
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | A. H. Janaka Sampath | -- | 1892 | 2023-05-17 10:01:13 | | | |
| 2 | Wendy Huang | Meta information modification | 1892 | 2023-05-17 12:14:53 | | |
Video Upload Options
Among a diverse range of dense mineral reserves found across the world, only ilmenite and rutile ores are capable of yielding titanium compounds, specifically titanium dioxide, through industrial processes. Although ilmenite and rutile are extensively used to extract TiO2 at the industrial level, through the sulphate and chloride processes, they can also be recognized to possess the potential to be employed as the raw material to synthesize other titanium compounds as well. Since titanium containing compounds possess the capability to be applied in numerous applications, such as environmental remediation, energy technologies, the pharmaceutical industry, paint industry and textile industry, exploration of the ability of these ore materials to yield titanium species is highly significant in the field of research as well as the industrial sector.
1. Introduction
2. Sulphate Process
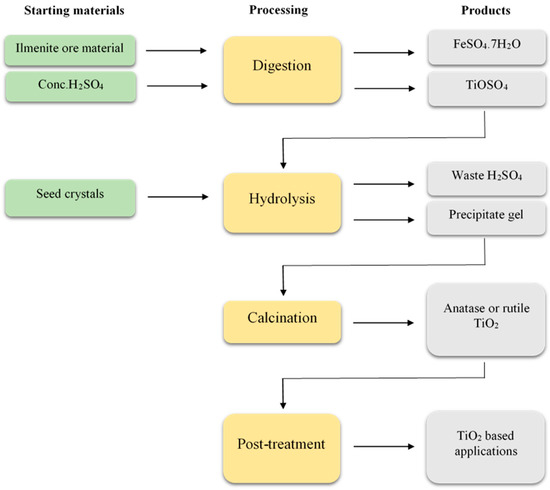
- 1.
-
Acid digestion
- 2.
-
Hydrolysis
- 3.
-
Calcination
- 4.
-
Post-treatment
2.1. Advantages of the Sulphate Process
2.2. Disadvantages of the Sulphate Process
3. Chloride Process

- 5.
-
Chlorination
- 6.
-
Purification (condensation)
- 7.
-
Oxidation
- 8.
-
Post-treatment
3.1. Advantages of the Chloride Process
3.2. Disadvantages of the Chloride Process
References
- Armakovic, S.J.; Savanovic, M.M.; Armakovic, S. Titanium dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26, https://doi.org/10.3390/catal13010026..
- Landman, M.; Rauls, E.; Schmidt, W.G. The electronic structure and optical response of rutile, anatase and brookite TiO2. J. Phys. Condens. Matter 2012, 24, 195503, https://doi.org/10.1088/0953-8984/24/19/195503.
- Lan, Y.; Lu, Y.; Ren, Z. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2013, 2, 1031–1045, https://doi.org/10.1016/j.nanoen.2013.04.002.
- Choi, H.; Al-Abed, S.R.; Dionysiou, D.D.; Stathatos, E.; Lianos, P. . TiO2-Based Advanced Oxidation Nanotechnologies for Water Purification and Reuse; Choi, H.; Al-Abed, S.R.; Dionysiou, D.D.; Stathatos, E.; Lianos, P. , Eds.; Elsvier Science BV: Amsterdam, The Netherland, 2010; pp. 229–254.
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Role of Titanium Dioxide (TiO2) Structural Design/Morphology in Photocatalytic Air Purification. J. Solid State Chem. 2003, 174, 104-110, https://doi.org/10.1016/j.apcatb.2020.118735.
- Shang, J.; Chai, M.; Zhu, Y. Solid-Phase Photocatalytic Degradation of Polystyrene Plastic with TiO2 as Photocatalyst. J. Solid State Chem. 2003, 174, 104–110, https://doi.org/10.1016/S0022-4596(03)00183-X.
- Zhai, Y.; Hunting, E.R.; Liu, G.; Baas, E.; Peijnenburg, W.J.G.M.; Vijver, M.G. Compositional Alterations in Soil Bacterial Communities Exposed to TiO2 Nanoparticles Are Not Reflected in Functional Impacts. Environ. Res. 2019, 178, 108713, https://doi.org/10.1016/j.envres.2019.108713.
- Singh, R.S.; Rangari, V.K.; Sanagapalli, S.; Jayaraman, V.; Mahendra, S.; Singh, V.P. Nano-Structured CdTe, CdS and TiO2 for Thin Film Solar Cell Applications. Sol. Energy Mater. Sol. Cells 2004, 82, 315–330, https://doi.org/10.1016/j.solmat.2004.02.006.
- Ismael, M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nanoparticles. Sol. Energy 2020, 211, 522–546, https://doi.org/10.1016/j.solener.2020.09.073.
- Carlucci, C.; Degennaro, L.; Luisi, R. Titanium Dioxide as a Catalyst in Biodiesel Production. Catalysts 2019, 9, 75, https://doi.org/10.3390/catal9010075.
- Siddle, G.R. The Prospects for Titanium Dioxide in the Paint Industry. Pigm. Resin Technol. 1975, 4, 4–12, https://doi.org/10.1108/eb041106.
- Li, G.; Liu, H.; Zhao, H.; Gao, Y.; Wang, J.; Jiang, H.; Boughton, R.I. Chemical Assembly of TiO2 and TiO2/Ag Nanoparticles on Silk Fiber to Produce Multifunctional Fabrics.. J. Colloid Interface Sci. 2011, 358, 307–315, https://doi.org/10.1016/j.jcis.2011.02.053.
- Sriwong, C.; Wongnawa, S.; Patarapaiboolchai, O. Rubber Sheet Strewn with TiO2 Particles: Photocatalytic Activity and Recyclability. J. Environ. Sci. 2012, 24, 464–472, https://doi.org/10.1016/S1001-0742(11)60794-8.
- Morsella, M.; d’Alessandro, N.; Lanterna, A.E.; Scaiano, J.C. Improving the Sunscreen Properties of TiO2 through an Understanding of Its Catalytic Properties. ACS Omega 2016, 1, 464–469, https://doi.org/10.1021/acsomega.6b00177.
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Antimicrobial Effects of TiO2 and Ag2O Nanoparticles against Drug-Resistant Bacteria Andleishmaniaparasites. Future Microbiol. 2011, 6, 933–940, https://doi.org/10.2217/fmb.11.78.
- Zhu, X.; Zheng, S.; Zhang, Y.; Fang, Z.Z.; Zhang, M.; Sun, P.; Li, Q.; Zhang, Y.; Li, P.; Jin, W.; et al. Potentially More Ecofriendly Chemical Pathway for Production of High-Purity TiO2 from Titanium Slag. ACS Sustain. Chem. Eng. 2019, 7, 4821–4830, https://doi.org/10.1021/acssuschemeng.8b05102.
- Ramos-Delgado, N.A.; Gracia-Pinilla, M.Á.; Mangalaraja, R.V.; O’Shea, K.; Dionysiou, D.D. Industrial Synthesis and Characterization of Nanophotocatalysts Materials: Titania. Nanotechnol. Rev. 2016, 5, 467–479, https://doi.org/10.1515/ntrev-2016-0007.
- Abdelfattah, N.A. Preparation of Titanium Dioxide Anatase Pigment from Rosetta Ilmenite Concentrate via the Sulfate Route. In Proceedings of the 4th International Conference on Radiation Sciences and Applications , Vienna, Austria, 8–11 June 2015 ; Volume 10, pp. 193–199.
- Sahu, K.K.; Alex, T.C.; Mishra, D.; Agrawal, A. An Overview on the Production of Pigment Grade Titania from Titania-Rich Slag. Waste Manag. Res. 2006, 24, 74–79, https://doi.org/10.1177/0734242 × 06061016.
- Tang, S.; Zhang, Y.; Yuan, S.; Yue, H.; Liu, C.; Li, C.; Liang, B. Microwave-Assisted Seed Preparation for Producing Easily Phase-Transformed Anatase to Rutile. RSC Adv. 2017, 7, 45607–45614, https://doi.org/10.1039/C7RA07385B.
- Thasirisap, E.; Vittayakorn, N.; Seeharaj, P. Surface modification of TiO2 particles with the sono-assisted exfoliation method. Ultrason. Sonochem. 2017, 39, 733–740, https://doi.org/10.1016/j.ultsonch.2017.06.002.
- Goncalves, G.; Marques, P.A.A.P.; Pinto, R.J.B.; Trindade, T.; Neto, C.P. Surface modification of cellulosic fibres for multi-purpose TiO2 based nanocomposites. Compos. Sci. Technol. 2009, 69, 1051–1056, https://doi.org/10.1016/j.compsitech.2009.01.020.
- Al-Sarray, E.; Jabbar, A. Investigate the Ability of the Eggshell to Attenuate the Gamma and Beta Rays as Compared with Composite FeSO4.7H2O. Nucl. Sci. 2018, 3, 16–22, https://doi.org/10.11648/j.ns.20180301.13.
- Zhu, G.; Zheng, H.; Chen, W.; Fan, W.; Zhang, P.; Tshukudu, T. Preparation of a Composite Coagulant: Polymeric Aluminum Ferric Sulfate (PAFS) for Wastewater Treatment. Desalination 2012, 285, 315–323, https://doi.org/10.1016/j.desal.2011.10.019.
- Yang, F.; Hlavacek, V. Effective Extraction of Titanium from Rutile by a Low-Temperature Chloride Process. AIChE J. 2000, 46, 355–360, https://doi.org/10.1002/aic.690460213.
- Zhang, W.; Zhu, Z.; Cheng, C.Y. A literature review of titanium metallurgical processes. Hydrometallurgy 2011, 108, 177–188, https://doi.org/10.1016/j.hydromet.2011.04.005.
- Lynch, D.C. Conversion of VOCl3 to VOCl2 in Liquid TiCl4. Metall. Mater. Trans. B 2002, 33, 142–146, https://doi.org/10.1007/S11663-002-0096-0.




