
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sepiso Kenias Masenga | -- | 3844 | 2023-05-17 13:14:55 | | | |
| 2 | Lindsay Dong | -6 word(s) | 3838 | 2023-05-18 09:00:53 | | |
Video Upload Options
The development of antiretroviral drugs (ARVs) was a great milestone in the management of human immunodeficiency virus (HIV) infection. ARVs suppress viral activity in the host cell, thus minimizing injury to the cells and prolonging life. However, an effective treatment has remained elusive for four decades due to the successful immune evasion mechanisms of the virus.
1. Introduction
Tremendous progress in the understanding of the HIV molecular interaction with the host cell, the host cell responses to the virus and potential therapeutic implications of this interaction has been made since its discovery [30][31]. Vigorous research heralded the development of antiretroviral drugs a very important milestone in controlling the HIV pandemic [32]. Despite much progress in understanding the HIV–host cell interactions, the cure for HIV infection has remained elusive for four decades now [31][33].
2. Structure of HIV
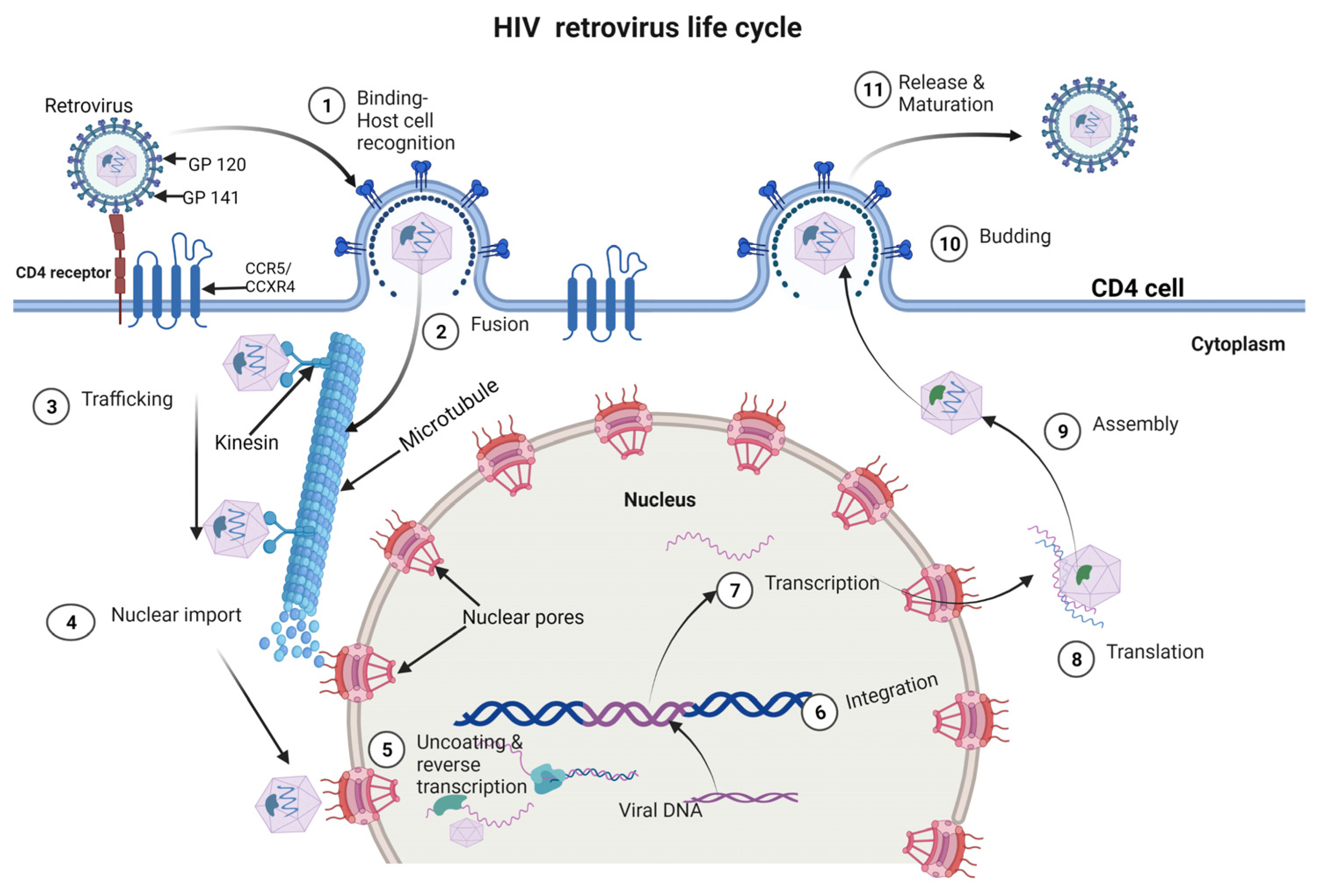
3. HIV Life Cycle
4. HIV-Related Factors Promoting Infection and Immune Evasion
4.1. Downregulation of MHC Class I and II
4.2. Production of Non-Neutralizing Antibodies
4.3. Induction of Immune Exhaustion
4.4. Destruction of Virus-Specific T Helper Cells
4.5. The Emergence of Antigenic Escape Variants
4.6. Expression of an Envelope Complex That Minimizes Antibody Access
4.7. Dysregulation of the JAK/STAT Pathway
4.8. Other Factors That Promote HIV Infection
5. Host Cell Mechanisms That Control Infection and Replication
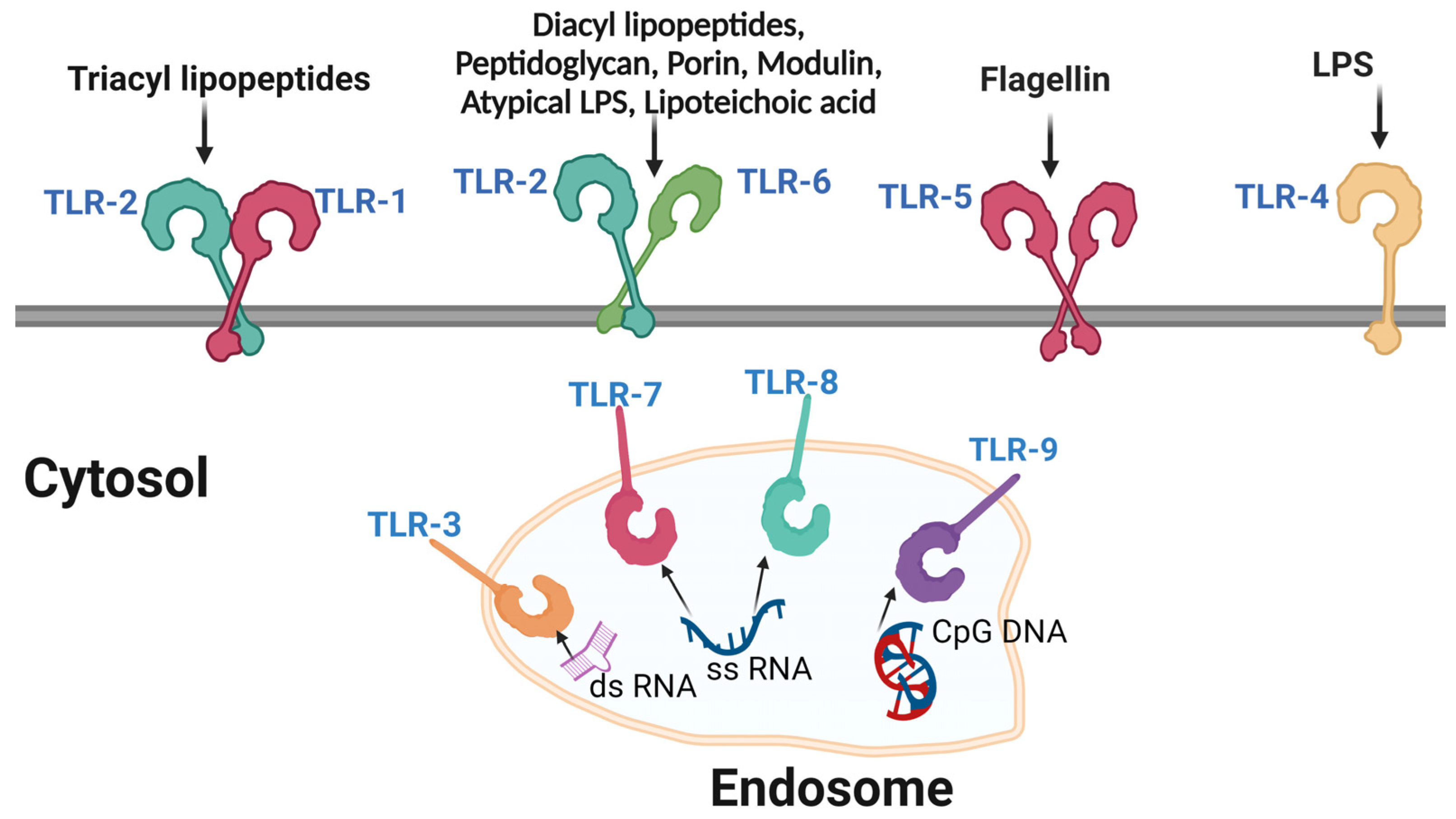
5.1. Pathogen Recognition Receptors (PRRs)
5.2. Dendritic Cells
5.2.1. Plasmacytoid Dendritic Cells (pDCs)
5.2.2. Conventional Dendritic Cells (cDCs)
5.3. Macrophages
5.4. CD4+ T Cells
5.5. CD8+ T Cells
6. Influence of Sex on HIV Transmission and Immune Responses
References
- German Advisory Committee Blood. Human Immunodeficiency Virus (HIV). Transfus. Med. Hemotherapy 2016, 43, 203–222.
- Moss, J.A. HIV/AIDS Review. Radiol. Technol. 2013, 84, 247–267.
- Faria, N.R.; Rambaut, A.; Suchard, M.A.; Baele, G.; Bedford, T.; Ward, M.J.; Tatem, A.J.; Sousa, J.D.; Arinaminpathy, N.; Pépin, J.; et al. HIV Epidemiology. The Early Spread and Epidemic Ignition of HIV-1 in Human Populations. Science 2014, 346, 56–61.
- Rife, B.; Salemi, M. On the Early Dynamics and Spread of HIV-1. Trends Microbiol. 2015, 23, 3–4.
- Visseaux, B.; Le Hingrat, Q.; Damond, F.; Charpentier, C.; Descamps, D. . Virol. Montrouge Fr. 2019, 23, 277–291.
- Kalinichenko, S.; Komkov, D.; Mazurov, D. HIV-1 and HTLV-1 Transmission Modes: Mechanisms and Importance for Virus Spread. Viruses 2022, 14, 152.
- Visseaux, B.; Damond, F.; Matheron, S.; Descamps, D.; Charpentier, C. Hiv-2 Molecular Epidemiology. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2016, 46, 233–240.
- Boswell, M.T.; Rowland-Jones, S.L. Delayed Disease Progression in HIV-2: The Importance of TRIM5α and the Retroviral Capsid. Clin. Exp. Immunol. 2019, 196, 305–317.
- Vidya Vijayan, K.K.; Karthigeyan, K.P.; Tripathi, S.P.; Hanna, L.E. Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections. Front. Immunol. 2017, 8, 580.
- Joseph, S.B.; Arrildt, K.T.; Sturdevant, C.B.; Swanstrom, R. HIV-1 Target Cells in the CNS. J. Neurovirol. 2015, 21, 276–289.
- Woodham, A.W.; Skeate, J.G.; Sanna, A.M.; Taylor, J.R.; Da Silva, D.M.; Cannon, P.M.; Kast, W.M. Human Immunodeficiency Virus Immune Cell Receptors, Coreceptors, and Cofactors: Implications for Prevention and Treatment. AIDS Patient Care STDs 2016, 30, 291–306.
- Fenwick, C.; Joo, V.; Jacquier, P.; Noto, A.; Banga, R.; Perreau, M.; Pantaleo, G. T-Cell Exhaustion in HIV Infection. Immunol. Rev. 2019, 292, 149–163.
- Ma, T.; Luo, X.; George, A.F.; Mukherjee, G.; Sen, N.; Spitzer, T.L.; Giudice, L.C.; Greene, W.C.; Roan, N.R. HIV Efficiently Infects T Cells from the Endometrium and Remodels Them to Promote Systemic Viral Spread. eLife 2020, 9, e55487.
- Renault, C.; Veyrenche, N.; Mennechet, F.; Bedin, A.-S.; Routy, J.-P.; Van de Perre, P.; Reynes, J.; Tuaillon, E. Th17 CD4+ T-Cell as a Preferential Target for HIV Reservoirs. Front. Immunol. 2022, 13, 822576.
- Ng’eno, B.N.; Kellogg, T.A.; Kim, A.A.; Mwangi, A.; Mwangi, M.; Wamicwe, J.; Rutherford, G.W. Modes of HIV Transmission among Adolescents and Young Adults Aged 10-24 Years in Kenya. Int. J. STD AIDS 2018, 29, 800–805.
- del Rio, C. The Global HIV Epidemic: What the Pathologist Needs to Know. Semin. Diagn. Pathol. 2017, 34, 314–317.
- Kassa, G.M. Mother-to-Child Transmission of HIV Infection and Its Associated Factors in Ethiopia: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2018, 18, 216.
- Osório, D.; Munyangaju, I.; Nacarapa, E.; Muhiwa, A.; Nhangave, A.V.; Ramos, J.M. Mother-to-Child Transmission of HIV Infection and Its Associated Factors in the District of Bilene, Gaza Province-Mozambique. PLoS ONE 2021, 16, e0260941.
- Morar, M.M.; Pitman, J.P.; McFarland, W.; Bloch, E.M. The Contribution of Unsafe Blood Transfusion to Human Immunodeficiency Virus Incidence in Sub-Saharan Africa: Reexamination of the 5% to 10% Convention. Transfusion 2016, 56, 3121–3132.
- Belov, A.; Yang, H.; Forshee, R.A.; Whitaker, B.I.; Eder, A.F.; Chancey, C.; Anderson, S.A. Modeling the Risk of HIV Transfusion Transmission. J. Acquir. Immune Defic. Syndr. 1999 2023, 92, 173–179.
- Ball, L.J.; Puka, K.; Speechley, M.; Wong, R.; Hallam, B.; Wiener, J.C.; Koivu, S.; Silverman, M.S. Sharing of Injection Drug Preparation Equipment Is Associated With HIV Infection: A Cross-Sectional Study. J. Acquir. Immune Defic. Syndr. 1999 2019, 81, e99–e103.
- Kumar, P.; Aridoss, S.; Mathiyazhakan, M.; Balasubramanian, G.; Jaganathasamy, N.; Natesan, M.; Padmapriya, V.M.; David, J.K.; Rajan, S.; Adhikary, R.; et al. Substance Use and Risk of HIV Infection among Men Who Have Sex with Men in India. Medicine 2020, 99, e21360.
- Scheim, A.I.; Nosova, E.; Knight, R.; Hayashi, K.; Kerr, T. HIV Incidence Among Men Who Have Sex with Men and Inject Drugs in a Canadian Setting. AIDS Behav. 2018, 22, 3957–3961.
- El-Bassel, N.; Shaw, S.A.; Dasgupta, A.; Strathdee, S.A. Drug Use as a Driver of HIV Risks: Re-Emerging and Emerging Issues. Curr. Opin. HIV AIDS 2014, 9, 150–155.
- Edeza, A.; Bazzi, A.; Salhaney, P.; Biancarelli, D.; Childs, E.; Mimiaga, M.J.; Drainoni, M.-L.; Biello, K. HIV Pre-Exposure Prophylaxis for People Who Inject Drugs: The Context of Co-Occurring Injection- and Sexual-Related HIV Risk in the U.S. Northeast. Subst. Use Misuse 2020, 55, 525–533.
- Bruxelle, J.-F.; Trattnig, N.; Mureithi, M.W.; Landais, E.; Pantophlet, R. HIV-1 Entry and Prospects for Protecting against Infection. Microorganisms 2021, 9, 228.
- Jewanraj, J.; Ngcapu, S.; Liebenberg, L.J.P. Semen: A Modulator of Female Genital Tract Inflammation and a Vector for HIV-1 Transmission. Am. J. Reprod. Immunol. 2021, 86, e13478.
- Cavarelli, M.; Le Grand, R. The Importance of Semen Leukocytes in HIV-1 Transmission and the Development of Prevention Strategies. Hum. Vaccines Immunother. 2020, 16, 2018–2032.
- Kaul, R.; Liu, C.M.; Park, D.E.; Galiwango, R.M.; Tobian, A.A.R.; Prodger, J.L. The Penis, the Vagina and HIV Risk: Key Differences (Aside from the Obvious). Viruses 2022, 14, 1164.
- Jones, J.; Sullivan, P.S.; Curran, J.W. Progress in the HIV Epidemic: Identifying Goals and Measuring Success. PLoS Med. 2019, 16, e1002729.
- Shcherbatova, O.; Grebennikov, D.; Sazonov, I.; Meyerhans, A.; Bocharov, G. Modeling of the HIV-1 Life Cycle in Productively Infected Cells to Predict Novel Therapeutic Targets. Pathogens 2020, 9, 255.
- Nelson, A.G.; Zhang, X.; Ganapathi, U.; Szekely, Z.; Flexner, C.W.; Owen, A.; Sinko, P.J. Drug Delivery Strategies and Systems for HIV/AIDS Pre-Exposure Prophylaxis and Treatment. J. Control. Release 2015, 219, 669–680.
- Sankaranantham, M. HIV—Is a Cure Possible? Indian J. Sex. Transm. Dis. AIDS 2019, 40, 1–5.
- Sakuragi, J. Morphogenesis of the Infectious HIV-1 Virion. Front. Microbiol. 2011, 2, 242.
- McGettigan, J.P.; Naper, K.; Orenstein, J.; Koser, M.; McKenna, P.M.; Schnell, M.J. Functional Human Immunodeficiency Virus Type 1 (HIV-1) Gag-Pol or HIV-1 Gag-Pol and Env Expressed from a Single Rhabdovirus-Based Vaccine Vector Genome. J. Virol. 2003, 77, 10889–10899.
- Hill, M.; Tachedjian, G.; Mak, J. The Packaging and Maturation of the HIV-1 Pol Proteins. Curr. HIV Res. 2005, 3, 73–85.
- Staudt, R.P.; Alvarado, J.J.; Emert-Sedlak, L.A.; Shi, H.; Shu, S.T.; Wales, T.E.; Engen, J.R.; Smithgall, T.E. Structure, Function, and Inhibitor Targeting of HIV-1 Nef-Effector Kinase Complexes. J. Biol. Chem. 2020, 295, 15158–15171.
- NIH HIV Replication Cycle|NIH: National Institute of Allergy and Infectious Diseases. Available online: https://www.niaid.nih.gov/diseases-conditions/hiv-replication-cycle (accessed on 23 February 2023).
- Greta Hughson HIV Lifecycle. Available online: https://www.aidsmap.com/about-hiv/hiv-lifecycle (accessed on 3 March 2023).
- Xiao, T.; Cai, Y.; Chen, B. HIV-1 Entry and Membrane Fusion Inhibitors. Viruses 2021, 13, 735.
- Wilen, C.B.; Tilton, J.C.; Doms, W. HIV: Cell Binding and Entry. Cold Spring Harb. Perspect. Med. 2012, 2, a006866.
- Negi, G.; Sharma, A.; Dey, M.; Dhanawat, G.; Parveen, N. Membrane Attachment and Fusion of HIV-1, Influenza A, and SARS-CoV-2: Resolving the Mechanisms with Biophysical Methods. Biophys. Rev. 2022, 14, 1109–1140.
- Doms, R.W.; Moore, J.P. HIV-1 Membrane Fusion. J. Cell. Biol. 2000, 151, f9–f14.
- Sapp, N.; Burge, N.; Cox, K.; Prakash, P.; Balasubramaniam, M.; Thapa, S.; Christensen, D.; Li, M.; Linderberger, J.; Kvaratskhelia, M.; et al. HIV-1 Preintegration Complex Preferentially Integrates the Viral DNA into Nucleosomes Containing Trimethylated Histone 3-Lysine 36 Modification and Flanking Linker DNA. J. Virol. 2022, 96, e0101122.
- Blanco-Rodriguez, G.; Gazi, A.; Monel, B.; Frabetti, S.; Scoca, V.; Mueller, F.; Schwartz, O.; Krijnse-Locker, J.; Charneau, P.; Di Nunzio, F. Remodeling of the Core Leads HIV-1 Preintegration Complex into the Nucleus of Human Lymphocytes. J. Virol. 2020, 94, e00135-20.
- Rozina, A.; Anisenko, A.; Kikhai, T.; Silkina, M.; Gottikh, M. Complex Relationships between HIV-1 Integrase and Its Cellular Partners. Int. J. Mol. Sci. 2022, 23, 12341.
- Elliott, J.L.; Kutluay, S.B. Going beyond Integration: The Emerging Role of HIV-1 Integrase in Virion Morphogenesis. Viruses 2020, 12, 1005.
- PubChem HIV Life Cycle. Available online: https://pubchem.ncbi.nlm.nih.gov/pathway/Reactome:R-HSA-162587 (accessed on 3 March 2023).
- Goulder, P.J.R.; Watkins, D.I. Impact of MHC Class I Diversity on Immune Control of Immunodeficiency Virus Replication. Nat. Rev. Immunol. 2008, 8, 619–630.
- Swigut, T.; Alexander, L.; Morgan, J.; Lifson, J.; Mansfield, K.G.; Lang, S.; Johnson, R.P.; Skowronski, J.; Desrosiers, R. Impact of Nef-Mediated Downregulation of Major Histocompatibility Complex Class I on Immune Response to Simian Immunodeficiency Virus. J. Virol. 2004, 78, 13335–13344.
- Kerkau, T.; Bacik, I.; Bennink, J.R.; Yewdell, J.W.; Hünig, T.; Schimpl, A.; Schubert, U. The Human Immunodeficiency Virus Type 1 (HIV-1) Vpu Protein Interferes with an Early Step in the Biosynthesis of Major Histocompatibility Complex (MHC) Class I Molecules. J. Exp. Med. 1997, 185, 1295–1306.
- Koyanagi, N.; Kawaguchi, Y. Evasion of the Cell-Mediated Immune Response by Alphaherpesviruses. Viruses 2020, 12, 1354.
- Stumptner-Cuvelette, P.; Morchoisne, S.; Dugast, M.; Le Gall, S.; Raposo, G.; Schwartz, O.; Benaroch, P. HIV-1 Nef Impairs MHC Class II Antigen Presentation and Surface Expression. Proc. Natl. Acad. Sci. USA 2001, 98, 12144–12149.
- Wonderlich, E.R.; Leonard, J.A.; Collins, K.L. HIV Immune Evasion: Disruption of Antigen Presentation by the HIV Nef Protein. Adv. Virus Res. 2011, 80, 103–127.
- Roeth, J.F.; Collins, K.L. Human Immunodeficiency Virus Type 1 Nef: Adapting to Intracellular Trafficking Pathways. Microbiol. Mol. Biol. Rev. 2006, 70, 548–563.
- Blagoveshchenskaya, A.D.; Thomas, L.; Feliciangeli, S.F.; Hung, C.-H.; Thomas, G. HIV-1 Nef Downregulates MHC-I by a PACS-1- and PI3K-Regulated ARF6 Endocytic Pathway. Cell 2002, 111, 853–866.
- Khan, N.; Geiger, J.D. Role of Viral Protein U (Vpu) in HIV-1 Infection and Pathogenesis. Viruses 2021, 13, 1466.
- Dubé, M.; Bego, M.G.; Paquay, C.; Cohen, É.A. Modulation of HIV-1-Host Interaction: Role of the Vpu Accessory Protein. Retrovirology 2010, 7, 114.
- Guha, D.; Ayyavoo, V. Innate Immune Evasion Strategies by Human Immunodeficiency Virus Type 1. ISRN AIDS 2013, 2013, 954806.
- Carrington, M.; Alter, G. Innate Immune Control of HIV. Cold Spring Harb. Perspect. Med. 2012, 2, a007070.
- Tomescu, C.; Law, W.K.; Kedes, D.H. Surface Downregulation of Major Histocompatibility Complex Class I, PE-CAM, and ICAM-1 Following De Novo Infection of Endothelial Cells with Kaposi’s Sarcoma-Associated Herpesvirus. J. Virol. 2003, 77, 9669–9684.
- Dirk, B.S.; Pawlak, E.N.; Johnson, A.L.; Van Nynatten, L.R.; Jacob, R.A.; Heit, B.; Dikeakos, J.D. HIV-1 Nef Sequesters MHC-I Intracellularly by Targeting Early Stages of Endocytosis and Recycling. Sci. Rep. 2016, 6, 37021.
- Mielke, D.; Bandawe, G.; Zheng, J.; Jones, J.; Abrahams, M.-R.; Bekker, V.; Ochsenbauer, C.; Garrett, N.; Abdool Karim, S.; Moore, P.L.; et al. ADCC-Mediating Non-Neutralizing Antibodies Can Exert Immune Pressure in Early HIV-1 Infection. PLoS Pathog. 2021, 17, e1010046.
- Martín, J.; LaBranche, C.C.; González-Scarano, F. Differential CD4/CCR5 Utilization, Gp120 Conformation, and Neutralization Sensitivity between Envelopes from a Microglia-Adapted Human Immunodeficiency Virus Type 1 and Its Parental Isolate. J. Virol. 2001, 75, 3568–3580.
- Raja, A.; Venturi, M.; Kwong, P.; Sodroski, J. CD4 Binding Site Antibodies Inhibit Human Immunodeficiency Virus Gp120 Envelope Glycoprotein Interaction with CCR5. J. Virol. 2003, 77, 713–718.
- Sachdeva, M.; Fischl, M.A.; Pahwa, R.; Sachdeva, N.; Pahwa, S. Immune Exhaustion Occurs Concomitantly with Immune Activation and Decrease in Regulatory T Cells in Viremic Chronically HIV-1 Infected Patients. J. Acquir. Immune Defic. Syndr. 1999 2010, 54, 447–454.
- Balasubramaniam, M.; Pandhare, J.; Dash, C. Immune Control of HIV. J. Life Sci. Westlake Village Calif. 2019, 1, 4–37.
- Walker, B.; McMichael, A. The T-Cell Response to HIV. Cold Spring Harb. Perspect. Med. 2012, 2, a007054.
- Cummins, N.W.; Badley, A.D. Mechanisms of HIV-Associated Lymphocyte Apoptosis: 2010. Cell. Death Dis. 2010, 1, e99.
- Oyaizu, N.; McCloskey, T.; Than, S.; Hu, R.; Kalyanaraman, V.; Pahwa, S. Cross-Linking of CD4 Molecules Upregulates Fas Antigen Expression in Lymphocytes by Inducing Interferon-Gamma and Tumor Necrosis Factor- Alpha Secretion. Blood 1994, 84, 2622–2631.
- Chandrasekar, A.P.; Cummins, N.W.; Badley, A.D. The Role of the BCL-2 Family of Proteins in HIV-1 Pathogenesis and Persistence. Clin. Microbiol. Rev. 2019, 33, e00107-19.
- Xu, H.; Wang, X.; Veazey, R.S. Mucosal Immunology of HIV Infection. Immunol. Rev. 2013, 254, 10–33.
- McMichael, A.J.; Borrow, P.; Tomaras, G.D.; Goonetilleke, N.; Haynes, B.F. The Immune Response during Acute HIV-1 Infection: Clues for Vaccine Development. Nat. Rev. Immunol. 2010, 10, 11–23.
- Batorsky, R.; Sergeev, R.A.; Rouzine, I.M. The Route of HIV Escape from Immune Response Targeting Multiple Sites Is Determined by the Cost-Benefit Tradeoff of Escape Mutations. PLoS Comput. Biol. 2014, 10, e1003878.
- Cuevas, J.M.; Geller, R.; Garijo, R.; López-Aldeguer, J.; Sanjuán, R. Extremely High Mutation Rate of HIV-1 In Vivo. PLoS Biol. 2015, 13, e1002251.
- Abram, M.E.; Ferris, A.L.; Das, K. Mutations in HIV-1 Reverse Transcriptase Affect the Errors Made in a Single Cycle of Viral Replication. J. Virol. 2014, 88, 7589–7601.
- Burton, D.R.; Mascola, J.R. Antibody Responses to Envelope Glycoproteins in HIV-1 Infection. Nat. Immunol. 2015, 16, 571–576.
- Prabakaran, P.; Dimitrov, A.S.; Fouts, T.R.; Dimitrov, D.S. Structure and Function of the HIV Envelope Glycoprotein as Entry Mediator, Vaccine Immunogen, and Target for Inhibitors. Adv. Pharmacol. San. Diego Calif. 2007, 55, 33–97.
- Swiecki, M.; Colonna, M. Type I Interferons: Diversity of Sources, Production Pathways and Effects on Immune Responses. Curr. Opin. Virol. 2011, 1, 463–475.
- Marziali, F.; Delpeuch, M.; Kumar, A.; Appourchaux, R.; Dufloo, J.; Tartour, K.; Etienne, L.; Cimarelli, A. Functional Heterogeneity of Mammalian IFITM Proteins against HIV-1. J. Virol. 2021, 95, e0043921.
- Ali, S.; Mann-Nüttel, R.; Schulze, A.; Richter, L.; Alferink, J.; Scheu, S. Sources of Type I Interferons in Infectious Immunity: Plasmacytoid Dendritic Cells Not Always in the Driver’s Seat. Front. Immunol. 2019, 10, 778.
- Ivashkiv, L.B.; Donlin, L.T. Regulation of Type I Interferon Responses. Nat. Rev. Immunol. 2014, 14, 36–49.
- Hermant, P.; Michiels, T. Interferon-λ in the Context of Viral Infections: Production, Response and Therapeutic Implications. J. Innate Immun. 2014, 6, 563–574.
- Lackner, A.A.; Lederman, M.M.; Rodriguez, B. HIV Pathogenesis: The Host. Cold Spring Harb. Perspect. Med. 2012, 2, a007005.
- Azimi, F.C.; Lee, J.E. Structural Perspectives on HIV-1 Vif and APOBEC3 Restriction Factor Interactions. Protein Sci. Publ. Protein Soc. 2020, 29, 391–406.
- Kung, W.-W.; Ramachandran, S.; Makukhin, N.; Bruno, E.; Ciulli, A. Structural Insights into Substrate Recognition by the SOCS2 E3 Ubiquitin Ligase. Nat. Commun. 2019, 10, 2534.
- Stanley, B.J.; Ehrlich, E.S.; Short, L.; Yu, Y.; Xiao, Z.; Yu, X.-F.; Xiong, Y. Structural Insight into the Human Immunodeficiency Virus Vif SOCS Box and Its Role in Human E3 Ubiquitin Ligase Assembly. J. Virol. 2008, 82, 8656.
- Lata, S.; Mishra, R.; Banerjea, A.C. Proteasomal Degradation Machinery: Favorite Target of HIV-1 Proteins. Front. Microbiol. 2018, 9, 2738.
- Du, Y.; Hu, Z.; Luo, Y.; Wang, H.Y.; Yu, X.; Wang, R.-F. Function and Regulation of CGAS-STING Signaling in Infectious Diseases. Front. Immunol. 2023, 14, 1130423.
- Sivro, A.; Su, R.-C.; Plummer, F.A.; Ball, T.B. Interferon Responses in HIV Infection: From Protection to Disease. AIDS Rev. 2014, 16, 43–51.
- Ananworanich, J.; Sacdalan, C.P.; Pinyakorn, S.; Chomont, N.; Souza, M.; Luekasemsuk, T.; Schuetz, A.; Krebs, S.J.; Dewar, R.; Jagodzinski, L.; et al. Virological and Immunological Characteristics of HIV-Infected Individuals at the Earliest Stage of Infection. J. Virus Erad. 2016, 2, 43–48.
- Robb, M.L.; Eller, L.A.; Kibuuka, H.; Rono, K.; Maganga, L.; Nitayaphan, S.; Kroon, E.; Sawe, F.K.; Sinei, S.; Sriplienchan, S.; et al. Prospective Study of Acute HIV-1 Infection in Adults in East Africa and Thailand. N. Engl. J. Med. 2016, 374, 2120–2130.
- Martín-Moreno, A.; Muñoz-Fernández, M.A. Dendritic Cells, the Double Agent in the War Against HIV-1. Front. Immunol. 2019, 10, 2485.
- Lee, H.-C.; Chathuranga, K.; Lee, J.-S. Intracellular Sensing of Viral Genomes and Viral Evasion. Exp. Mol. Med. 2019, 51, 1–13.
- Shi, Y.; Su, J.; Chen, R.; Wei, W.; Yuan, Z.; Chen, X.; Wang, X.; Liang, H.; Ye, L.; Jiang, J. The Role of Innate Immunity in Natural Elite Controllers of HIV-1 Infection. Front. Immunol. 2022, 13, 780922.
- Radoshevich, L.; Dussurget, O. Cytosolic Innate Immune Sensing and Signaling upon Infection. Front. Microbiol. 2016, 7, 313.
- Goubau, D.; Deddouche, S.; Reis e Sousa, C. Cytosolic Sensing of Viruses. Immunity 2013, 38, 855–869.
- Kell, A.M.; Gale, M. RIG-I in RNA Virus Recognition. Virology 2015, 479–480, 110–121.
- Abbas, F.; Cenac, C.; Youness, A.; Azar, P.; Delobel, P.; Guéry, J.-C. HIV-1 Infection Enhances Innate Function and TLR7 Expression in Female Plasmacytoid Dendritic Cells. Life Sci. Alliance 2022, 5, e202201452.
- Scagnolari, C.; Antonelli, G. Type I Interferon and HIV: Subtle Balance between Antiviral Activity, Immunopathogenesis and the Microbiome. Cytokine Growth Factor. Rev. 2018, 40, 19.
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 291.
- Amarante-Mendes, G.P.; Adjemian, S.; Branco, L.M.; Zanetti, L.C.; Weinlich, R.; Bortoluci, K.R. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379.
- Kawai, T.; Akira, S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-like Receptors. Nat. Immunol. 2010, 11, 373–384.
- Jang, J.-H.; Shin, H.W.; Lee, J.M.; Lee, H.-W.; Kim, E.-C.; Park, S.H. An Overview of Pathogen Recognition Receptors for Innate Immunity in Dental Pulp. Mediat. Inflamm. 2015, 2015, 794143.
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273.
- Schoenemeyer, A.; Barnes, B.J.; Mancl, M.E.; Latz, E.; Goutagny, N.; Pitha, P.M.; Fitzgerald, K.A.; Golenbock, D.T. The Interferon Regulatory Factor, IRF5, Is a Central Mediator of Toll-like Receptor 7 Signaling. J. Biol. Chem. 2005, 280, 17005–17012.
- Kwa, M.Q.; Nguyen, T.; Huynh, J.; Ramnath, D.; De Nardo, D.; Lam, P.Y.; Reynolds, E.C.; Hamilton, J.A.; Sweet, M.J.; Scholz, G.M. Interferon Regulatory Factor 6 Differentially Regulates Toll-like Receptor 2-Dependent Chemokine Gene Expression in Epithelial Cells. J. Biol. Chem. 2014, 289, 19758–19768.
- Manches, O.; Frleta, D.; Bhardwaj, N. Dendritic Cells in Progression and Pathology of HIV Infection. Trends Immunol. 2014, 35, 114–122.
- Chathuranga, K.; Weerawardhana, A.; Dodantenna, N.; Lee, J.-S. Regulation of Antiviral Innate Immune Signaling and Viral Evasion Following Viral Genome Sensing. Exp. Mol. Med. 2021, 53, 1647–1668.
- Collin, M.; Bigley, V. Human Dendritic Cell Subsets: An Update. Immunology 2018, 154, 3–20.
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92.
- Parihar, A.; Eubank, T.D.; Doseff, A.I. Monocytes and Macrophages Regulate Immunity through Dynamic Networks of Survival and Cell Death. J. Innate Immun. 2010, 2, 204–215.
- Mass, E.; Nimmerjahn, F.; Kierdorf, K.; Schlitzer, A. Tissue-Specific Macrophages: How They Develop and Choreograph Tissue Biology. Nat. Rev. Immunol. 2023, 1–17.
- Schulz, C.; Perdiguero, E.G.; Chorro, L.; Szabo-Rogers, H.; Cagnard, N.; Kierdorf, K.; Prinz, M.; Wu, B.; Jacobsen, S.E.W.; Pollard, J.W.; et al. A Lineage of Myeloid Cells Independent of Myb and Hematopoietic Stem Cells. Science 2012, 336, 86–90.
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-Resident Macrophages Self-Maintain Locally throughout Adult Life with Minimal Contribution from Circulating Monocytes. Immunity 2013, 38, 792–804.
- Bajgar, A.; Krejčová, G. On the Origin of the Functional Versatility of Macrophages. Front. Physiol. 2023, 14, 246.
- Koppensteiner, H.; Brack-Werner, R.; Schindler, M. Macrophages and Their Relevance in Human Immunodeficiency Virus Type I Infection. Retrovirology 2012, 9, 82.
- Wu, L. Biology of HIV Mucosal Transmission. Curr. Opin. HIV AIDS 2008, 3, 534–540.
- Shen, R.; Richter, H.E.; Smith, P.D. Interactions between HIV-1 and Mucosal Cells in the Female Reproductive Tract. Am. J. Reprod. Immunol. 2014, 71, 608–617.
- Teer, E.; Joseph, D.E.; Glashoff, R.H.; Faadiel Essop, M. Monocyte/Macrophage-Mediated Innate Immunity in HIV-1 Infection: From Early Response to Late Dysregulation and Links to Cardiovascular Diseases Onset. Virol. Sin. 2021, 36, 565–576.
- Bertram, K.M.; Tong, O.; Royle, C.; Turville, S.G.; Nasr, N.; Cunningham, A.L.; Harman, A.N. Manipulation of Mononuclear Phagocytes by HIV: Implications for Early Transmission Events. Front. Immunol. 2019, 10, 2263.
- Okoye, A.A.; Picker, L.J. CD4+ T Cell Depletion in HIV Infection: Mechanisms of Immunological Failure. Immunol. Rev. 2013, 254, 54–64.
- Abbas, W.; Herbein, G. T-Cell Signaling in HIV-1 Infection. Open Virol. J. 2013, 7, 57–71.
- Courtney, A.H.; Lo, W.-L.; Weiss, A. TCR signaling: Mechanisms of initiation and propagation. Trends Biochem. Sci. 2018, 43, 108–123.
- Pino, S.C.; O’Sullivan-Murphy, B.; Lidstone, E.A.; Thornley, T.B.; Jurczyk, A.; Urano, F.; Greiner, D.L.; Mordes, J.P.; Rossini, A.A.; Bortell, R. Protein Kinase C Signaling during T Cell Activation Induces the Endoplasmic Reticulum Stress Response. Cell. Stress. Chaperones 2008, 13, 421–434.
- Perdomo-Celis, F.; Taborda, N.A.; Rugeles, M.T. CD8+ T-Cell Response to HIV Infection in the Era of Antiretroviral Therapy. Front. Immunol. 2019, 10, 1896.
- McBrien, J.B.; Kumar, N.A.; Silvestri, G. Mechanisms of CD8+ T Cell-Mediated Suppression of HIV/SIV Replication. Eur. J. Immunol. 2018, 48, 898–914.
- Juno, J.A.; van Bockel, D.; Kent, S.J.; Kelleher, A.D.; Zaunders, J.J.; Munier, C.M.L. Cytotoxic CD4 T Cells—Friend or Foe during Viral Infection? Front. Immunol. 2017, 8, 19.
- Ziegler, S.; Altfeld, M. Sex Differences in HIV-1-Mediated Immunopathology. Curr. Opin. HIV AIDS 2016, 11, 209.
- Santinelli, L.; Ceccarelli, G.; Borrazzo, C.; Innocenti, G.P.; Frasca, F.; Cavallari, E.N.; Celani, L.; Nonne, C.; Mastroianni, C.M.; d’Ettorre, G. Sex-Related Differences in Markers of Immune Activation in Virologically Suppressed HIV-Infected Patients. Biol. Sex. Differ. 2020, 11, 23.
- Anastos, K.; Gange, S.J.; Lau, B.; Weiser, B.; Detels, R.; Giorgi, J.V.; Margolick, J.B.; Cohen, M.; Phair, J.; Melnick, S.; et al. Association of Race and Gender with HIV-1 RNA Levels and Immunologic Progression. J. Acquir. Immune Defic. Syndr. 2000, 24, 218–226.
- Portales, P.; Clot, J.; Corbeau, P. Sex Differences in HIV-1 Viral Load Due to Sex Difference in CCR5 Expression. Ann. Intern. Med. 2001, 134, 81–82.
- Prodger, J.L.; Gray, R.; Kigozi, G.; Nalugoda, F.; Galiwango, R.; Hirbod, T.; Wawer, M.; Hofer, S.O.P.; Sewankambo, N.; Serwadda, D.; et al. Foreskin T Cell Subsets Differ Substantially from Blood with Respect to HIV Co-Receptor Expression, Inflammatory Profile and Memory Status. Mucosal Immunol. 2012, 5, 121–128.
- Ballweber, L.; Robinson, B.; Kreger, A.; Fialkow, M.; Lentz, G.; McElrath, M.J.; Hladik, F. Vaginal Langerhans Cells Nonproductively Transporting HIV-1 Mediate Infection of T Cells. J. Virol. 2011, 85, 13443–13447.
- Rasheed, Z. Male Circumcision and Human Immunodeficiency Virus Infection: An Update on Randomized Controlled Trials and Molecular Evidences. Int. J. Health Sci. 2018, 12, 1–3.
- Prodger, J.L.; Kaul, R. The Biology of How Circumcision Reduces HIV Susceptibility: Broader Implications for the Prevention Field. AIDS Res. Ther. 2017, 14, 49.
- Weiss, H.A.; Dickson, K.E.; Agot, K.; Hankins, C.A. Male Circumcision for HIV Prevention: Current Research and Programmatic Issues. AIDS Lond. Engl. 2010, 24 (Suppl. 4), S61–S69.
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol. 2016, 16, 626–638.
- Moran, J.A.; Turner, S.R.; Marsden, M.D. Contribution of Sex Differences to HIV Immunology, Pathogenesis, and Cure Approaches. Front. Immunol. 2022, 13, 905773.
- Addo, M.M.; Altfeld, M. Sex-Based Differences in HIV Type 1 Pathogenesis. J. Infect. Dis. 2014, 209, S86–S92.
- Youness, A.; Miquel, C.-H.; Guéry, J.-C. Escape from X Chromosome Inactivation and the Female Predominance in Autoimmune Diseases. Int. J. Mol. Sci. 2021, 22, 1114.
- Young, N.A.; Wu, L.-C.; Burd, C.J.; Friedman, A.K.; Kaffenberger, B.H.; Rajaram, M.V.S.; Schlesinger, L.S.; James, H.; Shupnik, M.A.; Jarjour, W.N. Estrogen Modulation of Endosome-Associated Toll-like Receptor 8: An IFNα-Independent Mechanism of Sex-Bias in Systemic Lupus Erythematosus. Clin. Immunol. 2014, 151, 66–77.
- Justina, V.D.; Giachini, F.R.; Sullivan, J.C.; Webb, R.C. Toll-Like Receptors Contribute to Sex Differences in Blood Pressure Regulation. J. Cardiovasc. Pharmacol. 2020, 76, 255–266.
- Zandieh, Z.; Amjadi, F.; Ashrafi, M.; Aflatoonian, A.; Fazeli, A.; Aflatoonian, R. The Effect of Estradiol and Progesterone on Toll Like Receptor Gene Expression in A Human Fallopian Tube Epithelial Cell Line. Cell. J. Yakhteh 2016, 17, 678–691.
- Harding, A.T.; Heaton, N.S. The Impact of Estrogens and Their Receptors on Immunity and Inflammation during Infection. Cancers 2022, 14, 909.
- Alesci, A.; Nicosia, N.; Fumia, A.; Giorgianni, F.; Santini, A.; Cicero, N. Resveratrol and Immune Cells: A Link to Improve Human Health. Molecules 2022, 27, 424.
- Liao, Z.H.; Huang, T.; Xiao, J.W.; Gu, R.C.; Ouyang, J.; Wu, G.; Liao, H. Estrogen Signaling Effects on Muscle-Specific Immune Responses through Controlling the Recruitment and Function of Macrophages and T Cells. Skelet. Muscle 2019, 9, 20.
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295.
- Cai, Y.; Zhou, J.; Webb, D.C. Estrogen Stimulates Th2 Cytokine Production and Regulates the Compartmentalisation of Eosinophils during Allergen Challenge in a Mouse Model of Asthma. Int. Arch. Allergy Immunol. 2012, 158, 252–260.
- Joseph, K.; Tholanikunnel, B.G.; Kaplan, A.P. Cytokine and Estrogen Stimulation of Endothelial Cells Augments Activation of the Prekallikrein-High Molecular Weight Kininogen Complex: Implications for Hereditary Angioedema. J. Allergy Clin. Immunol. 2017, 140, 170–176.
- Shivers, K.-Y.; Amador, N.; Abrams, L.; Hunter, D.; Jenab, S.; Quiñones-Jenab, V. Estrogen Alters Baseline and Inflammatory-Induced Cytokine Levels Independent from Hypothalamic–Pituitary–Adrenal Axis Activity. Cytokine 2015, 72, 121–129.
- Cutolo, M.; Sulli, A.; Straub, R.H. Estrogen Metabolism and Autoimmunity. Autoimmun. Rev. 2012, 11, A460–A464.
- Javadian, A.; Salehi, E.; Bidad, K.; Sahraian, M.A.; Izad, M. Effect of Estrogen on Th1, Th2 and Th17 Cytokines Production by Proteolipid Protein and PHA Activated Peripheral Blood Mononuclear Cells Isolated from Multiple Sclerosis Patients. Arch. Med. Res. 2014, 45, 177–182.
- Gao, H.; Liu, L.; Zhao, Y.; Hara, H.; Chen, P.; Xu, J.; Tang, J.; Wei, L.; Li, Z.; Cooper, D.K.C.; et al. Human IL-6, IL-17, IL-1β, and TNF-α Differently Regulate the Expression of pro-Inflammatory Related Genes, Tissue Factor, and Swine Leukocyte Antigen Class I in Porcine Aortic Endothelial Cells. Xenotransplantation 2017, 24, e12291.
- Hagman, S.; Mäkinen, A.; Ylä-Outinen, L.; Huhtala, H.; Elovaara, I.; Narkilahti, S. Effects of Inflammatory Cytokines IFN-γ, TNF-α and IL-6 on the Viability and Functionality of Human Pluripotent Stem Cell-Derived Neural Cells. J. Neuroimmunol. 2019, 331, 36–45.
- Theofilopoulos, A.N.; Koundouris, S.; Kono, D.H.; Lawson, B.R. The Role of IFN-Gamma in Systemic Lupus Erythematosus: A Challenge to the Th1/Th2 Paradigm in Autoimmunity. Arthritis Res. Ther. 2001, 3, 136.
- Chaouali, M.; Azaiez, M.B.; Tezeghdenti, A.; Yacoubi-Oueslati, B.; Ghazouani, E.; Kochkar, R. High Levels of Proinflammatory Cytokines IL-6, IL-8, TNF-A, IL-23, and IFN-γ in Tunisian Patients with Type 1 Autoimmune Hepatitis. Eur. Cytokine Netw. 2020, 31, 94–103.
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen Receptors Alpha (ERα) and Beta (ERβ): Subtype-Selective Ligands and Clinical Potential. Steroids 2014, 0, 13–29.
- Atanaskova, N.; Keshamouni, V.G.; Krueger, J.S.; Schwartz, J.A.; Miller, F.; Reddy, K.B. MAP Kinase/Estrogen Receptor Cross-Talk Enhances Estrogen-Mediated Signaling and Tumor Growth but Does Not Confer Tamoxifen Resistance. Oncogene 2002, 21, 4000–4008.
- Liu, A.; Zhang, D.; Yang, X.; Song, Y. Estrogen Receptor Alpha Activates MAPK Signaling Pathway to Promote the Development of Endometrial Cancer. J. Cell. Biochem. 2019, 120, 17593–17601.
- Filardo, E.J.; Quinn, J.A.; Frackelton, A.R.; Bland, K.I. Estrogen Action via the G Protein-Coupled Receptor, GPR30: Stimulation of Adenylyl Cyclase and CAMP-Mediated Attenuation of the Epidermal Growth Factor Receptor-to-MAPK Signaling Axis. Mol. Endocrinol. 2002, 16, 70–84.
- Petrova, T.; Pesic, J.; Pardali, K.; Gaestel, M.; Arthur, J.S.C. P38 MAPK Signalling Regulates Cytokine Production in IL-33 Stimulated Type 2 Innate Lymphoid Cells. Sci. Rep. 2020, 10, 3479.
- Morrison, D.K. MAP Kinase Pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254.




