Among a diverse range of dense mineral reserves found across the world, only ilmenite and rutile ores are capable of yielding titanium compounds, specifically titanium dioxide, through industrial processes. Although ilmenite and rutile are extensively used to extract TiO2 at the industrial level, through the sulphate and chloride processes, they can also be recognized to possess the potential to be employed as the raw material to synthesize other titanium compounds as well. Since titanium containing compounds possess the capability to be applied in numerous applications, such as environmental remediation, energy technologies, the pharmaceutical industry, paint industry and textile industry, exploration of the ability of these ore materials to yield titanium species is highly significant in the field of research as well as the industrial sector.
- rutile
- ilmenite
- TiO2
- sulphate
- chloride
1. Introduction
2. Sulphate Process
Statistical data reveal that 98% of manufacturers in China use the sulphate process for the production of TiO2 .[16]. The process can be categorized into four main stages, namely, acid digestion, hydrolysis, calcination and post-treatment, which are illustrated in Figure 1. In short, the initial step of the process involves converting the raw material, ilmenite, into titanium-sulphate through acid digestion. The resulting compound is then hydrolysed to yield hydrous titania, TiO2·H2O. Finally, the hydrated form is subjected to calcination to form TiO2 .[17][18][19].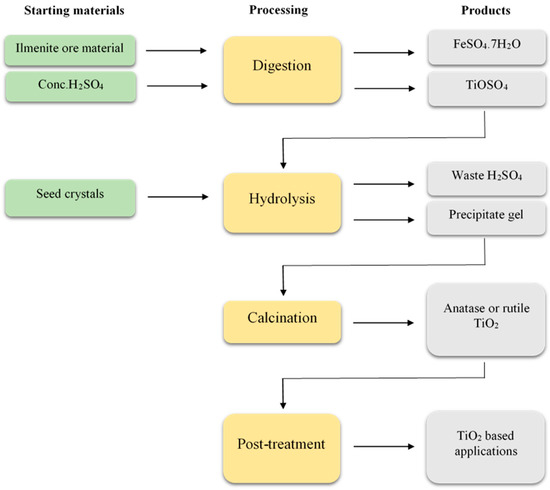
- 1.
-
Acid digestionTo ensure a 94% efficiency in this step, a 60% excess of 96% concentrated sulphuric acid (H2SO4) relative to the TiO2 content in the raw material is required. The excess acid is added to purified ilmenite, which is then digested at 150 °C .[17]. The reaction occurring during the digestion of ilmenite, i.e., FeTiO3, is revealed below.2H2SO4 (aq) + FeTiO3 (s) → TiOSO4 (aq) + 2H2O (l) + FeSO4 (aq)Due to the high temperature used in the digestion step, the ferrous content in ilmenite, which is considered to be an impurity for the final product, undergoes oxidation into the ferric form. Hence, by incorporating scrap iron (Fe) into the mixture, it is possible to convert all of the Fe3+ ions into Fe2+ that can be conveniently separated as crystalline iron(II) sulphate heptahydrate (FeSO4·7H2O) at a relatively low temperature of approximately 15 °C .[17].Fe3+ (aq) + Fe (s) → 2 Fe2+ (aq)
- 2.
-
HydrolysisHydrolysis of the titanium salt (TiOSO4) occurs upon heating the digestion yield at 109 °C, resulting in the formation of a gelatinous precipitate and a significant amount of residual sulphuric acid. To eliminate any residual acid, the gelatinous precipitate is subjected to multiple washes during the purification process. Adding different types of seeds during the process allows the formation of the final product (TiO2) in different phases, i.e., anatase or rutile crystalline phases .[17][20].TiOSO4 (aq) + (n + 1) H2O (l) → H2SO4 (aq) + TiO2·nH2O (s)
- 3.
-
CalcinationThe hydrolysis product (TiO2·nH2O, hydrous titania) is subjected to calcination in rotary kilns, where the reaction temperature plays a crucial role in determining the final outcome. At temperatures around 200–300 °C the hydrolysed material undergoes dehydration, followed by the formation of crystalline TiO2 at approximately 480 °C. Moreover, heating up to 750–850 °C results in the production of anatase TiO2, while the rutile phase is yielded at temperatures ranging from 900–930 °C .[17].TiO2·nH2O (s) → nH2O (l) + TiO2 (s)It is important to avoid raising the temperature unnecessarily in order to preserve the appropriate structural and chemical propertied of TiO2 that are advantageous for industrial applications.
- 4.
-
Post-treatmentThe calcined product (either anatase or rutile TiO2), is subjected to suitable surface modifications in preparation for further applications .[21][22].
2.1. Advantages of the Sulphate Process
The main advantage of the sulphate process is the feasibility of isolating TiO2 either in the anatase or rutile crystalline phase, depending on the calcination temperature. The capital investment required for the sulphate process is comparatively lower than that of the chloride process. In the same way, since the energy demand and starting materials are cheaper, operating costs are also lower for the sulphate process compared to the chloride process. In terms of waste generation, the remaining portion of dilute sulphuric acid can be reconcentrated and reused for the ilmenite digestion, enhancing the productivity of the sulphate process. Additionally, waste sulphuric acid can be reacted with limestone (CaCO3), as shown below, in order to produce gypsum (CaSO4·2H2O) .[16].H2SO4 (aq) + CaCO3 (s) → CaSO4·2H2O (s) + CO2 (g)Moreover, the by-product, FeSO4·7H2O, can be used either in the exact form or in an altered form for any other industries. For instance, FeSO4·7H2O can be oxidized into the ferric form and can then be used for water purification .[23][24].2.2. Disadvantages of the Sulphate Process
The main drawback is the environmental damage caused by the waste of the sulphate process. For instance, highly concentrated (98%) sulphuric acid is used to collapse the ore material. Furthermore, the use of water in the hydrolysis step consequently generates a huge amount diluted sulphuric acid with a lower concentration of approximately 20%. According to the reports, the production of one ton of titania using this process generates approximately 25,000 m3 of exhaust gas, 200 tons of acidic water and 7–11 tons of spent acid .[16]. Therefore, environmental pollution control becomes more expensive in the sulphate process. Furthermore, being a batch process, the sulphate process can be utilized to extract only small amounts of TiO2, where the reactants are allowed to react in the reaction chamber and once the reaction is completed the products are removed and proceeded to the next step .[17].3. Chloride Process
Based on statistical data from the industry, the chloride process accounts for 60% of worldwide TiO2 production .[16]. Figure 2 provides a depiction of the major steps involved in the chloride process, which is a continuous process and utilizes higher-grade rutile as the starting material to produce TiO2. These stages include chlorination, purification (condensation), oxidation and post-treatment. The basic steps of the process involve reacting the ore material with chlorine gas to form TiCl4 in the gaseous state, followed by a reaction with oxygen to produce TiO2 .[17][19][25]. Because of the high sensitivity of TiCl4 vapour to moisture, it is crucial to maintain an extremely dry environment in the reaction chamber. Figure 2.Illustration showing the key stages of the chloride process.
Figure 2.Illustration showing the key stages of the chloride process.- 5.
-
ChlorinationDuring this step, the dry titanium ore material is heated to a temperature of 950 °C while providing Cl2 vapour to the reaction chamber, where a coke-assisted exothermic reaction is driven to yield TiCl4 vapour along with CO, CO2 and other impurity chlorides .[17].2Cl2 (g) + TiO2 (s) + C (s) → CO2 (g) + TiCl4 (g)
- 6.
-
Purification (condensation)With the purpose of removing volatile impurity chlorides with higher molecular masses from the reaction mixture, the gaseous mixture generated during chlorination is cooled, allowing for their efficient condensation .[25]. Moreover, it should be noted that vanadium chloride, because it has a similar boiling point to titanium tetrachloride, cannot be eliminated through condensation like other impurity chlorides .[26][27].
- 7.
-
OxidationWhen purified TiCl4 is burned with molecular oxygen, it produces only the rutile phase of TiO2, and the anatase phase does not form. In order to obtain the highest possible yield in this step, the oxidation reaction should be carried out at a temperature around 1000 °C .[17][25].O2 (g) + TiCl4 (g) → 2Cl2 (g) + TiO2 (s)
- 8.
-
Post-treatmentAfter obtaining pure rutile TiO2, it can be subjected to proper surface modifications using appropriate functionalization techniques to make it suitable for particular applications .[21][22].
3.1. Advantages of the Chloride Process
Titanium dioxide can be obtained through the chloride process with a higher purity only in the rutile phase without anatase contaminants. Moreover, when compared to the sulphate process, the waste generation is also lower in the chloride process, where the by-product, chlorine gas, can be reused to feed the chlorinator at the beginning of the process .[17]. Being a continuous process, the chloride process is endowed with a higher production capability over the sulphate process.3.2. Disadvantages of the Chloride Process
Since the chloride process is able to produce only the rutile form of TiO2, the industries which require the pure anatase form will not be benefited from this extraction process. Furthermore, the raw material of this process should be higher grade natural or synthetic rutile, with low contents of MgO and CaO, which is more expensive and rarely available compared to ilmenite .[16]. Thus, the capital investment required to establish a chloride process plant is 1.7 times higher than that of a sulphate process plant .[19]. Since extremely dry conditions and very high temperatures are essential requirements, the operating cost is also greater for the chloride process. Because of the use of very high temperatures, around 1000 °C, impurity chlorides, such as MnCl2 (melting point: 650 °C), CaCl2 (melting point: 782 °C) and MgCl2 (melting point: 714 °C), can accumulate in the chlorinator in their liquid state, eventually retarding the process .[25]. Although the waste generation is relatively lower compared to the sulphate process, a number of environmental challenges are associated with the chloride process as well. Most significantly, TiCl4 is highly corrosive and its leakage should be necessarily avoided. Moreover, in accordance with the chlorination reaction, 550 kg of CO2 is produced along with the production of one ton of TiO2. It has been further estimated that around 2 million tons of CO2 are generated annually through this chlorination reaction alone regarding the chloride process .[16].
References
- Armakovic, S.J.; Savanovic, M.M.; Armakovic, S. Titanium dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26, https://doi.org/10.3390/catal13010026..
- Landman, M.; Rauls, E.; Schmidt, W.G. The electronic structure and optical response of rutile, anatase and brookite TiO2. J. Phys. Condens. Matter 2012, 24, 195503, https://doi.org/10.1088/0953-8984/24/19/195503.
- Lan, Y.; Lu, Y.; Ren, Z. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2013, 2, 1031–1045, https://doi.org/10.1016/j.nanoen.2013.04.002.
- Choi, H.; Al-Abed, S.R.; Dionysiou, D.D.; Stathatos, E.; Lianos, P. . TiO2-Based Advanced Oxidation Nanotechnologies for Water Purification and Reuse; Choi, H.; Al-Abed, S.R.; Dionysiou, D.D.; Stathatos, E.; Lianos, P. , Eds.; Elsvier Science BV: Amsterdam, The Netherland, 2010; pp. 229–254.
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Role of Titanium Dioxide (TiO2) Structural Design/Morphology in Photocatalytic Air Purification. J. Solid State Chem. 2003, 174, 104-110, https://doi.org/10.1016/j.apcatb.2020.118735.
- Shang, J.; Chai, M.; Zhu, Y. Solid-Phase Photocatalytic Degradation of Polystyrene Plastic with TiO2 as Photocatalyst. J. Solid State Chem. 2003, 174, 104–110, https://doi.org/10.1016/S0022-4596(03)00183-X.
- Zhai, Y.; Hunting, E.R.; Liu, G.; Baas, E.; Peijnenburg, W.J.G.M.; Vijver, M.G. Compositional Alterations in Soil Bacterial Communities Exposed to TiO2 Nanoparticles Are Not Reflected in Functional Impacts. Environ. Res. 2019, 178, 108713, https://doi.org/10.1016/j.envres.2019.108713.
- Singh, R.S.; Rangari, V.K.; Sanagapalli, S.; Jayaraman, V.; Mahendra, S.; Singh, V.P. Nano-Structured CdTe, CdS and TiO2 for Thin Film Solar Cell Applications. Sol. Energy Mater. Sol. Cells 2004, 82, 315–330, https://doi.org/10.1016/j.solmat.2004.02.006.
- Ismael, M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nanoparticles. Sol. Energy 2020, 211, 522–546, https://doi.org/10.1016/j.solener.2020.09.073.
- Carlucci, C.; Degennaro, L.; Luisi, R. Titanium Dioxide as a Catalyst in Biodiesel Production. Catalysts 2019, 9, 75, https://doi.org/10.3390/catal9010075.
- Siddle, G.R. The Prospects for Titanium Dioxide in the Paint Industry. Pigm. Resin Technol. 1975, 4, 4–12, https://doi.org/10.1108/eb041106.
- Li, G.; Liu, H.; Zhao, H.; Gao, Y.; Wang, J.; Jiang, H.; Boughton, R.I. Chemical Assembly of TiO2 and TiO2/Ag Nanoparticles on Silk Fiber to Produce Multifunctional Fabrics.. J. Colloid Interface Sci. 2011, 358, 307–315, https://doi.org/10.1016/j.jcis.2011.02.053.
- Sriwong, C.; Wongnawa, S.; Patarapaiboolchai, O. Rubber Sheet Strewn with TiO2 Particles: Photocatalytic Activity and Recyclability. J. Environ. Sci. 2012, 24, 464–472, https://doi.org/10.1016/S1001-0742(11)60794-8.
- Morsella, M.; d’Alessandro, N.; Lanterna, A.E.; Scaiano, J.C. Improving the Sunscreen Properties of TiO2 through an Understanding of Its Catalytic Properties. ACS Omega 2016, 1, 464–469, https://doi.org/10.1021/acsomega.6b00177.
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Antimicrobial Effects of TiO2 and Ag2O Nanoparticles against Drug-Resistant Bacteria Andleishmaniaparasites. Future Microbiol. 2011, 6, 933–940, https://doi.org/10.2217/fmb.11.78.
- Zhu, X.; Zheng, S.; Zhang, Y.; Fang, Z.Z.; Zhang, M.; Sun, P.; Li, Q.; Zhang, Y.; Li, P.; Jin, W.; et al. Potentially More Ecofriendly Chemical Pathway for Production of High-Purity TiO2 from Titanium Slag. ACS Sustain. Chem. Eng. 2019, 7, 4821–4830, https://doi.org/10.1021/acssuschemeng.8b05102.
- Ramos-Delgado, N.A.; Gracia-Pinilla, M.Á.; Mangalaraja, R.V.; O’Shea, K.; Dionysiou, D.D. Industrial Synthesis and Characterization of Nanophotocatalysts Materials: Titania. Nanotechnol. Rev. 2016, 5, 467–479, https://doi.org/10.1515/ntrev-2016-0007.
- Abdelfattah, N.A. Preparation of Titanium Dioxide Anatase Pigment from Rosetta Ilmenite Concentrate via the Sulfate Route. In Proceedings of the 4th International Conference on Radiation Sciences and Applications , Vienna, Austria, 8–11 June 2015 ; Volume 10, pp. 193–199.
- Sahu, K.K.; Alex, T.C.; Mishra, D.; Agrawal, A. An Overview on the Production of Pigment Grade Titania from Titania-Rich Slag. Waste Manag. Res. 2006, 24, 74–79, https://doi.org/10.1177/0734242 × 06061016.
- Tang, S.; Zhang, Y.; Yuan, S.; Yue, H.; Liu, C.; Li, C.; Liang, B. Microwave-Assisted Seed Preparation for Producing Easily Phase-Transformed Anatase to Rutile. RSC Adv. 2017, 7, 45607–45614, https://doi.org/10.1039/C7RA07385B.
- Thasirisap, E.; Vittayakorn, N.; Seeharaj, P. Surface modification of TiO2 particles with the sono-assisted exfoliation method. Ultrason. Sonochem. 2017, 39, 733–740, https://doi.org/10.1016/j.ultsonch.2017.06.002.
- Goncalves, G.; Marques, P.A.A.P.; Pinto, R.J.B.; Trindade, T.; Neto, C.P. Surface modification of cellulosic fibres for multi-purpose TiO2 based nanocomposites. Compos. Sci. Technol. 2009, 69, 1051–1056, https://doi.org/10.1016/j.compsitech.2009.01.020.
- Al-Sarray, E.; Jabbar, A. Investigate the Ability of the Eggshell to Attenuate the Gamma and Beta Rays as Compared with Composite FeSO4.7H2O. Nucl. Sci. 2018, 3, 16–22, https://doi.org/10.11648/j.ns.20180301.13.
- Zhu, G.; Zheng, H.; Chen, W.; Fan, W.; Zhang, P.; Tshukudu, T. Preparation of a Composite Coagulant: Polymeric Aluminum Ferric Sulfate (PAFS) for Wastewater Treatment. Desalination 2012, 285, 315–323, https://doi.org/10.1016/j.desal.2011.10.019.
- Yang, F.; Hlavacek, V. Effective Extraction of Titanium from Rutile by a Low-Temperature Chloride Process. AIChE J. 2000, 46, 355–360, https://doi.org/10.1002/aic.690460213.
- Zhang, W.; Zhu, Z.; Cheng, C.Y. A literature review of titanium metallurgical processes. Hydrometallurgy 2011, 108, 177–188, https://doi.org/10.1016/j.hydromet.2011.04.005.
- Lynch, D.C. Conversion of VOCl3 to VOCl2 in Liquid TiCl4. Metall. Mater. Trans. B 2002, 33, 142–146, https://doi.org/10.1007/S11663-002-0096-0.
