Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sergey Alferov | -- | 1880 | 2023-05-19 08:19:30 | | | |
| 2 | Lindsay Dong | Meta information modification | 1880 | 2023-05-19 08:43:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Oskin, P.; Demkina, I.; Dmitrieva, E.; Alferov, S. Functionalization of Carbon Nanotubes Surface by Aryl Groups. Encyclopedia. Available online: https://encyclopedia.pub/entry/44549 (accessed on 07 February 2026).
Oskin P, Demkina I, Dmitrieva E, Alferov S. Functionalization of Carbon Nanotubes Surface by Aryl Groups. Encyclopedia. Available at: https://encyclopedia.pub/entry/44549. Accessed February 07, 2026.
Oskin, Pavel, Iraida Demkina, Elena Dmitrieva, Sergey Alferov. "Functionalization of Carbon Nanotubes Surface by Aryl Groups" Encyclopedia, https://encyclopedia.pub/entry/44549 (accessed February 07, 2026).
Oskin, P., Demkina, I., Dmitrieva, E., & Alferov, S. (2023, May 19). Functionalization of Carbon Nanotubes Surface by Aryl Groups. In Encyclopedia. https://encyclopedia.pub/entry/44549
Oskin, Pavel, et al. "Functionalization of Carbon Nanotubes Surface by Aryl Groups." Encyclopedia. Web. 19 May, 2023.
Copy Citation
Arylated nanotubes are characterized by extended solubility, and are widely used in photoelectronics, semiconductor technology, and bioelectrocatalysis. The main emphasis is on arylation methods according to the radical mechanism, such as the Gomberg–Bachmann and Billups reactions, and the decomposition of peroxides.
carbon nanomaterials
semiconductor nanotubes
metal nanotubes
carbon nanotube functionalization
1. Introduction
Carbon nanotubes (CNTs), a present-day material discovered in 1993 [1], are widely used in various fields of human activity. Numbers of different CNTs forms have been obtained [2]. CNTs division is of the utmost interest, that depends on the number of layers (single-walled, double-walled, and multi-walled), as well as on the structure type (armchair”-like and zigzag), which affects the conductivity type (metallic and semiconductor, respectively) [3][4][5]. These types of nanotubes are the most notable for their properties. Due to the strong van der Waals interaction between aromatic systems, CNTs “stick together” into dense aggregates, making them almost insoluble, thus it significantly complicates the composite materials generation based on them. The use of various methods of CNT surface functionalization has been proposed to solve this problem, thereby weakening intermolecular interactions. The development of CNT functionalization methods has also enabled the extension of the application area due to a greater variety of CNT properties.
2. Methods of Introducing Aryl Fragments to the CNT Surface
2.1. The Gomberg–Bachmann Reaction
Arylation by the Gomberg–Bachmann reaction with diazonium salts (Figure 1) is the most common method of modifying the surface of carbon nanomaterials.
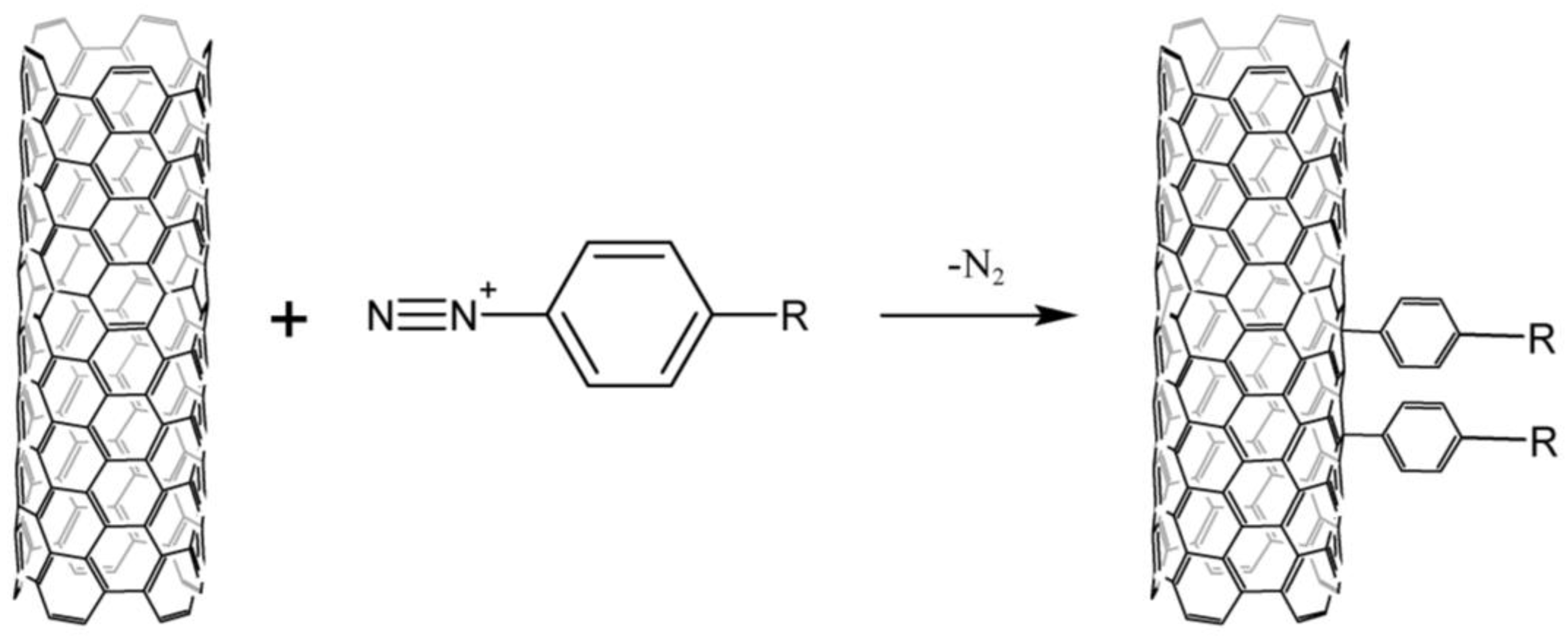
Figure 1. Scheme of CNTs arylation by the Gomberg–Bachmann reaction with diazonium salts.
Diazonium salts are unstable in solution and extremely explosive in dry form, excluding some complex fluorides, aryl sulfonates [6], and o-benzene disulfimides [7]. Aqueous solutions of arylenediazonium borofluorides are most commonly used for the arylation of CNTs. Their functionalization is carried out either electrochemically [8] or due to the spontaneous decomposition of diazonium cations [9]. However, this approach has a number of disadvantages. CNTs are dispersed in water with the use of SDS, which certainly bind to nanotubes due to the strong hydrophobic interactions, and this is not always acceptable in a number of areas, such as electrochemistry and bioelectrocatalysis. Furthermore, arylenediazonium borofluorides are poorly soluble in water.
The production of arylenediazonium in non-aqueous media in situ is also widely used, this is achieved by the reaction between isoamyl nitrite and aromatic amine. However, this approach is not environmentally friendly, as well as the dispersibility of native nanotubes in organic solvents does not meet the requirements. The reaction has been proposed to be carried out in a two-phase water-isoamyl nitrite system [10], but this approach does not solve most of the problems.
The occurring aryl radicals attach to nanotubes, reacting as electrophiles. Due to this fact, there can be observed both the selectivity to CNTs with a metallic type of conductivity and a higher reaction rate of aryl radicals with acceptor substituents [11]. The resulting aryl-CNT radicals react with diazonium salt, while the structure is stabilized. In the acidic media, a nucleophile from the solution should be attached to the CNT; however, no data confirming this could be found in the literature (Figure 2). The aryl fragment is split off from the nanotube if there occurs no addition of the diazonium cation to the aryl-CNT radical. This has been shown by measuring the electrical conductivity of nanotubes during the reaction. Electrical conductivity increases after the termination of the reaction, which is explained by the cleavage of a part of the aryl groups [12].
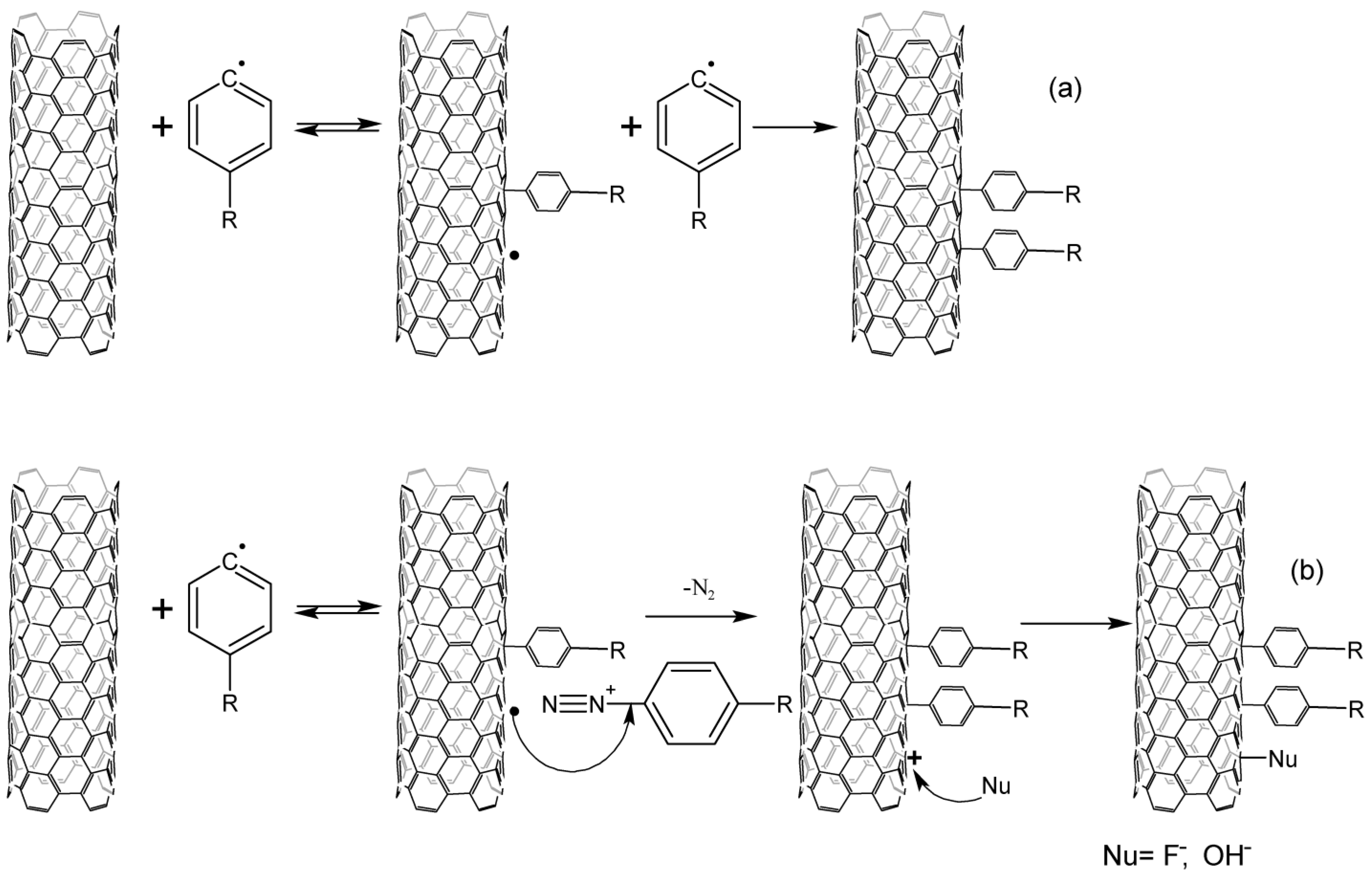
Figure 2. Interaction of CNTs with aryl radicals and arylenediazonium cations, (a)—alkaline media, (b)—acidic media.
2.2. Billups Reaction
The reductive arylation of CNT salts with various reagents (the Billups reaction and its modifications) is of great interest. It has been shown since 1997 that an electron is transferred from a metal atom to carbon when CNTs are fused with alkali metals [13]. Due to Coulomb repulsion, CNT salts with alkali metals have high spontaneous solubility—up to 2 mg/g in DMSO and DMF, and up to 4 mg/g in sulfolane; moreover, the solutions are stable for up to a year and resemble properties of polyelectrolytes in an inert atmosphere [14][15]. The addition of dibenzo-18-crown-6 increases solubility, due to complexation with sodium ions [16]. Further, there is no need to use ultrasound, which destroys the structure, when preparing solutions of CNT salts [17][18][19][20].
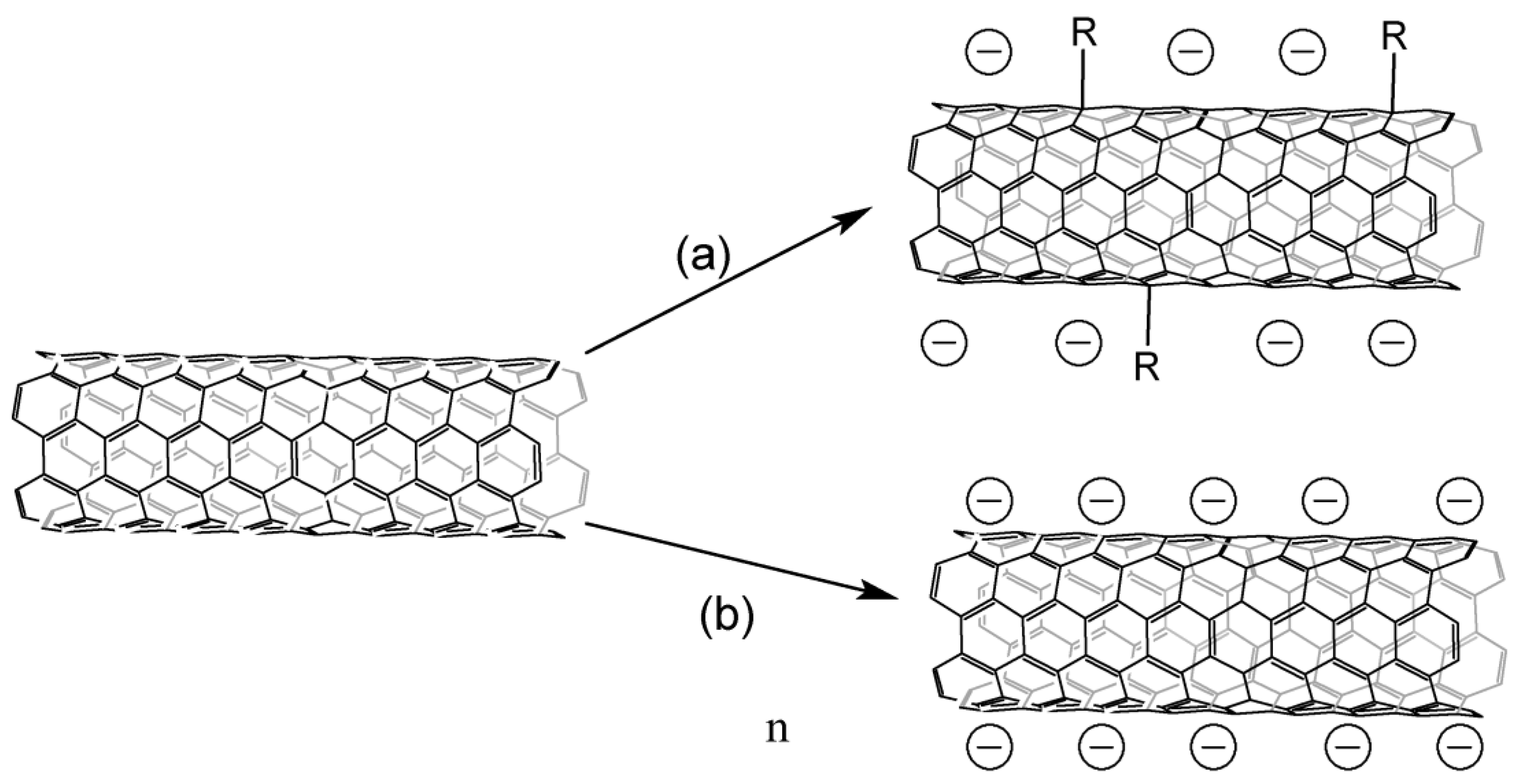
CNT salts can be obtained by several methods (Figure 3), interaction with an alkali metal solution in liquid ammonia is the most common [17][18][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35], and lithium is mostly used. Alkali metal is embedded inside CNTs, lithium can also form covalent bonds with carbon atoms [34], and alkali metal is possible to be embedded between the layers of tubes with subsequent arylation/hydrogenation of the inner tubes in the case of CNTS [36]. The functionalization degree may be controlled by maintaining an optimal metal-carbon ratio [37].

Figure 3. Obtaining CNT salts: (a)—interaction with lithium alkylides, (b)—interaction with alkali metals in solvents of various nature in the presence or absence of electronic transport mediators.
The reaction in THF with alkali metals in the presence of electron–carrying catalysts, such as naphthalene [26][37][38][39][40][41], benzophenone [39], or 4,4’-di-tert-butylbiphenyl is another commonly used method of obtaining CNT salts [42]. However, the reaction product turns out to be a contaminated catalyst since it is difficult to remove it due to the strong stack interactions with CNT. This can lead to deterioration of the electrochemical properties of CNTs. Several studies use butyllithium or another lithium alkylide in cyclohexane or THF to produce CNT salts [31][43][44][45][46][47][48][49]. However, in this case, alkyl radicals are also sewn onto the surface of CNTs in addition to aryl ones. The fusion of CNTs with alkali metals in an argon atmosphere [20][50][51][52] or interaction with sodium amalgam in toluene in the presence of dibenzo-18-crown-6 is less common due to the technical complexity [16]. It has also been proposed to replace liquid ammonia with ethylenediamine, but this approach has not been widely used [23][53].
Aryliodides are mostly used for the arylation of CNT salts [20][21][22][37][38]; therefore, their interaction with CNT has been studied in full detail. Thus, it is shown that the degree of functionalization is higher if there are donor substituents in the aryl radical [21]. Reducing arylation proceeds more easily for nanotubes of smaller diameters [27]. The study [30] proposes a reactor to produce alkylated and arylated nanotubes by the Billups–Birch reaction on a semi-industrial scale.
Using aryliodides as arylating agents leads to the fact that functionalized CNTs turn out to be contaminated by-products of a combination of radicals that are difficult to remove. This has been shown by analysis of GC-MS filtrate after alkylation of CNT [17][24]. Alkylation is carried out with other reagents, such as peroxides [17][29][39], cyclic halides [29], sulfides and disulfides [33], carbonyl compounds [31], acetylenes [45], diazonium salts [20][50][54] and iodonium [41] to solve this problem. The free radicals formed from these compounds give unstable combination products that immediately disintegrate.
2.3. Reactions with Peroxides and Related Compounds
Thermal decomposition of benzoyl peroxide is widely used for CNT phenylation [55][56][57][58][59][60]. The reaction has been carried out in boiling benzene (o-chlorobenzene) [55][56][58] in an argon atmosphere or boiling toluene [57] in the air. Despite the higher boiling point of toluene and the same ratio of reagents, the study [57] shows a lower functionalization degree than others.
A solid-phase reaction is also described [60]. Other peroxides can be used in addition to benzoyl peroxides, such as p-methoxybenzoyl peroxide or phthaloyl peroxide [56]. Peroxides can also be used as initiators of radical reactions with other compounds, for example, aryl and alkyl iodides [55]. Phenylated nanotubes can also undergo further functionalization, e.g., sulfonation in oleum to increase their solubility in water [58].
2.4. Other Arylation Methods
There is a report on the use of the Ullmann reaction for the arylation of CNTs [61]. Initially, CNT has been chlorinated by reacting with iodine trichloride in tetrachlorocarbon for 3 hours. Chlorinated CNTs have reacted with iodobenzene, phenol, or aniline in the presence of cesium carbonate, copper chloride 1, and phenanthroline in DMF, at 120 degrees for 2 days. The functionalization degree has reached 3.5 mmol/g. It is shown that functionalization proceeds best for CNTs of smaller diameters.
CNT has been proposed to be arylated with 4-methoxyphenylhydrazine hydrochloride by boiling in toluene in an oxygen medium [62] or irradiating with microwave radiation. This turns out to be more effective [63]. Arylated CNTs are better soluble in o-chlorobenzene. CNT modification with phenylhydrazine by interaction in an aqueous solution in the presence of SDS for 2 days has also been described [64].
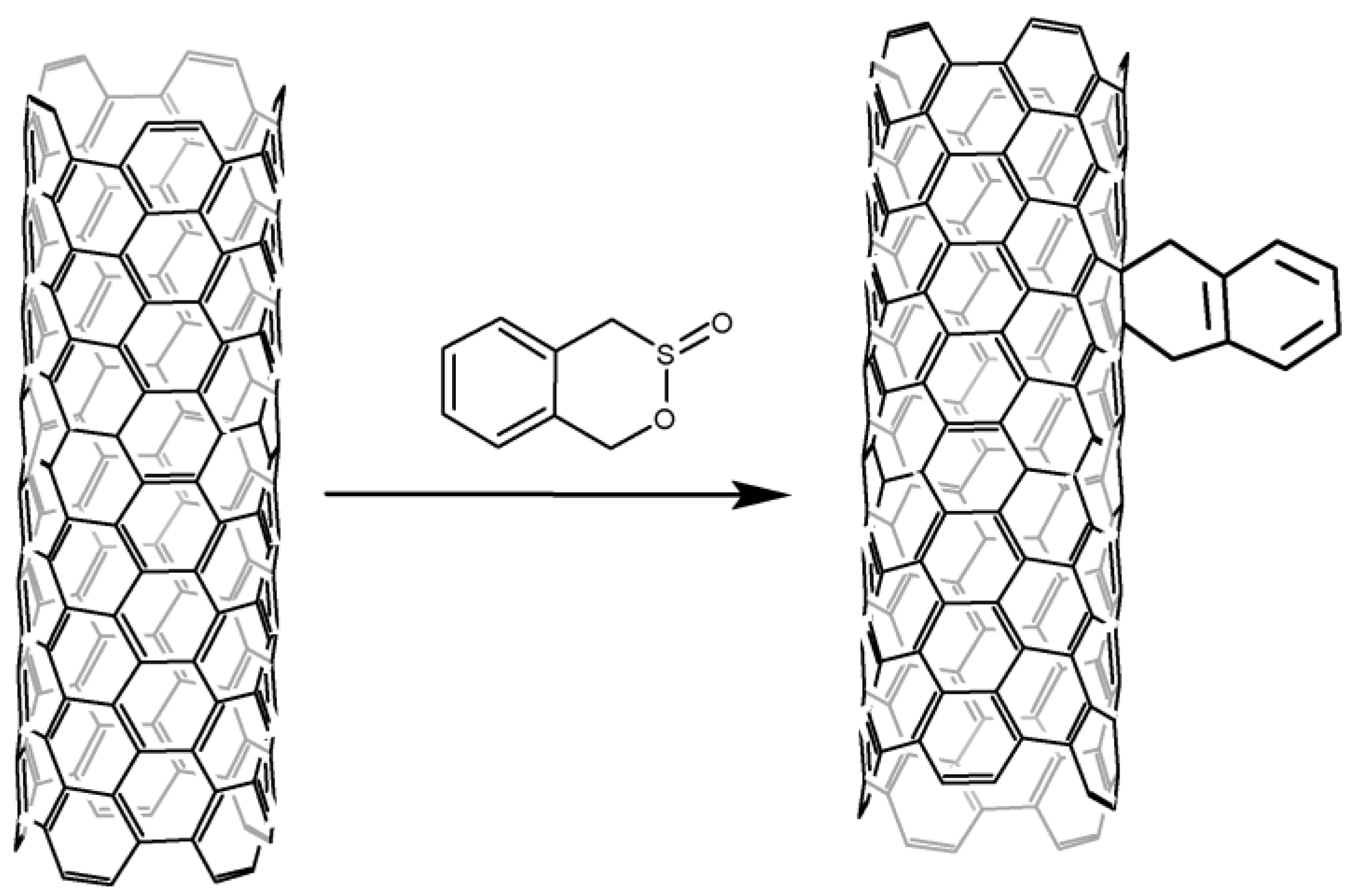
2.5. Alternative Methods of Introducing Aryl Groups to the CNT Surface
The cross-linking of aromatic hydrocarbons with nanotubes via a carboxamide bond and cycloaddition reactions do not belong to traditional arylation methods, nevertheless, they allow introducing aromatic fragments onto the CNT surface. Therefore, they can be used to solve the same problems. The production of aromatic amides from CNTs has a number of disadvantages: the multi-stage nature of the process, the strong destruction of the CNT structure, as well as the crosslinking of aryl groups mainly with edge carbon atoms (Figure 4).

Figure 4. Cycloaddition to CNT under microwave irradiation.
2.6. Fractionation of Carbon Nanotubes
All scalable methods of CNT synthesis give a mixture with a wide range of properties: length, diameter, and type of conductivity. The isolation of individual CNT fractions is important for the most effective use of their extraordinary properties. The most acute problem is the separation of metal nanotubes from semiconductor ones [65]. Semiconductor CNTs are used to create transistors [66]; moreover, they have photoluminescence [67][68][69][70]. The admixture of metal CNTs worsens their properties significantly, causing incorrect transistors’ functioning. Gomberg–Bachmann arylation proceeds selectively to metallic nanotubes [71][72][73][74][75].
3. Properties and Application of Aryl-Group Functionalized CNTs
3.1. Solubility of Arylated Carbon Nanotubes
A dispersibility increase in different solvents is one of the most important properties of CNTs functionalized by aryl groups. The solubility of functionalized CNTs is not quantified in most studies. As usual, the scholars show that native nanotubes, generally, are not capable of forming stable dispersions in a particular solvent. The research [76] shows that the CNT solubility differs from one author to another due to the use of different methods for its determination.
3.2. Photoluminescence of Arylated Carbon Nanotubes
It is widely known that semiconductor carbon nanotubes are capable of photoluminescence in the near IR range [67][68][69][70]. Arylation makes it possible to introduce small defects in the CNT structure in a targeted manner, which makes it possible to achieve more intense luminescence [67][68][69][77][78][79][80][81][82][83][84][85][86][87][88][89][90]. Further, unlike alkylation and oxygen doping, arylation provides more opportunities to influence the luminescence spectrum: the use of aryl radicals of different structures [77][78][81][88][90], subsequent modification of aryl radicals [85][88], selective arylation under the action of light [87][89], biarylation with different bridge lengths between sewn aryl groups [86]. It is important to note that arylated CNTs have the rare property of luminescent solvatochromism, which may have unusual applications in the future [80].
3.3. Development of Bioelectrodes with Surface-Oriented Immobilization of Enzymes
Bioelectrocatalysis plays an important role in modern chemistry [91]. The creation of electrodes with oriented immobilized enzymes is necessary for the development of biofuel cells [92][93][94][95][96][97][98][99][100][101][102][103], hybrid batteries [95][104][105][106][107][108], and biosensors [109]. CNTs are a promising basis for the creation of bioelectrodes due to their high electrical conductivity and surface area. Enzymes having hydrophobic pockets, such as laccase, bilirubin oxidase, and fructose dehydrogenase, can bind to aryl radicals on the electrode surface, which facilitates electron transfer. Aryl groups are often introduced through the acylation of aromatic amines with oxidized CNTs [92][94][104][110][111][112] or Gomberg–Bachmann arylation [92][93][98][103][104][105][113][114]. A non-covalent modification of CNT by pyrene derivatives has also been proposed [101][115][116]. Comparing the effectiveness of these functionalization methods is difficult due to the small amount of information and its inconsistency.
References
- Iijima, S.; Ichihashi, T. Single-Shell Carbon Nanotubes of 1-nm Diameter. Nature 1993, 363, 603–605.
- Yakobson, B.I.; Avouris, P. Mechanical Properties of Carbon Nanotubes. In Carbon Nanotubes; Springer: Berlin/Heidelberg, Germany, 2007; pp. 287–327.
- Radosavljević, M.; Lefebvre, J.; Johnson, A.T. High-Field Electrical Transport and Breakdown in Bundles of Single-Wall Carbon Nanotubes. Phys. Rev. B 2001, 64, 241307.
- Yao, Z.; Kane, C.L.; Dekker, C. High-Field Electrical Transport in Single-Wall Carbon Nanotubes. Phys. Rev. Lett. 2000, 84, 2941–2944.
- Collins, P.G.; Hersam, M.; Arnold, M.; Martel, R.; Avouris, P. Current Saturation and Electrical Breakdown in Multiwalled Carbon Nanotubes. Phys. Rev. Lett. 2001, 86, 3128–3131.
- Trusova, M.E.; Postnikov, P.S.; Krasnokutskaya, E.A.; Filimonov, V.D.; Chi, K.-W. A New Approach to the Synthesis of Stable Aryldiazonium Tosylates, Their Structure and Application in Organic Synthesis. News Tomsk Polytech. Univ. 2008, 312, 83–86.
- Barbero, M.; Crisma, M.; Degani, I.; Fochi, R.; Perracino, P. New Dry Arenediazonium Salts, Stabilized to an Exceptionally High Degree by the Anion of o-Benzenedisulfonimide. Synthesis 1998, 1998, 1171–1175.
- Bahr, J.L.; Yang, J.; Kosynkin, D.V.; Bronikowski, M.J.; Smalley, R.E.; Tour, J.M. Functionalization of Carbon Nanotubes by Electrochemical Reduction of Aryl Diazonium Salts: A Bucky Paper Electrode. J. Am. Chem. Soc. 2001, 123, 6536–6542.
- Chakraborty, A.K.; Coleman, K.S.; Dhanak, V.R. The Electronic Fine Structure of 4-Nitrophenyl Functionalized Single-Walled Carbon Nanotubes. Nanotechnology 2009, 20, 155704.
- Price, B.K.; Tour, J.M. Functionalization of Single-Walled Carbon Nanotubes “on Water”. J. Am. Chem. Soc. 2006, 128, 12899–12904.
- Schmidt, G.; Gallon, S.; Esnouf, S.; Bourgoin, J.P.; Chenevier, P. Mechanism of the Coupling of Diazonium to Single-Walled Carbon Nanotubes and Its Consequences. Chem.–A Eur. J. 2009, 15, 2101–2110.
- Wilson, H.; Ripp, S.; Prisbrey, L.; Brown, M.A.; Sharf, T.; Myles, D.J.T.; Blank, K.G.; Minot, E.D. Electrical Monitoring of Sp3 Defect Formation in Individual Carbon Nanotubes. J. Phys. Chem. C 2016, 120, 1971–1976.
- Rao, A.M.; Eklund, P.C.; Bandow, S.; Thess, A.; Smalley, R.E. Evidence for Charge Transfer in Doped Carbon Nanotube Bundles from Raman Scattering. Nature 1997, 388, 257–259.
- Pénicaud, A.; Poulin, P.; Derré, A.; Anglaret, E.; Petit, P. Spontaneous Dissolution of a Single-Wall Carbon Nanotube Salt. J. Am. Chem. Soc. 2005, 127, 8–9.
- Fogden, S.; Howard, C.A.; Heenan, R.K.; Skipper, N.T.; Shaffer, M.S.P. Scalable Method for the Reductive Dissolution, Purification, and Separation of Single-Walled Carbon Nanotubes. ACS Nano 2012, 6, 54–62.
- Anderson, R.E.; Barron, A.R. Solubilization of Single-Wall Carbon Nanotubes in Organic Solvents without Sidewall Functionalization. J. Nanosci. Nanotechnol. 2007, 7, 3436–3440.
- Billups, W.E.; Liang, F.; Chattopadhyay, J.; Beach, J. Uses of Single Wall Carbon Nanotube Salts in Organic Syntheses. ECS Trans. 2007, 2, 65–76.
- Liang, F.; Alemany, L.B.; Beach, J.M.; Billups, W.E. Structure Analyses of Dodecylated Single-Walled Carbon Nanotubes. Am. Chem. Soc. 2005, 127, 13941–13948.
- Badaire, S.; Poulin, P.; Maugey, M.; Zakri, C. In Situ Measurements of Nanotube Dimensions in Suspensions by Depolarized Dynamic Light Scattering. Langmuir 2004, 20, 10367–10370.
- Schirowski, M.; Abellán, G.; Nuin, E.; Pampel, J.; Dolle, C.; Wedler, V.; Fellinger, T.P.; Spiecker, E.; Hauke, F.; Hirsch, A. Fundamental Insights into the Reductive Covalent Cross-Linking of Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2018, 140, 3352–3360.
- Chattopadhyay, J.; Sadana, A.K.; Liang, F.; Beach, J.M.; Xiao, Y.; Hauge, R.H.; Billups, W.E. Carbon Nanotube Salts. Arylation of Single-Wall Carbon Nanotubes. Org. Lett. 2005, 7, 4067–4069.
- Stephenson, J.J.; Sadana, A.K.; Higginbotham, A.L.; Tour, J.M. Highly Functionalized and Soluble Multiwalled Carbon Nanotubes by Reductive Alkylation and Arylation: The Billups Reaction. Chem. Mater. 2006, 18, 4658–4661.
- Chen, Y.; Haddon, R.C.; Fang, S.; Rao, A.M.; Eklund, P.C.; Lee, W.H.; Dickey, E.C.; Grulke, E.A.; Pendergrass, J.C.; Chavan, A.; et al. Chemical Attachment of Organic Functional Groups to Single-Walled Carbon Nanotube Material. J. Mater. Res. 1998, 13, 2423–2431.
- Liang, F.; Sadana, A.K.; Peera, A.; Chattopadhyay, J.; Gu, Z.; Hauge, R.H.; Billups, W.E. A Convenient Route to Functionalized Carbon Nanotubes. Nano Lett. 2004, 4, 1257–1260.
- Liang, F.; Beach, J.M.; Kobashi, K.; Sadana, A.K.; Vega-Cantu, Y.I.; Tour, J.M.; Billups, W.E. In Situ Polymerization Initiated by Single-Walled Carbon Nanotube Salts. Chem. Mater. 2006, 18, 4764–4767.
- Borondics, F.; Jakab, E.; Pekker, S. Functionalization of Carbon Nanotubes via Dissolving Metal Reductions. J. Nanosci. Nanotechnol. 2007, 7, 1551–1559.
- Wunderlich, D.; Hauke, F.; Hirsch, A. Preferred Functionalization of Metallic and Small-Diameter Single Walled Carbon Nanotubes via Reductive Alkylation. J. Mater. Chem. 2008, 18, 1493–1497.
- Zhang, K.S.; Pham, D.; Lawal, O.; Ghosh, S.; Gangoli, V.S.; Smalley, P.; Kennedy, K.; Brinson, B.E.; Billups, W.E.; Hauge, R.H.; et al. Overcoming Catalyst Residue Inhibition of the Functionalization of Single-Walled Carbon Nanotubes via the Billups-Birch Reduction. ACS Appl. Mater. Interfaces 2017, 9, 37972–37980.
- Mukherjee, A.; Combs, R.; Chattopadhyay, J.; Abmayr, D.W.; Engel, P.S.; Billups, W.E. Attachment of Nitrogen and Oxygen Centered Radicals to Single-Walled Carbon Nanotube Salts. Chem. Mater. 2008, 20, 7339–7343.
- Pham, D.; Zhang, K.; Lawal, O.; Ghosh, S.; Gangoli, V.; Ainscough, T.; Kellogg, B.; Hauge, R.; Adams, W.; Barron, A. Apparatus for Scalable Functionalization of Single-Walled Carbon Nanotubes via the Billups-Birch Reduction. J. Carbon Res. 2017, 3, 19.
- Gebhardt, B.; Syrgiannis, Z.; Backes, C.; Graupner, R.; Hauke, F.; Hirsch, A. Carbon Nanotube Sidewall Functionalization with Carbonyl Compounds-Modified Birch Conditions vs the Organometallic Reduction Approach. J. Am. Chem. Soc. 2011, 133, 7985–7995.
- Syrgiannis, Z.; Gebhardt, B.; Dotzer, C.; Hauke, F.; Graupner, R.; Hirsch, A. Reductive Retrofunctionalization of Single-Walled Carbon Nanotubes. Angew. Chem. Int. Ed. 2010, 49, 3322–3325.
- Chattopadhyay, J.; Chakraborty, S.; Mukherjee, A.; Runtang, W.; Engel, P.S.; Billups, W.E. SET Mechanism in the Functionalization of Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2007, 111, 17928–17932.
- Gu, Z.; Liang, F.; Chen, Z.; Sadana, A.; Kittrell, C.; Billups, W.E.; Hauge, R.H.; Smalley, R.E. In Situ Raman Studies on Lithiated Single-Wall Carbon Nanotubes in Liquid Ammonia. Chem. Phys. Lett. 2005, 410, 467–470.
- Clark, M.D.; Krishnamoorti, R. Dispersion of Functionalized Multiwalled Carbon Nanotubes. J. Phys. Chem. C 2009, 113, 20861–20868.
- Pekker, S.; Salvetat, J.P.; Jakab, E.; Bonard, J.M.; Forró, L. Hydrogenation of Carbon Nanotubes and Graphite in Liquid Ammonia. J. Phys. Chem. B 2001, 105, 7938–7943.
- Voiry, D.; Roubeau, O. Stoichiometric Control of Single Walled Carbon Nanotubes Functionalization. J. Mater. Chem. 2010, 20, 4385–4391.
- Borondics, F.; Bokor, M.; Matus, P.; Tompa, K.; Pekker, S.; Jakab, E. Reductive Functionalization of Carbon Nanotubes. Fuller. Nanotub. Carbon Nanostructures 2005, 13, 375–382.
- Martínez-Rubí, Y.; Guan, J.; Lin, S.; Scriver, C.; Sturgeon, R.E.; Simard, B. Rapid and Controllable Covalent Functionalization of Single-Walled Carbon Nanotubes at Room Temperature. Chem. Commun. 2007, 48, 5146–5148.
- Voiry, D.; Vallés, C.; Roubeau, O.; Pénicaud, A. Dissolution and Alkylation of Industrially Produced Multi-Walled Carbon Nanotubes. Carbon 2011, 49, 170–175.
- He, M.; Swager, T.M. Covalent Functionalization of Carbon Nanomaterials with Iodonium Salts. Chem. Mater. 2016, 28, 8542–8549.
- García-Gallastegui, A.; Obieta, I.; Bustero, I.; Imbuluzqueta, G.; Arbiol, J.; Miranda, J.I.; Aizpurua, J.M. Reductive Functionalization of Single-Walled Carbon Nanotubes with Lithium Metal Catalyzed by Electron Carrier Additives. Chem. Mater. 2008, 20, 4433–4438.
- Viswanathan, G.; Chakrapani, N.; Yang, H.; Wei, B.; Chung, H.; Cho, K.; Ryu, C.Y.; Ajayan, P.M. Single-Step in Situ Synthesis of Polymer-Grafted Single-Wall Nanotube Composites. J. Am. Chem. Soc. 2003, 125, 9258–9259.
- Graupner, R.; Abraham, J.; Wunderlich, D.; Vencelová, A.; Lauffer, P.; Röhrl, J.; Hundhausen, M.; Ley, L.; Hirsch, A. Nucleophilic-Alkylation-Reoxidation: A Functionalization Sequence for Single-Wall Carbon Nanotubes. J. Am. Chem. Soc. 2006, 128, 6683–6689.
- Gebhardt, B.; Graupner, R.; Hauke, F.; Hirsch, A. A Novel Diameter-Selective Functionalization of SWCNTs with Lithium Alkynylides. Eur. J. Org. Chem. 2010, 2010, 1494–1501.
- Chen, S.; Shen, W.; Wu, G.; Chen, D.; Jiang, M. A New Approach to the Functionalization of Single-Walled Carbon Nanotubes with Both Alkyl and Carboxyl Groups. Chem. Phys. Lett. 2005, 402, 312–317.
- Maeda, Y.; Kato, T.; Hasegawa, T.; Kako, M.; Akasaka, T.; Lu, J.; Nagase, S. Two-Step Alkylation of Single-Walled Carbon Nanotubes: Substituent Effect on Sidewall Functionalization. Org. Lett. 2010, 12, 996–999.
- Roubeau, O.; Lucas, A.; Pénicaud, A.; Derré, A. Covalent Functionalization of Carbon Nanotubes Through Organometallic Reduction and Electrophilic Attack. J. Nanosci. Nanotechnol. 2007, 7, 3509–3513.
- Bayazit, M.K.; Suri, A.; Coleman, K.S. Formylation of Single-Walled Carbon Nanotubes. Carbon 2010, 48, 3412–3419.
- Schirowski, M.; Tyborski, C.; Maultzsch, J.; Hauke, F.; Hirsch, A.; Goclon, J. Reductive Diazotation of Carbon Nanotubes: An Experimental and Theoretical Selectivity Study. Chem. Sci. 2019, 10, 706–717.
- Duclaux, L. Review of the Doping of Carbon Nanotubes (Multiwalled and Single-Walled). Carbon 2002, 40, 1751–1764.
- Hof, F.; Bosch, S.; Eigler, S.; Hauke, F.; Hirsch, A. New Basic Insight into Reductive Functionalization Sequences of Single Walled Carbon Nanotubes (SWCNTs). J. Am. Chem. Soc. 2013, 135, 18385–18395.
- Tang, X.; Jiao, Q.; Cao, Y.; Zhang, P.; Liu, H.; Wu, H.; Zhou, M.; Li, X.; Zhao, Y. Reductive Alkylation and Arylation of Single-Walled Carbon Nanotubes in Ethylenediamine via Benkeser Reaction. Chem. Lett. 2009, 38, 220–221.
- Hof, F.; Bosch, S.; Englert, J.M.; Hauke, F.; Hirsch, A. Statistical Raman Spectroscopy: An Method for the Characterization of Covalently Functionalized Single-Walled Carbon Nanotubes. Angew. Chem. Int. Ed. 2012, 51, 11727–11730.
- Ying, Y.; Saini, R.K.; Liang, F.; Sadana, A.K.; Billups, W.E. Functionalization of Carbon Nanotubes by Free Radicals. Org. Lett. 2003, 5, 1471–1473.
- Engel, P.S.; Billups, W.E.; Abmayr, D.W.; Tsvaygboym, K.; Wang, R. Reaction of Single-Walled Carbon Nanotubes with Organic Peroxides. J. Phys. Chem. C 2008, 112, 695–700.
- Umek, P.; Seo, J.W.; Hernadi, K.; Mrzel, A.; Pechy, P.; Mihailovic, D.D.; Forró, L. Addition of Carbon Radicals Generated from Organic Peroxides to Single Wall Carbon Nanotubes. Chem. Mater. 2003, 15, 4751–4755.
- Liang, F.; Beach, J.M.; Rai, P.K.; Guo, W.; Hauge, R.H.; Pasquali, M.; Smalley, R.E.; Billups, W.E. Highly Exfoliated Water-Soluble Single-Walled Carbon Nanotubes. Chem. Mater. 2006, 18, 1520–1524.
- Xu, X.; Yang, Y.; Guan, Y.; Wei, D.; Zheng, A. Persistent Free Radicals on Carbon Nanotubes and Their Catalytic Effect on Benzoyl Peroxide Decomposition. Carbon 2023, 201, 473–482.
- Peng, H.; Reverdy, P.; Khabashesku, V.N.; Margrave, J.L. Sidewall Functionalization of Single-Walled Carbon Nanotubes with Organic Peroxides. Chem. Commun. 2003, 3, 362–363.
- Kolanowska, A.; Kuziel, A.W.; Jędrysiak, R.G.; Krzywiecki, M.; Korczeniewski, E.; Wiśniewski, M.; Terzyk, A.P.; Boncel, S. Ullmann Reactions of Carbon Nanotubes—Advantageous and Unexplored Functionalization toward Tunable Surface Chemistry. Nanomaterials 2019, 9, 1619.
- Liu, J.; Zubiri, M.R.I.; Dossot, M.; Vigolo, B.; Hauge, R.H.; Fort, Y.; Ehrhardt, J.J.; McRae, E. Sidewall Functionalization of Single-Wall Carbon Nanotubes (SWNTs) through Aryl Free Radical Addition. Chem. Phys. Lett. 2006, 430, 93–96.
- Liu, J.; i Zubiri, M.R.; Vigolo, B.; Dossot, M.; Fort, Y.; Ehrhardt, J.J.; McRae, E. Efficient Microwave-Assisted Radical Functionalization of Single-Wall Carbon Nanotubes. Carbon 2007, 45, 885–891.
- Yokoi, T.; Iwamatsu, S.I.; Komai, S.I.; Hattori, T.; Murata, S. Chemical Modification of Carbon Nanotubes with Organic Hydrazines. Carbon 2005, 43, 2869–2874.
- Janas, D. Towards Monochiral Carbon Nanotubes: A Review of Progress in the Sorting of Single-Walled Carbon Nanotubes. Mater. Chem. Front. 2018, 2, 36–63.
- Klinke, C.; Hannon, J.B.; Afzali, A.; Avouris, P. Field-Effect Transistors Assembled from Functionalized Carbon Nanotubes. Nano Lett. 2006, 6, 906–910.
- Nanot, S.; Hároz, E.H.; Kim, J.H.; Hauge, R.H.; Kono, J. Optoelectronic Properties of Single-Wall Carbon Nanotubes. Adv. Mater. 2012, 24, 4977–4994.
- O’Connell, M.J.; Bachilo, S.H.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band Gap Fluorescence from Individual Single-Walled Carbon Nanotubes. Science 2002, 297, 593–596.
- Janas, D. Perfectly Imperfect: A Review of Chemical Tools for Exciton Engineering in Single-Walled Carbon Nanotubes. Mater. Horiz. 2020, 7, 2860–2881.
- Zaumseil, J. Luminescent Defects in Single-Walled Carbon Nanotubes for Applications. Adv. Opt. Mater. 2022, 10, 2101576.
- Strano, M.S.; Dyke, C.A.; Usrey, M.L.; Barone, P.W.; Allen, M.J.; Shan, H.; Kittrell, C.; Hauge, R.H.; Tour, J.M.; Smalley, R.E. Electronic Structure Control of Single-Waited Carbon Nanotube Functionalization. Science 2003, 301, 1519–1522.
- An, L.; Fu, Q.; Lu, C.; Liu, J. A Simple Chemical Route to Selectively Eliminate Metallic Carbon Nanotubes in Nanotube Network Devices. J. Am. Chem. Soc. 2004, 126, 10520–10521.
- Kim, W.J.; Usrey, M.L.; Strano, M.S. Selective Functionalization and Free Solution Electrophoresis of Single-Walled Carbon Nanotubes: Separate Enrichment of Metallic and Semiconducting SWNT. Chem. Mater. 2007, 19, 1571–1576.
- Toyoda, S.; Yamaguchi, Y.; Hiwatashi, M.; Tomonari, Y.; Murakami, H.; Nakashima, N. Separation of Semiconducting Single-Walled Carbon Nanotubes by Using a Long-Alkyl-Chain Benzenediazonium Compound. Chem. Asian J. 2007, 2, 145–149.
- Usrey, M.L.; Nair, N.; Agnew, D.E.; Pina, C.F.; Strano, M.S. Controlling the Electrophoretic Mobility of Single-Walled Carbon Nanotubes: A Comparison of Theory and Experiment. Langmuir 2007, 23, 7768–7776.
- Dyke, C.A.; Tour, J.M. Overcoming the Insolubility of Carbon Nanotubes Through High Degrees of Sidewall Functionalization. Chem.–A Eur. J. 2004, 10, 812–817.
- Shiraki, T.; Uchimura, S.; Shiraishi, T.; Onitsuka, H.; Nakashima, N. Near Infrared Photoluminescence Modulation by Defect Site Design Using Aryl Isomers in Locally Functionalized Single-Walled Carbon Nanotubes. Chem. Commun. 2017, 53, 12544–12547.
- Piao, Y.; Meany, B.; Powell, L.R.; Valley, N.; Kwon, H.; Schatz, G.C.; Wang, Y. Brightening of Carbon Nanotube Photoluminescence through the Incorporation of sp3 Defects. Nat. Chem. 2013, 5, 840–845.
- Kwon, H.; Kim, M.; Meany, B.; Piao, Y.; Powell, L.R.; Wang, Y. Optical Probing of Local PH and Temperature in Complex Fluids with Covalently Functionalized, Semiconducting Carbon Nanotubes. J. Phys. Chem. C 2015, 119, 3733–3739.
- Shiraki, T.; Niidome, Y.; Toshimitsu, F.; Shiraishi, T.; Shiga, T.; Yu, B.; Fujigaya, T. Solvatochromism of near Infrared Photoluminescence from Doped Sites of Locally Functionalized Single-Walled Carbon Nanotubes. Chem. Commun. 2019, 55, 3662–3665.
- Kilina, S.; Ramirez, J.; Tretiak, S. Brightening of the Lowest Exciton in Carbon Nanotubes via Chemical Functionalization. Nano Lett. 2012, 12, 2306–2312.
- Shiraishi, T.; Shiraki, T.; Nakashima, N. Substituent Effects on the Redox States of Locally Functionalized Single-Walled Carbon Nanotubes Revealed by in Situ Photoluminescence Spectroelectrochemistry. Nanoscale 2017, 9, 16900–16907.
- Shiraki, T.; Shiga, T.; Shiraishi, T.; Onitsuka, H.; Nakashima, N.; Fujigaya, T. Multistep Wavelength Switching of Near-Infrared Photoluminescence Driven by Chemical Reactions at Local Doped Sites of Single-Walled Carbon Nanotubes. Chem.–A Eur. J. 2018, 24, 19162–19165.
- Hartmann, N.F.; Yalcin, S.E.; Adamska, L.; Hároz, E.H.; Ma, X.; Tretiak, S.; Htoon, H.; Doorn, S.K. Photoluminescence Imaging of Solitary Dopant Sites in Covalently Doped Single-Wall Carbon Nanotubes. Nanoscale 2015, 7, 20521–20530.
- Onitsuka, H.; Fujigaya, T.; Nakashima, N.; Shiraki, T. Control of the Near Infrared Photoluminescence of Locally Functionalized Single-Walled Carbon Nanotubes via Doping by Azacrown-Ether Modification. Chem.–A Eur. J. 2018, 24, 9393–9398.
- Shiraki, T.; Yu, B.; Shiraishi, T.; Shiga, T.; Fujigaya, T. Meta-Linkage Design of Bis-Aryldiazonium Modifiers for Wavelength Tuning of near Infrared Photoluminescence from Locally Functionalized Single-Walled Carbon Nanotubes. Chem. Lett. 2019, 48, 791–794.
- Powell, L.R.; Piao, Y.; Wang, Y. Optical Excitation of Carbon Nanotubes Drives Localized Diazonium Reactions. J. Phys. Chem. Lett. 2016, 7, 3690–3694.
- Kim, M.; Adamska, L.; Hartmann, N.F.; Kwon, H.; Liu, J.; Velizhanin, K.A.; Piao, Y.; Powell, L.R.; Meany, B.; Doorn, S.K.; et al. Fluorescent Carbon Nanotube Defects Manifest Substantial Vibrational Reorganization. J. Phys. Chem. C 2016, 120, 11268–11276.
- Kwon, H.; Furmanchuk, A.; Kim, M.; Meany, B.; Guo, Y.; Schatz, G.C.; Wang, Y. Molecularly Tunable Fluorescent Quantum Defects. J. Am. Chem. Soc. 2016, 138, 6878–6885.
- Shiraki, T.; Shiraishi, T.; Juhász, G.; Nakashima, N. Emergence of New Red-Shifted Carbon Nanotube Photoluminescence Based on Proximal Doped-Site Design. Sci. Rep. 2016, 6, 28393.
- Dey, B.; Dutta, T. Laccases: Thriving the Domain of Bio-Electrocatalysis. Bioelectrochemistry 2022, 146, 108144.
- Zelechowska, K.; Stolarczyk, K.; Łyp, D.; Rogalski, J.; Roberts, K.P.; Bilewicz, R.; Biernat, J.F. Aryl and N-Arylamide Carbon Nanotubes for Electrical Coupling of Laccase to Electrodes in Biofuel Cells and Biobatteries. Biocybern. Biomed. Eng. 2013, 33, 235–245.
- Karaśkiewicz, M.; Nazaruk, E.; Zelechowska, K.; Biernat, J.F.; Rogalski, J.; Bilewicz, R. Fully Enzymatic Mediatorless Fuel Cell with Efficient Naphthylated Carbon Nanotube-Laccase Composite Cathodes. Electrochem. Commun. 2012, 20, 124–127.
- Stolarczyk, K.; Łyp, D.; Zelechowska, K.; Biernat, J.F.; Rogalski, J.; Bilewicz, R. Arylated Carbon Nanotubes for Biobatteries and Biofuel Cells. Electrochim. Acta 2012, 79, 74–81.
- Aquino Neto, S.; Milton, R.D.; Hickey, D.P.; de Andrade, A.R.; Minteer, S.D. Membraneless Ethanol/O2 Biofuel Cell Using PQQ-Dependent Alcohol and Aldehyde Dehydrogenase Along with Au Nanoparticles. ECS Meet. Abstr. 2016, MA2016-01, 1847.
- Milton, R.D.; Giroud, F.; Thumser, A.E.; Minteer, S.D.; Slade, R.C.T. Glucose Oxidase Progressively Lowers Bilirubin Oxidasebioelectrocatalytic Cathode Performance in Single-Compartmentglucose/Oxygen Biological Fuel Cells. Electrochim. Acta 2014, 140, 59–64.
- Minson, M.; Meredith, M.T.; Shrier, A.; Giroud, F.; Hickey, D.; Glatzhofer, D.T.; Minteer, S.D. High Performance Glucose/O 2 Biofuel Cell: Effect of Utilizing Purified Laccase with Anthracene-Modified Multi-Walled Carbon Nanotubes. J. Electrochem. Soc. 2012, 159, G166–G170.
- Sorrentino, I.; Gentil, S.; Nedellec, Y.; Cosnier, S.; Piscitelli, A.; Giardina, P.; le Goff, A. POXC Laccase from Pleurotus Ostreatus: A High-Performance Multicopper Enzymefor Direct Oxygen Reduction Reaction Operating in a Proton-Exchange Membrane Fuel Cell. ChemElectroChem 2019, 6, 1023–1027.
- Ortiz-Ortega, E.; Escalona-Villalpando, R.A.; Galindo-de-la-Rosa, J.; Ledesma-García, J.; Minteer, S.D.; Arriaga, L.G. Sweat as Energy Source Using an Enzymatic Microfluidic Fuel Cell. J. Phys. Conf. Ser. 2018, 1052, 012142.
- Liang, Y.; Cai, R.; Hickey, D.P.; Kitt, J.P.; Harris, J.M.; Minteer, S.D.; Korzeniewski, C. Infrared Microscopy as a Probe of Composition within a Model Biofuel Cell Electrode Prepared from Trametes Versicolor Laccase. ChemElectroChem 2019, 6, 818–826.
- Escalona-Villalpando, R.A.; Reid, R.C.; Milton, R.D.; Arriaga, L.G.; Minteer, S.D.; Ledesma-García, J. Improving the Performance of Lactate/Oxygen Biofuel Cells Using a Microfluidic Design. J. Power Sources 2017, 342, 546–552.
- Shrier, A.; Giroud, F.; Rasmussen, M.; Minteer, S.D. Operational Stability Assays for Bioelectrodes for Biofuel Cells: Effect of Immobilization Matrix on Laccase Biocathode Stability. J. Electrochem. Soc. 2014, 161, H244–H248.
- Zumpano, R.; Lambertini, L.; Tortolini, C.; Bollella, P.; Favero, G.; Antiochia, R.; Mazzei, F. A Glucose/Oxygen Enzymatic Fuel Cell Exceeding 1.5 V Based on Glucose Dehydrogenase Immobilized onto PolyMethylene Blue-Carbon Nanotubes Modified Double-Sided Screen Printed Electrodes: Proof-of-Concept in Human Serum and Saliva. J. Power Sources 2020, 476, 228615.
- Stolarczyk, K.; Sepelowska, M.; Lyp, D.; Żelechowska, K.; Biernat, J.F.; Rogalski, J.; Farmer, K.D.; Roberts, K.N.; Bilewicz, R. Hybrid Biobattery Based on Arylated Carbon Nanotubes and Laccase. Bioelectrochemistry 2012, 87, 154–163.
- Majdecka, D.; Draminska, S.; Stolarczyk, K.; Kizling, M.; Krysinski, P.; Golimowski, J.; Biernat, J.F.; Bilewicz, R. Sandwich Biobattery with Enzymatic Cathode and Zinc Anode Integrated with Sensor. J. Electrochem. Soc. 2015, 162, F555–F559.
- Knoche, K.L.; Hickey, D.P.; Milton, R.D.; Curchoe, C.L.; Minteer, S.D. Hybrid Glucose/O2 Biobattery and Supercapacitor Utilizing a Pseudocapacitive Dimethylferrocene Redox Polymer at the Bioanode. ACS Energy Lett. 2016, 1, 380–385.
- Quah, T.; Milton, R.D.; Abdellaoui, S.; Minteer, S.D. Bioelectrocatalytic NAD+/NADH Inter-Conversion: Transformation of an Enzymatic Fuel Cell into an Enzymatic Redox Flow Battery. Chem. Commun. 2017, 53, 8411–8414.
- Giroud, F.; Hickey, D.P.; Schmidtke, D.W.; Glatzhofer, D.T.; Minteer, S.D. A Monosaccharide-Based Coin-Cell Biobattery. ChemElectroChem 2014, 1, 1880–1885.
- Gorton, L.; Bollella, P.; Kano, K.; Hibino, Y.; Antiochia, R.; Rojas-Carrillo, O. (Invited) Fructose Biosensors Based on Direct Electron Transfer between Fructose Dehydrogenase and Electrodes. ECS Meet. Abstr. 2019, MA2019-04, 384.
- Meredith, M.T.; Minson, M.; Hickey, D.; Artyushkova, K.; Glatzhofer, D.T.; Minteer, S.D. Anthracene-Modified Multi-Walled Carbon Nanotubes as Direct Electron Transfer Scaffolds for Enzymatic Oxygen Reduction. ACS Catal. 2011, 1, 1683–1690.
- Aquino Neto, S.; da Silva, R.G.; Milton, R.D.; Minteer, S.D.; de Andrade, A.R. Hybrid Bioelectrocatalytic Reduction of Oxygen at Anthracene-Modified Multi-Walled Carbon Nanotubes Decorated with Ni90Pd10 Nanoparticles. Electrochim. Acta 2017, 251, 195–202.
- Holmberg, S.; Rodriguez-Delgado, M.; Milton, R.D.; Ornelas-Soto, N.; Minteer, S.D.; Parra, R.; Madou, M.J. Bioelectrochemical Study of Thermostable Pycnoporus Sanguineus CS43 Laccase Bioelectrodes Based on Pyrolytic Carbon Nanofibers for Bioelectrocatalytic O2 Reduction. ACS Catal. 2015, 5, 7507–7518.
- Bollella, P.; Hibino, Y.; Kano, K.; Gorton, L.; Antiochia, R. Enhanced Direct Electron Transfer of Fructose Dehydrogenase Rationally Immobilized on a 2-Aminoanthracene Diazonium Cation Grafted Single-Walled Carbon Nanotube Based Electrode. ACS Catal. 2018, 8, 10279–10289.
- Ben Tahar, A.; Żelechowska, K.; Biernat, J.F.; Paluszkiewicz, E.; Cinquin, P.; Martin, D.; Zebda, A. High Catalytic Performance of Laccase Wired to Naphthylated Multiwall Carbon Nanotubes. Biosens. Bioelectron. 2020, 151, 111961.
- Milton, R.D.; Wang, T.; Knoche, K.L.; Minteer, S.D. Tailoring Biointerfaces for Electrocatalysis. Langmuir 2016, 32, 2291–2301.
- Giroud, F.; Minteer, S.D. Anthracene-Modified Pyrenes Immobilized on Carbon Nanotubes for Direct Electroreduction of O2 by Laccase. Electrochem. Commun. 2013, 34, 157–160.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
19 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




