Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sergey Alferov and Version 2 by Lindsay Dong.
Arylated nanotubes are characterized by extended solubility, and are widely used in photoelectronics, semiconductor technology, and bioelectrocatalysis. The main emphasis is on arylation methods according to the radical mechanism, such as the Gomberg–Bachmann and Billups reactions, and the decomposition of peroxides.
- carbon nanomaterials
- semiconductor nanotubes
- metal nanotubes
- carbon nanotube functionalization
1. Introduction
Carbon nanotubes (CNTs), a present-day material discovered in 1993 [1], are widely used in various fields of human activity. Numbers of different CNTs forms have been obtained [2]. CNTs division is of the utmost interest, that depends on the number of layers (single-walled, double-walled, and multi-walled), as well as on the structure type (armchair”-like and zigzag), which affects the conductivity type (metallic and semiconductor, respectively) [3][4][5][3,4,5]. These types of nanotubes are the most notable for their properties. Due to the strong van der Waals interaction between aromatic systems, CNTs “stick together” into dense aggregates, making them almost insoluble, thus it significantly complicates the composite materials generation based on them. The use of various methods of CNT surface functionalization has been proposed to solve this problem, thereby weakening intermolecular interactions. The development of CNT functionalization methods has also enabled the extension of the application area due to a greater variety of CNT properties.
2. Methods of Introducing Aryl Fragments to the CNT Surface
2.1. The Gomberg–Bachmann Reaction
Arylation by the Gomberg–Bachmann reaction with diazonium salts (Figure 1) is the most common method of modifying the surface of carbon nanomaterials.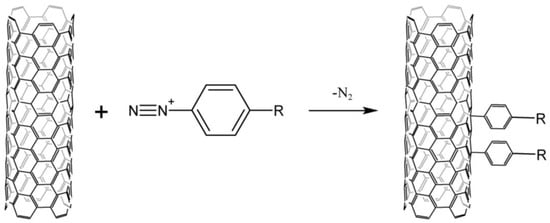
Figure 1.
Scheme of CNTs arylation by the Gomberg–Bachmann reaction with diazonium salts.
Diazonium salts are unstable in solution and extremely explosive in dry form, excluding some complex fluorides, aryl sulfonates [6][25], and o-benzene disulfimides [7][26]. Aqueous solutions of arylenediazonium borofluorides are most commonly used for the arylation of CNTs. Their functionalization is carried out either electrochemically [8][27] or due to the spontaneous decomposition of diazonium cations [9][28]. However, this approach has a number of disadvantages. CNTs are dispersed in water with the use of SDS, which certainly bind to nanotubes due to the strong hydrophobic interactions, and this is not always acceptable in a number of areas, such as electrochemistry and bioelectrocatalysis. Furthermore, arylenediazonium borofluorides are poorly soluble in water.
The production of arylenediazonium in non-aqueous media in situ is also widely used, this is achieved by the reaction between isoamyl nitrite and aromatic amine. However, this approach is not environmentally friendly, as well as the dispersibility of native nanotubes in organic solvents does not meet the requirements. The reaction has been proposed to be carried out in a two-phase water-isoamyl nitrite system [10][29], but this approach does not solve most of the problems.
The occurring aryl radicals attach to nanotubes, reacting as electrophiles. Due to this fact, there can be observed both the selectivity to CNTs with a metallic type of conductivity and a higher reaction rate of aryl radicals with acceptor substituents [11][39]. The resulting aryl-CNT radicals react with diazonium salt, while the structure is stabilized. In the acidic media, a nucleophile from the solution should be attached to the CNT; however, no data confirming this could be found in the literature (Figure 23). The aryl fragment is split off from the nanotube if there occurs no addition of the diazonium cation to the aryl-CNT radical. This has been shown by measuring the electrical conductivity of nanotubes during the reaction. Electrical conductivity increases after the termination of the reaction, which is explained by the cleavage of a part of the aryl groups [12][40].
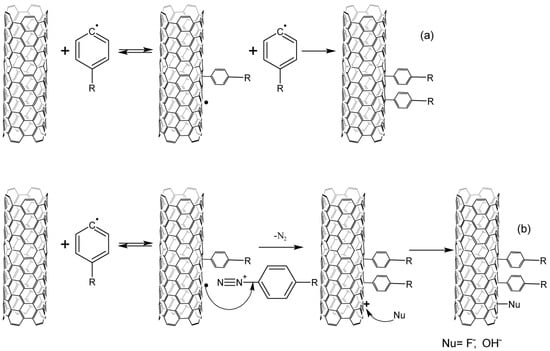

Figure 23.
Interaction of CNTs with aryl radicals and arylenediazonium cations, (
a
)—alkaline media, (
b)—acidic media.
2.2. Billups Reaction
The reductive arylation of CNT salts with various reagents (the Billups reaction and its modifications) is of great interest. It has been shown since 1997 that an electron is transferred from a metal atom to carbon when CNTs are fused with alkali metals [13]. Due to Coulomb repulsion, CNT salts with alkali metals have high spontaneous solubility—up to 2 mg/g in DMSO and DMF, and up to 4 mg/g in sulfolane; moreover, the solutions are stable for up to a year and resemble properties of polyelectrolytes in an inert atmosphere [14][15]. The addition of dibenzo-18-crown-6 increases solubility, due to complexation with sodium ions [16]. Further, there is no need to use ultrasound, which destroys the structure, when preparing solutions of CNT salts [17][18][19][20].
CNT salts can be obtained by several methods (
)—acidic media.
2.2. Billups Reaction
The reductive arylation of CNT salts with various reagents (the Billups reaction and its modifications) is of great interest. It has been shown since 1997 that an electron is transferred from a metal atom to carbon when CNTs are fused with alkali metals [48]. Due to Coulomb repulsion, CNT salts with alkali metals have high spontaneous solubility—up to 2 mg/g in DMSO and DMF, and up to 4 mg/g in sulfolane; moreover, the solutions are stable for up to a year and resemble properties of polyelectrolytes in an inert atmosphere [49,50]. The addition of dibenzo-18-crown-6 increases solubility, due to complexation with sodium ions [51]. Further, there is no need to use ultrasound, which destroys the structure, when preparing solutions of CNT salts [52,53,54,55].
CNT salts can be obtained by several methods (
Figure 3), interaction with an alkali metal solution in liquid ammonia is the most common [17][18][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35], and lithium is mostly used. Alkali metal is embedded inside CNTs, lithium can also form covalent bonds with carbon atoms [34], and alkali metal is possible to be embedded between the layers of tubes with subsequent arylation/hydrogenation of the inner tubes in the case of CNTS [36]. The functionalization degree may be controlled by maintaining an optimal metal-carbon ratio [37].
4), interaction with an alkali metal solution in liquid ammonia is the most common [52,53,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70], and lithium is mostly used. Alkali metal is embedded inside CNTs, lithium can also form covalent bonds with carbon atoms [69], and alkali metal is possible to be embedded between the layers of tubes with subsequent arylation/hydrogenation of the inner tubes in the case of CNTS [71]. The functionalization degree may be controlled by maintaining an optimal metal-carbon ratio [72].
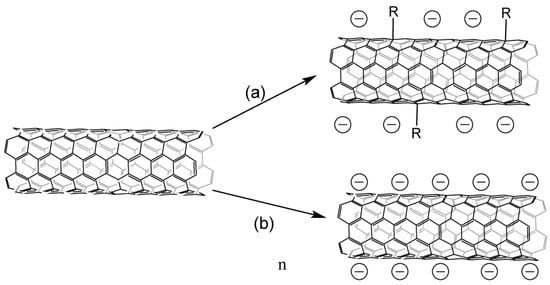

Figure 34.
Obtaining CNT salts: (
a
)—interaction with lithium alkylides, (
b
)—interaction with alkali metals in solvents of various nature in the presence or absence of electronic transport mediators.
The reaction in THF with alkali metals in the presence of electron–carrying catalysts, such as naphthalene [26][37][38][39][40][41], benzophenone [39], or 4,4’-di-tert-butylbiphenyl is another commonly used method of obtaining CNT salts [42]. However, the reaction product turns out to be a contaminated catalyst since it is difficult to remove it due to the strong stack interactions with CNT. This can lead to deterioration of the electrochemical properties of CNTs. Several studies use butyllithium or another lithium alkylide in cyclohexane or THF to produce CNT salts [31][43][44][45][46][47][48][49]. However, in this case, alkyl radicals are also sewn onto the surface of CNTs in addition to aryl ones. The fusion of CNTs with alkali metals in an argon atmosphere [20][50][51][52] or interaction with sodium amalgam in toluene in the presence of dibenzo-18-crown-6 is less common due to the technical complexity [16]. It has also been proposed to replace liquid ammonia with ethylenediamine, but this approach has not been widely used [23][53].
The reaction in THF with alkali metals in the presence of electron–carrying catalysts, such as naphthalene [61,72,73,74,75,76], benzophenone [74], or 4,4’-di-tert-butylbiphenyl is another commonly used method of obtaining CNT salts [77]. However, the reaction product turns out to be a contaminated catalyst since it is difficult to remove it due to the strong stack interactions with CNT. This can lead to deterioration of the electrochemical properties of CNTs. Several studies use butyllithium or another lithium alkylide in cyclohexane or THF to produce CNT salts [66,78,79,80,81,82,83,84]. However, in this case, alkyl radicals are also sewn onto the surface of CNTs in addition to aryl ones. The fusion of CNTs with alkali metals in an argon atmosphere [32,55,85,86] or interaction with sodium amalgam in toluene in the presence of dibenzo-18-crown-6 is less common due to the technical complexity [51]. It has also been proposed to replace liquid ammonia with ethylenediamine, but this approach has not been widely used [58,87].
Aryliodides are mostly used for the arylation of CNT salts [20][21][22][37][38]; therefore, their interaction with CNT has been studied in full detail. Thus, it is shown that the degree of functionalization is higher if there are donor substituents in the aryl radical [21]. Reducing arylation proceeds more easily for nanotubes of smaller diameters [27]. The study [30] proposes a reactor to produce alkylated and arylated nanotubes by the Billups–Birch reaction on a semi-industrial scale.
Aryliodides are mostly used for the arylation of CNT salts [55,56,57,72,73]; therefore, their interaction with CNT has been studied in full detail. Thus, it is shown that the degree of functionalization is higher if there are donor substituents in the aryl radical [56]. Reducing arylation proceeds more easily for nanotubes of smaller diameters [62]. The study [65] proposes a reactor to produce alkylated and arylated nanotubes by the Billups–Birch reaction on a semi-industrial scale.
Using aryliodides as arylating agents leads to the fact that functionalized CNTs turn out to be contaminated by-products of a combination of radicals that are difficult to remove. This has been shown by analysis of GC-MS filtrate after alkylation of CNT [17][24]. Alkylation is carried out with other reagents, such as peroxides [17][29][39], cyclic halides [29], sulfides and disulfides [33], carbonyl compounds [31], acetylenes [45], diazonium salts [20][50][54] and iodonium [41] to solve this problem. The free radicals formed from these compounds give unstable combination products that immediately disintegrate.
2.3. Reactions with Peroxides and Related Compounds
Using aryliodides as arylating agents leads to the fact that functionalized CNTs turn out to be contaminated by-products of a combination of radicals that are difficult to remove. This has been shown by analysis of GC-MS filtrate after alkylation of CNT [52,59]. Alkylation is carried out with other reagents, such as peroxides [52,64,74], cyclic halides [64], sulfides and disulfides [68], carbonyl compounds [66], acetylenes [80], diazonium salts [32,55,89] and iodonium [76] to solve this problem. The free radicals formed from these compounds give unstable combination products that immediately disintegrate.
2.3. Reactions with Peroxides and Related Compounds
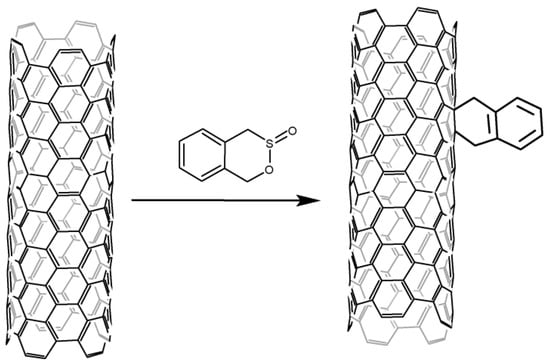
Thermal decomposition of benzoyl peroxide is widely used for CNT phenylation [55][56][57][58][59][60][91,92,93,94,95,96]. The reaction has been carried out in boiling benzene (o-chlorobenzene) [55][56][58][91,92,94] in an argon atmosphere or boiling toluene [57][93] in the air. Despite the higher boiling point of toluene and the same ratio of reagents, the study [57][93] shows a lower functionalization degree than others.
A solid-phase reaction is also described [60][96]. Other peroxides can be used in addition to benzoyl peroxides, such as p-methoxybenzoyl peroxide or phthaloyl peroxide [56][92]. Peroxides can also be used as initiators of radical reactions with other compounds, for example, aryl and alkyl iodides [55][91]. Phenylated nanotubes can also undergo further functionalization, e.g., sulfonation in oleum to increase their solubility in water [58][94].
2.4. Other Arylation Methods
There is a report on the use of the Ullmann reaction for the arylation of CNTs [61][100]. Initially, CNT has been chlorinated by reacting with iodine trichloride in tetrachlorocarbon for 3 hours. Chlorinated CNTs have reacted with iodobenzene, phenol, or aniline in the presence of cesium carbonate, copper chloride 1, and phenanthroline in DMF, at 120 degrees for 2 days. The functionalization degree has reached 3.5 mmol/g. It is shown that functionalization proceeds best for CNTs of smaller diameters. CNT has been proposed to be arylated with 4-methoxyphenylhydrazine hydrochloride by boiling in toluene in an oxygen medium [62][101] or irradiating with microwave radiation. This turns out to be more effective [63][102]. Arylated CNTs are better soluble in o-chlorobenzene. CNT modification with phenylhydrazine by interaction in an aqueous solution in the presence of SDS for 2 days has also been described [64][103].2.5. Alternative Methods of Introducing Aryl Groups to the CNT Surface
The cross-linking of aromatic hydrocarbons with nanotubes via a carboxamide bond and cycloaddition reactions do not belong to traditional arylation methods, nevertheless, they allow introducing aromatic fragments onto the CNT surface. Therefore, they can be used to solve the same problems. The production of aromatic amides from CNTs has a number of disadvantages: the multi-stage nature of the process, the strong destruction of the CNT structure, as well as the crosslinking of aryl groups mainly with edge carbon atoms (Figure 48).
Figure 48. Cycloaddition to CNT under microwave irradiation.
2.6. Fractionation of Carbon Nanotubes
All scalable methods of CNT synthesis give a mixture with a wide range of properties: length, diameter, and type of conductivity. The isolation of individual CNT fractions is important for the most effective use of their extraordinary properties. The most acute problem is the separation of metal nanotubes from semiconductor ones [65]. Semiconductor CNTs are used to create transistors [66]; moreover, they have photoluminescence [67][68][69][70]. The admixture of metal CNTs worsens their properties significantly, causing incorrect transistors’ functioning. Gomberg–Bachmann arylation proceeds selectively to metallic nanotubes [71][72][73][74][75].
Cycloaddition to CNT under microwave irradiation.
2.6. Fractionation of Carbon Nanotubes
All scalable methods of CNT synthesis give a mixture with a wide range of properties: length, diameter, and type of conductivity. The isolation of individual CNT fractions is important for the most effective use of their extraordinary properties. The most acute problem is the separation of metal nanotubes from semiconductor ones [113]. Semiconductor CNTs are used to create transistors [114]; moreover, they have photoluminescence [115,116,117,118]. The admixture of metal CNTs worsens their properties significantly, causing incorrect transistors’ functioning. Gomberg–Bachmann arylation proceeds selectively to metallic nanotubes [43,44,119,120,121].
