receive a course of 30 mg prednisolone once daily for 14 days or to receive an eosinophil-directed therapy in which the prednisolone course was only given to patients with blood eosinophil counts of ≥2% at the time of exacerbation [
43]. The eosinophil-guided regimen was found to be non-inferior to the usual standard of care and reduced corticosteroid use by approximately 50%. The STARR2 trial, which is as of now not published, but presented at the European Respiratory Society congress in 2022, examined whether or not point-of-care eosinophil measurements can be used to determine whether or not prednisolone is needed for exacerbations in general practice. In the study, patients in the intervention group received a placebo instead of prednisolone for their exacerbations if their blood eosinophil counts were below 2%, while the control group received prednisolone for 14 days irrespective of their eosinophil count. The study found no difference in antibiotic or steroid needs after 30 days [
44]. The eo-Drive trial (NCT04234360) is currently ongoing with a design that randomizes patients to receive either 40 mg prednisolone once daily for 5 days or a placebo. Patients will subsequently be grouped by blood eosinophil counts with a 2% cutoff in the analysis [
45].
4. Eosinophil-Guided ICS for Stable COPD
COPD in its stable form is treated with either long-acting beta-adrenergic agonists (LABA), long-acting muscarine antagonists (LAMA), or both [
39]. For some patients, this regime is insufficient, and they still have frequent exacerbations, so the addition of ICS is necessary. While ICS is generally safe, ICS can increase exacerbations if patients are not properly selected [
47]. A significant side effect of ICS is an increase in the risk of acute pneumonia and other respiratory infections such as infection with Pseudomonas aeruginosa and Hemophilus influenzae [
48,
49,
50,
51]. COPD patients with Pseudomonas infection have a highly increased risk of death [
49]. Other side effects known from OCS have been proposed to occur through the systemic uptake of ICS such as in the case of diabetes [
52], cataracts [
53], and psychiatric effects [
54,
55], though the evidence is less conclusive.
5. Future Perspectives of Eosinophil-Guided Treatment
While eosinophil-guided treatment has been shown to be a safe way to reduce corticosteroid exposure, very few of the current studies consider the significant variability of blood eosinophils over time [
27]. Most have relied on a single measurement to determine the inflammatory endotype and thus treatment. While this approach is effective, it is likely that time-updated treatment guidance can further improve patient selection for corticosteroids. The currently ongoing COPERNICOS trial (NCT04481555) is the first trial of blood eosinophils as a treatment response marker for ICS with a time-updated design. It uses a protocol in which patients in the control group will receive usual treatment with ICS/LAMA/LABA while patients in the intervention group will have their ICS switched on or off every three months based on a blood eosinophil count cutoff value of 300 cells/μL with the aim of reducing corticosteroid exposure and side-effects while being non-inferior to current treatment guidelines [
59].
6. Procalcitonin as a Biomarker in Respiratory Infections
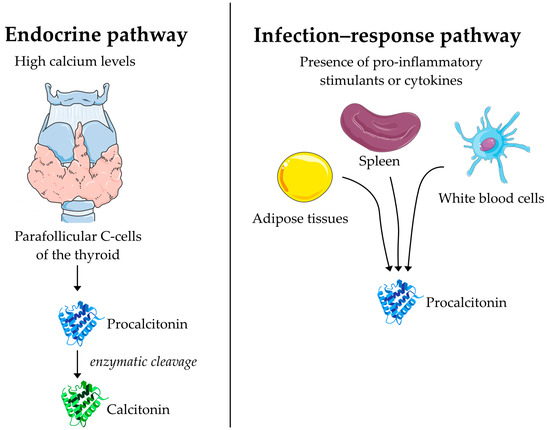
Procalcitonin (PCT) is a 116 amino acid peptide that was first discovered as the precursor to the peptide hormone calcitonin [
60,
61]. In its endocrine function, PCT is synthesized in the parafollicular C cells of the thyroid as a response to high calcium levels where it undergoes enzymatic processing and is secreted in the form of calcitonin acting on calcium homeostasis (
Figure 1) [
62]. Practically all PCT synthesized in the thyroid is converted to calcitonin, consequently leading to low levels of circulating PCT in the order of 0.01–0.05 ng/mL in the absence of disease [
63]. However, during bacterial infections, PCT was found to be elevated in blood with no effect on calcitonin and declined rapidly once the infection was cleared with antibiotic therapy [
64]. The mechanism of this increase in blood PCT is not entirely clear but sepsis was found to induce transcription of PCT mRNA in a range of tissues including the brain, colon, pancreas, white blood cells, spleen, and adipose tissue [
65].
Figure 1. The suggested mechanism for the synthesis and secretion of procalcitonin during infection and in its endocrine function as a precursor to calcitonin. In its endocrine function, procalcitonin is created by C-cells of the thyroid as a response to high calcium levels and immediately processed into calcitonin. During infection, procalcitonin is created in a range of tissues and directly secreted into the bloodstream.
7. Procalcitonin Guidance to Reduce Antibiotic Exposure in Acute Exacerbations of COPD
Antibiotics are generously used for patients hospitalized with AECOPD, and often, antibiotic exposure exceeds guidelines [
77]. Antibiotics should be used with caution, as it is associated with an increased risk of infections such as Clostridium difficile [
78] infection and adds to the global threat of microbial resistance [
79]. For this reason, there has been great interest in using PCT as a biomarker to reduce antibiotic exposure when treating respiratory infections (
Table 2).
The randomized controlled trial proHOSP included 1359 patients with acute lower respiratory tract infections, of which 533 (39.2%) had COPD. They measured PCT on days 1, 3, and 7 of the admission and adjusted antibiotics based on whether PCT was above or below 0.25 μg/L. There were no differences in mortality, rehospitalization, or intensive care admissions, neither in the total population, nor in the subset of COPD patients. However, antibiotic prescriptions for COPD exacerbations were reduced from 69.9% to 48.7% and the mean duration of intravenous therapy was reduced from 1.9 to 1.3 days. The trial had high adherence to the protocol, which was overruled in only 10.4% of COPD exacerbations [
80]. The proACT trial (
n = 1656) used a similar design with the same thresholds for PCT, also measuring on days 1, 3, and 7 [
81]. It also found no difference in adverse outcomes, but unlike proHOSP, there was no reduction in antibiotic exposure. A possible explanation of these differences could be that the protocol’s adherence was much lower in proACT compared to proHOSP. The adherence was close to 100% when the protocol recommended antibiotic treatment, but when the algorithm recommended against antibiotic treatment the adherence was generally around 60%. That was the case even with PCT <0.1, and the algorithm thus strongly recommends against antibiotics. The overall adherence for the treatment of COPD was 49.2%. The adherence was particularly low on days 3, 5, and 7.
Several other trials have explored this type of design in COPD patients [
82,
83,
84,
85]. Most have used a threshold of 0.25 μg/L, such as the proHOSP trial, repeatedly measuring PCT and encouraging antibiotics above the threshold and discouraging it below. A few trials additionally strongly favored antibiotics for PCT >0.5 and strongly discouraged it for PCT <0.1 [
80,
82,
83]. Some trials included only COPD patients, while others included a broader population of patients with respiratory tract infections but presented analyses in the subset of COPD patients. All trials were either open-label or single-blinded. Generally, this approach was successful in reducing antibiotic exposure with no effect on hospitalization or mortality. In one PCT-guided RCT of 120 patients hospitalized with AECOPD, there was a median reduction in antibiotic exposure of 5 days (3.5 days vs. 8.5 days) using a point-of-care PCT test and a treatment algorithm with four cut-offs [
83]. However, adherence to the protocol varied across trials. One trial followed the protocol recommendations in only 61% of patients in the PCT-guided group [
83]. The situation of physicians prescribing antibiotics against the algorithm is especially important, as these patients could have experienced adverse outcomes had the recommendation been followed.
8. Conclusions
There is an increasing body of evidence that supports that time-updated phenotypical treatment guidance can carry some pronounced benefits for patients and societies. Procalcitonin is a promising candidate for guiding the antibiotic therapy of AECOPD with evidence from several large well-designed trials using a time-updated design. Blood eosinophil measurements have proven to be successful in guiding the use of oral corticosteroids in moderate and severe COPD exacerbations, and post hoc analyses from large trials indicate that it could be a viable biomarker to use to guide ICS treatment as well. However, unlike procalcitonin, few studies account for the temporal variability of eosinophils levels. The CORTICO-COP trial has shown that daily monitoring of blood eosinophils is a safe and effective way to reduce OCS exposure and side effects for acute exacerbations.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11051395