
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jiangxia Zheng | -- | 2493 | 2023-05-16 03:36:29 | | | |
| 2 | Jessie Wu | Meta information modification | 2493 | 2023-05-16 05:25:16 | | |
Video Upload Options
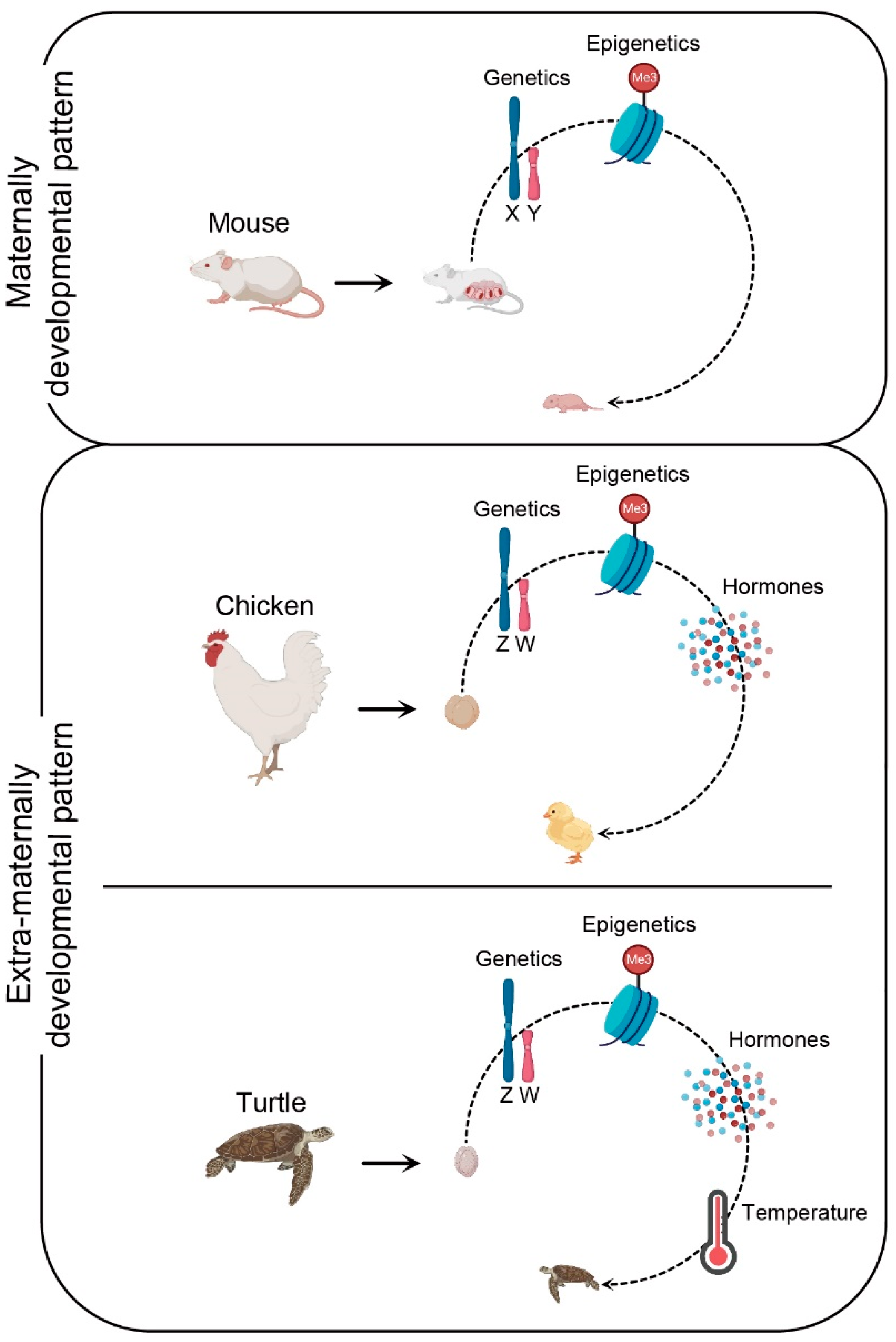
Sex determination and differentiation are processes by which a bipotential gonad adopts either a testicular or ovarian cell fate, and secondary sexual characteristics adopt either male or female developmental patterns. In birds, although genetic factors control the sex determination program, sex differentiation is sensitive to hormones, which can induce sex reversal when disturbed. Although these sex-reversed birds can form phenotypes opposite to their genotypes, none can experience complete sex reversal or produce offspring under natural conditions. Promising evidence indicates that the incomplete sex reversal is associated with cell autonomous sex identity (CASI) of avian cells, which is controlled by genetic factors.
1. Introduction
2. Occurrence of Vertebrate Sex Reversal
3. Mechanism of Avian Sex Reversal
4. Avian Sex Reversal Induced by Transplant Treatment
5. Avian Male-to-Female Sex Reversal Induced by Estrogens Treatment
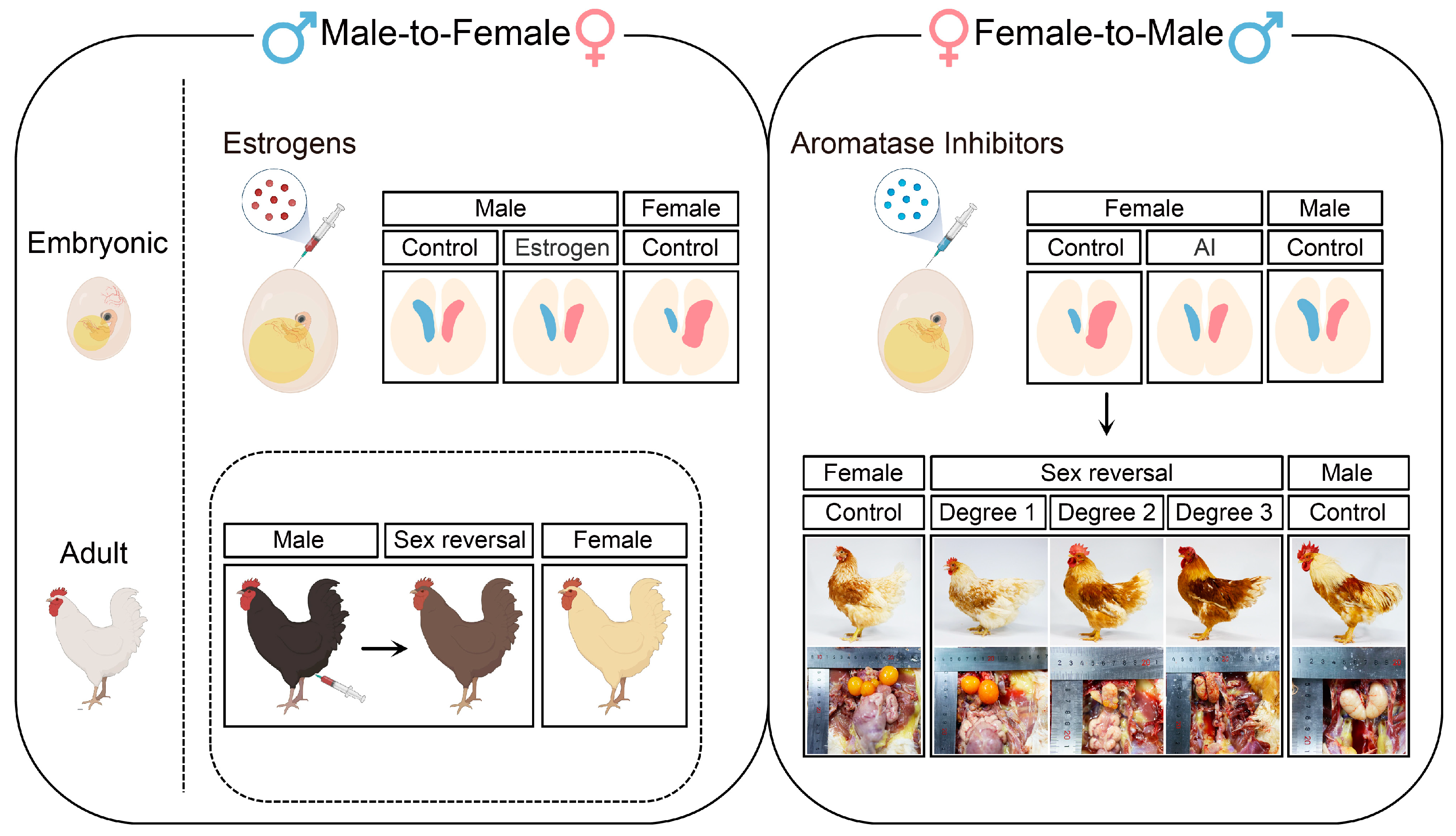
6. Avian Female-to-Male Sex Reversal Induced by Aromatase Inhibitors Treatment
7. Avian Sex Reversal and Cell Autonomous Sex Identity
References
- Tizard, M.L.; Jenkins, K.A.; Cooper, C.A.; Woodcock, M.E.; Challagulla, A.; Doran, T.J. Potential benefits of gene editing for the future of poultry farming. Transgenic Res. 2019, 28, 87–92.
- Zhu, R.; Fogelholm, M.; Jalo, E.; Poppitt, S.D.; Silvestre, M.P.; Møller, G.; Huttunen-Lenz, M.; Stratton, G.; Sundvall, J.; Macdonald, I.A.; et al. Animal-based food choice and associations with long-term weight maintenance and metabolic health after a large and rapid weight loss: The PREVIEW study. Clin. Nutr. 2022, 41, 817–828.
- Doran, T.J.; Morris, K.R.; Wise, T.G.; O’Neil, T.E.; Cooper, C.A.; Jenkins, K.A.; Tizard, M.L.V. Sex selection in layer chickens. Anim. Prod. Sci. 2018, 58, 476.
- Doran, T.J.; Cooper, C.A.; Jenkins, K.A.; Tizard, M.L. Advances in genetic engineering of the avian genome: “Realising the promise”. Transgenic Res. 2016, 25, 307–319.
- Sinclair, M.; Zhang, Y.; Descovich, K.; Phillips, C.J.C. Farm Animal Welfare Science in China-A Bibliometric Review of Chinese Literature. Animals 2020, 10, 540.
- McColl, K.A.; Clarke, B.; Doran, T.J. Role of genetically engineered animals in future food production. Aust. Vet. J. 2013, 91, 113–117.
- Cooper, C.A.; Doran, T.J.; Challagulla, A.; Tizard, M.L.V.; Jenkins, K.A. Innovative approaches to genome editing in avian species. J. Anim. Sci. Biotechnol. 2018, 9, 15.
- Hirst, C.E.; Serralbo, O.; Ayers, K.L.; Roeszler, K.N.; Smith, C.A. Genetic Manipulation of the Avian Urogenital System Using In Ovo Electroporation. Methods Mol. Biol. 2017, 1650, 177–190.
- Yoshino, T.; Murai, H.; Saito, D. Hedgehog-BMP signalling establishes dorsoventral patterning in lateral plate mesoderm to trigger gonadogenesis in chicken embryos. Nat. Commun. 2016, 7, 12561.
- Sekido, R.; Lovell-Badge, R. Mechanisms of gonadal morphogenesis are not conserved between chick and mouse. Dev. Biol. 2007, 302, 132–142.
- Guioli, S.; Zhao, D.; Nandi, S.; Clinton, M.; Lovell-Badge, R. Oestrogen in the chick embryo can induce chromosomally male ZZ left gonad epithelial cells to form an ovarian cortex that can support oogenesis. Development 2020, 147, dev181693.
- Chue, J.; Smith, C.A. Sex determination and sexual differentiation in the avian model. FEBS J. 2011, 278, 1027–1034.
- Smith, C.A.; Roeszler, K.N.; Ohnesorg, T.; Cummins, D.M.; Farlie, P.G.; Doran, T.J.; Sinclair, A.H. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 2009, 461, 267–271.
- Ioannidis, J.; Taylor, G.; Zhao, D.; Liu, L.; Idoko-Akoh, A.; Gong, D.; Lovell-Badge, R.; Guioli, S.; McGrew, M.J.; Clinton, M. Primary sex determination in birds depends on DMRT1 dosage, but gonadal sex does not determine adult secondary sex characteristics. Proc. Natl. Acad. Sci. USA 2021, 118, e2020909118.
- Major, A.T.; Ayers, K.; Chue, J.; Roeszler, K.; Smith, C. FOXL2 antagonises the male developmental pathway in embryonic chicken gonads. J. Endocrinol. 2019, 243, 211–228.
- Scheib, D. Effects and role of estrogens in avian gonadal differentiation. Differentiation 1983, 23, S87–S92.
- Elbrecht, A.; Smith, R.G. Aromatase enzyme activity and sex determination in chickens. Science 1992, 255, 467–470.
- George, F.W.; Wilson, J.D. pathogenesis of the henny feathering trait in the Sebright bantam chicken. Increased conversion of androgen to estrogen in skin. J. Clin. Investig. 1980, 66, 57–65.
- Warren, W.C.; Hillier, L.W.; Tomlinson, C.; Minx, P.; Kremitzki, M.; Graves, T.; Markovic, C.; Bouk, N.; Pruitt, K.D.; Thibaud-Nissen, F.; et al. A New Chicken Genome Assembly Provides Insight into Avian Genome Structure. G3 Genes Genomes Genet. 2017, 7, 109–117.
- Vaillant, S.; Dorizzi, M.; Pieau, C.; Richard-Mercier, N. Sex reversal and aromatase in chicken. J. Exp. Zool. 2001, 290, 727–740.
- Zhao, D.; McBride, D.; Nandi, S.; McQueen, H.A.; McGrew, M.J.; Hocking, P.M.; Lewis, P.D.; Sang, H.M.; Clinton, M. Somatic sex identity is cell autonomous in the chicken. Nature 2010, 464, 237–242.
- Godwin, J. Social determination of sex in reef fishes. Semin. Cell. Dev. Biol. 2009, 20, 264–270.
- Lamm, M.S.; Liu, H.; Gemmell, N.J.; Godwin, J.R. The Need for Speed: Neuroendocrine Regulation of Socially-controlled Sex Change. Integr. Comp. Biol. 2015, 55, 307–322.
- Liu, H.; Todd, E.V.; Lokman, P.M.; Lamm, M.S.; Godwin, J.R.; Gemmell, N.J. Sexual plasticity: A fishy tale. Mol. Reprod. Dev. 2017, 84, 171–194.
- Warner, R. Mating Behavior and Hermaphroditism in Coral Reef Fishes. Am. Sci. 1970, 72, 128–136.
- Warner, R.R.; Swearer, S.E. Social Control of Sex Change in the Bluehead Wrasse, Thalassoma bifasciatum (Pisces: Labridae). Biol. Bull. 1991, 181, 199–204.
- Major, A.T.; Smith, C.A. Sex Reversal in Birds. Sex. Dev. 2016, 10, 288–300.
- Matsumine, H.; Herbst, M.A.; Ou, S.H.; Wilson, J.D.; McPhaul, M.J. Aromatase mRNA in the extragonadal tissues of chickens with the henny-feathering trait is derived from a distinctive promoter structure that contains a segment of a retroviral long terminal repeat. Functional organization of the Sebright, Leghorn, and Campine aromatase genes. J. Biol. Chem. 1991, 266, 19900–19907.
- Balthazart, J.; Cornil, C.A.; Charlier, T.D.; Taziaux, M.; Ball, G.F. Estradiol, a key endocrine signal in the sexual differentiation and activation of reproductive behavior in quail. J. Exp. Zool. A Ecol. Genet. Physiol. 2009, 311, 323–345.
- Smith, C.A.; Sinclair, A.H. Sex determination: Insights from the chicken. Bioessays 2004, 26, 120–132.
- Pieau, C. Temperature variation and sex determination in reptiles. BioEssays 1996, 18, 19–26.
- Jost, A. Hormonal factors in the sex differentiation of the mammalian foetus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1970, 259, 119–130.
- Hu, M.C.; Hsu, N.C.; El Hadj, N.B.; Pai, C.I.; Chu, H.P.; Wang, C.K.; Chung, B.C. Steroid deficiency syndromes in mice with targeted disruption of Cyp11a1. Mol. Endocrinol. 2002, 16, 1943–1950.
- Wolf, U. Reorganization of the sex-determining pathway with the evolution of placentation. Hum. Genet. 1999, 105, 288–292.
- Maraud, R.; Rashedi, M.; Stoll, R. Influence of a temporary embryonic testis graft on the regression of Müllerian ducts in female chick embryo. Development 1982, 67, 81–87.
- Maraud, R.; Vergnaud, O.; Rashedi, M. New insights on the mechanism of testis differentiation from the morphogenesis of experimentally induced testes in genetically female chick embryos. Am. J. Anat. 1990, 188, 429–437.
- Halldin, K.; Berg, C.; Brandt, I.; Brunstrom, B. Sexual behavior in Japanese quail as a test end point for endocrine disruption: Effects of in ovo exposure to ethinylestradiol and diethylstilbestrol. Env. Health Perspect. 1999, 107, 861–866.
- Ellis, H.L.; Shioda, K.; Rosenthal, N.F.; Coser, K.R.; Shioda, T. Masculine epigenetic sex marks of the CYP19A1/aromatase promoter in genetically male chicken embryonic gonads are resistant to estrogen-induced phenotypic sex conversion. Biol. Reprod. 2012, 87, 1–12.
- Shioda, K.; Odajima, J.; Kobayashi, M.; Kobayashi, M.; Cordazzo, B.; Isselbacher, K.J.; Shioda, T. Transcriptomic and Epigenetic Preservation of Genetic Sex Identity in Estrogen-feminized Male Chicken Embryonic Gonads. Endocrinology 2021, 162, bqaa208.
- Widelitz, R.B.; Lin, G.W.; Lai, Y.C.; Mayer, J.A.; Tang, P.C.; Cheng, H.C.; Jiang, T.X.; Chen, C.F.; Chuong, C.M. Morpho-regulation in diverse chicken feather formation: Integrating branching modules and sex hormone-dependent morpho-regulatory modules. Dev. Growth Differ. 2019, 61, 124–138.
- Ellem, S.J.; Risbridger, G.P. Aromatase and regulating the estrogen:androgen ratio in the prostate gland. J. Steroid Biochem. Mol. Biol. 2010, 118, 246–251.
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Derm. 2001, 45, S116–S124.
- Bulun, S.E.; Lin, Z.; Imir, G.; Amin, S.; Demura, M.; Yilmaz, B.; Martin, R.; Utsunomiya, H.; Thung, S.; Gurates, B.; et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: From bench to treatment. Pharm. Rev. 2005, 57, 359–383.
- Hirst, C.E.; Major, A.T.; Ayers, K.L.; Brown, R.J.; Mariette, M.; Sackton, T.B.; Smith, C.A. Sex Reversal and Comparative Data Undermine the W Chromosome and Support Z-linked DMRT1 as the Regulator of Gonadal Sex Differentiation in Birds. Endocrinology 2017, 158, 2970–2987.
- Wartenberg, H.; Lenz, E.; Schweikert, H.U. Sexual differentiation and the germ cell in sex reversed gonads after aromatase inhibition in the chicken embryo. Andrologia 1992, 24, 1–6.
- Smith, C.A.; Katz, M.; Sinclair, A.H. DMRT1 is upregulated in the gonads during female-to-male sex reversal in ZW chicken embryos. Biol. Reprod. 2003, 68, 560–570.
- Testaz, S.; Duband, J.L. Central role of the alpha4beta1 integrin in the coordination of avian truncal neural crest cell adhesion, migration, and survival. Dev. Dyn. 2001, 222, 127–140.
- Cutting, A.D.; Ayers, K.; Davidson, N.; Oshlack, A.; Doran, T.; Sinclair, A.H.; Tizard, M.; Smith, C.A. Identification, expression, and regulation of anti-Mullerian hormone type-II receptor in the embryonic chicken gonad. Biol. Reprod. 2014, 90, 106.
- Burke, W.H.; Henry, M.H. Gonadal development and growth of chickens and turkeys hatched from eggs injected with an aromatase inhibitor. Poult. Sci. 1999, 78, 1019–1033.
- Estermann, M.A.; Smith, C.A. Fadrozole-mediated sex reversal in the embryonic chicken gonad involves a PAX2 positive undifferentiated supporting cell state. bioRxiv 2022.
- Yang, X.; Zheng, J.; Qu, L.; Chen, S.; Li, J.; Xu, G.; Yang, N. Methylation status of cMHM and expression of sex-specific genes in adult sex-reversed female chickens. Sex. Dev. 2011, 5, 147–154.
- Yang, X.; Zheng, J.; Na, R.; Li, J.; Xu, G.; Qu, L.; Yang, N. Degree of sex differentiation of genetic female chicken treated with different doses of an aromatase inhibitor. Sex. Dev. 2008, 2, 309–315.
- Sechman, A.; Rzasa, J.; Paczoska-Eliasiewicz, H. Effect of non-steroidal aromatase inhibitor on blood plasma ovarian steroid and thyroid hormones in laying hen (Gallus domesticus). J. Vet. Med. A Physiol. Pathol. Clin. Med. 2003, 50, 333–338.
- Mohammadrezaei, M.; Toghyani, M.; Gheisari, A.; Toghyani, M.; Eghbalsaied, S. Synergistic effect of fadrozole and insulin-like growth factor-I on female-to-male sex reversal and body weight of broiler chicks. PLoS ONE 2014, 9, e103570.
- Abinawanto; Zhang, C.; Saito, N.; Matsuda, Y.; Shimada, K. Identification of sperm-bearing female-specific chromosome in the sex-reversed chicken. J. Exp. Zool. 1998, 280, 65–72.
- Vaillant, S.; Guemene, D.; Dorizzi, M.; Pieau, C.; Richard-Mercier, N.; Brillard, J.P. Degree of sex reversal as related to plasma steroid levels in genetic female chickens (Gallus domesticus) treated with Fadrozole. Mol. Reprod. Dev. 2003, 65, 420–428.
- Takagi, S.; Tsukada, A.; Saito, N.; Shimada, K. Fertilizing ability of chicken sperm bearing the W chromosome. Poult. Sci. 2007, 86, 731–738.
- Brunstrom, B.; Axelsson, J.; Mattsson, A.; Halldin, K. Effects of estrogens on sex differentiation in Japanese quail and chicken. Gen. Comp. Endocrinol. 2009, 163, 97–103.
- Clinton, M.; Zhao, D.; Nandi, S.; McBride, D. Evidence for avian cell autonomous sex identity (CASI) and implications for the sex-determination process? Chromosome Res. 2012, 20, 177–190.
- Scholz, B.; Kultima, K.; Mattsson, A.; Axelsson, J.; Brunstrom, B.; Halldin, K.; Stigson, M.; Dencker, L. Sex-dependent gene expression in early brain development of chicken embryos. BMC Neurosci. 2006, 7, 12.
- Ayers, K.L.; Davidson, N.M.; Demiyah, D.; Roeszler, K.N.; Grutzner, F.; Sinclair, A.H.; Oshlack, A.; Smith, C.A. RNA sequencing reveals sexually dimorphic gene expression before gonadal differentiation in chicken and allows comprehensive annotation of the W-chromosome. Genome Biol. 2013, 14, R26.
- Morris, K.R.; Hirst, C.E.; Major, A.T.; Ezaz, T.; Ford, M.; Bibby, S.; Doran, T.J.; Smith, C.A. Gonadal and Endocrine Analysis of a Gynandromorphic Chicken. Endocrinology 2018, 159, 3492–3502.
- Zhang, X.; Li, J.; Wang, X.; Jie, Y.; Sun, C.; Zheng, J.; Li, J.; Yang, N.; Chen, S. ATAC-seq and RNA-seq analysis unravel the mechanism of sex differentiation and infertility in sex reversal chicken. Epigenetics Chromatin 2023, 16, 2.
- Briganti, F.; Papeschi, A.; Mugnai, T.; Dessì-Fulgheri, F. Effect of testosterone on male traits and behaviour in juvenile pheasants. Ethol. Ecol. Evol. 1999, 11, 171–178.
- Fennell, M.J.; Scanes, C.G. Inhibition of growth in chickens by testosterone, 5 alpha-dihydrotestosterone, and 19-nortestosterone. Poult. Sci. 1992, 71, 357–366.
- Agate, R.J.; Grisham, W.; Wade, J.; Mann, S.; Wingfield, J.; Schanen, C.; Palotie, A.; Arnold, A.P. Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proc. Natl. Acad. Sci. USA 2003, 100, 4873–4878.




