1. Introduction
Birds are important agricultural species and long-standing development model animals
[1][2][1,2]. For a long time, the global poultry industry has sought effective methods to control the sex of hatchlings to achieve different purposes. In the layer industry, for example, only female chickens are required, and day-old male hatchlings are cruelly culled, which seriously increases ethical and legal concerns
[3][4][5][6][7][3,4,5,6,7]. Deciphering the mechanisms of avian sex determination and differentiation can contribute to achieving sex control in chickens and avoiding such issues. Furthermore, owing to the high accessibility of chicken embryos, which can be genetically manipulated in vitro, they are ideal models for gonadal research
[8][9][10][11][8,9,10,11]. Given the conservation of gonadal morphogenesis, studies on chicken embryos have shed light on the developmental patterns of the human reproductive system and the causes of sex disorders
[12]. Thus, understanding the cell biology and genetics of gonadal formation during avian embryogenesis is crucial for improving our knowledge in diverse areas, from livestock breeding to human sex development.
In birds, the sex of offspring is determined by genetic factors. Male determinant genes activate and maintain the cascade reaction of testicular development, whereas female sex-related pathways are responsible for the proliferation and differentiation of the ovary
[13][14][15][13,14,15]. However, avian sex differentiation is regulated by multiple factors, including genetics, epigenetics, and sex hormones
[16][17][16,17]. Under natural conditions, for example, disease-induced gene mutation and alteration of estrogen levels can significantly divert the direction of sex differentiation, leading to sex reversal
[17][18][19][17,18,19]. Although adult sex-reversed chickens form almost identical phenotypes to their opposite genetic sexes, they cannot experience complete sex reversal and produce offspring, which is caused by the cell autonomous sex identity (CASI) of avian cells
[16][17][20][21][16,17,20,21]. Promising investigations indicates that hereditary factors are responsible for avian CASI. The research on the sex development of gynandromorphic birds suggests that the CASI is governed by the cellular sex chromosome combination, and is vital to the maintenance of genetic sex
[21]. Until now,
rwe
searchers have failed to clarify the underlying basis of this biological feature in birds, which needs further research.
2. Occurrence of Vertebrate Sex Reversal
In birds and some lower vertebrates, sex differentiation is often affected by various factors, leading to sex reversal. In chicken flocks, the population’s social structure can influence the sex of individuals, similar to the case of fish
[22][23][24][25][26][200,201,202,203,204]. For instance, when there is no rooster in a chicken flock, a hen may experience sex reversal to maintain the reproduction of the population
[27][135]. This is an adaptive change that arises during the long-term evolutionary process. However, no further research has been conducted to prove this phenomenon. In addition, diseases are a major cause of sex reversal. Ectopic activation of aromatase and accumulation of estrogens as a result of the henny-feathering trait, an autosomal dominant mutation, can feminize the feathering patterns of male chicken
[18][28][18,205]. However, this disease-mediated sex reversal might not influence gonadal morphology because aromatase is mainly mis-expressed in extragonadal tissues
[18][28][18,205].
3. Mechanism of Avian Sex Reversal
Bulk instances of sex reversal in birds are associated with the high sensitivity to alterations in sex hormone levels
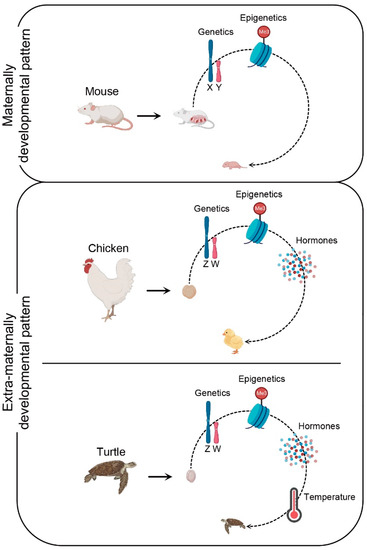
[16][29][16,206]. This biological characteristic of birds is closely related to their evolutionary processes. From the viewpoint of evolution, mechanisms of mammalian sex determination and differentiation evolved from synapsid reptiles, whereas diapsid reptiles gave rise to crocodiles, lizards, and birds 150 million years ago
[30][31][125,207]. In eutherian mammals, gonadal sex differentiation appears independent of sex steroid hormones and can proceed without steroidogenesis
[32][33][208,209]. Environmentally insensitive gonads of highly evolved mammalian embryos occur in the maternal womb, which is a potentially dangerous place rich in various hormones (
Figure 1)
[34][210]. Hence, the highly evolved placenta and maternal internal pregnancy pattern may have forced eutherians to abandon estrogens as components of the sex-determining cascade. However, sex differentiation of birds occurs in an extra-maternal environment and has characteristics similar to those of lower vertebrates (
Figure 1). Therefore, it is likely that they do not have the mechanism to resist the alteration of exogenous estrogen levels, which is usually unlikely to occur under normal hatching conditions. Therefore, the evolutionary position of birds and the biological characteristics of extra-maternal embryogenesis indicate that avian sex differentiation is prone to being affected by environmental hormones.
Figure 1. Schematic view of key factors influencing the embryonic sex determination and differentiation in maternal and extra-maternal developmental patterns. In mouse, the process of embryonic sex development occurs in the maternal environment, and is mainly regulated by genetic and epigenetic factors. In chicken, the process of embryonic sex development occurs in the extra-maternal environment, and is governed by genetic and epigenetic factors and sex hormones. Likewise, the process of turtle embryonic sex development occurs in the extra-maternal environment, and is controlled by genetic and epigenetic factors, sex hormones, and temperature.
4. Avian Sex Reversal Induced by Transplant Treatment
Sex-reversed birds can be obtained by altering endogenous hormone secretion through gonadal grafting. Transplantation of E13 (HH39) chicken whole testes into E3 female chicken extra-embryonic coelom can induce gonadal sex reversal
[35][36][211,212]. Under the stimulation of exogenous testes, the left gonad differentiates into a testis instead of an ovary, and germ cells migrate into sex cords. However, they do not experience a meiotic process
[36][212]. Moreover, grafting E2 female (male) sections of the presumptive mesoderm, which gives rise to gonads, to replace the equivalent tissue of male (female) at the same growth stage, induces the formation of mixed-sex chimeras
[21]. Therefore, artificial transplantation of exogenous tissues can affect avian sex differentiation, leading to sex reversal.
5. Avian Male-to-Female Sex Reversal Induced by Estrogens Treatment
In most cases, sex-reversed birds are created by adding estrogens or aromatase inhibitors to manipulate endogenous hormone levels (
Figure 2)
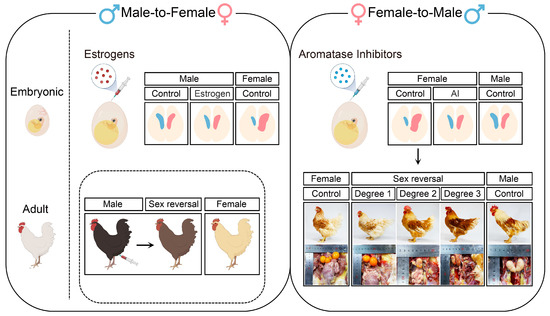
[16][17][16,17]. The administration of estrogen to quail eggs during the first half of embryonic life (before the onset of sex differentiation) can feminize genetic males, characterized by the hyperproliferation of the left gonadal cortex and vacuolized inner medulla
[16]. Moreover, injecting a 17a-Ethinylestradiol (EE2) emulsion into E3 quail eggs can demasculinize male individuals, which will lose typical masculine sexual behavior and form asymmetric testes with decreased areas of androgen-dependent cloacal glands after maturity
[37][213]. Recently, research has found that treating chicken eggs with this emulsion can feminize male chickens to different degrees
[38][39][177,178]. The left gonads of these individuals progressively form female-like cortical regions and fluid-filled medullae from low to high degrees of sex reversal, while the characterized testicular tissues are almost lost
[39][178]. However, the effect of estrogen-mediated male-to-female sex reversal is transient. Some reversed individuals revert to normal male phenotypes after hatching, whereas others can persist in female phenotypes for not more than 1 year
[30][125]. In addition, estrogen-regulated sex reversal is not limited to gonads but is also presented in secondary sexual characteristics. Injection of estradiol into leg muscles of adult male chickens can feminize feathering patterns, including reducing saddle feather length, increasing plumulaceous segments, and altering feathering colors (
Figure 2)
[40][214]. Similarly, these changes are not permanent and gradually disappear with decreased estrogen levels in the body. Taken together, the addition of estrogens can significantly affect the differentiation of gonadal structures during embryonic periods and influence the formation of secondary sexual characteristics in adult periods; however, these effects are only maintained for a short time.
Figure 2. Overview of gonadal phenotypes and secondary sexual characteristics in embryonic and adult sex-reversed chickens created by treatment with either estrogens or aromatase inhibitors. The left panel: Injection of estrogens into chicken eggs can lead to male-to-female sex reversal, characterized by the proliferation of the left gonad and degeneration of the right gonad. During adult period, injection of estrogens into leg muscles of male chicken can induce the feminization of feathering patterns. The right panel: Injection of aromatase inhibitors into chicken eggs can lead to female-to-male sex reversal, characterized by the symmetrical proliferation and differentiation of bilateral gonads. During the adult period, the secondary sexual characteristics and gonadal appearance show different degrees of sex reversal in aromatase inhibitors-treated female chicken.
6. Avian Female-to-Male Sex Reversal Induced by Aromatase Inhibitors Treatment
Injecting aromatase inhibitors, which reduce the production of gonadal estrogens, into avian eggs can induce female-to-male sex reversal (
Figure 2)
[17][41][42][43][17,215,216,217]. A previous study found that treating E3 chicken eggs with fadrozole, an aromatase inhibitor, can masculinize bilateral female embryonic gonads, which fail to form stratified cortexes, but develop functional medullae enclosing germ cells at E9.5
[44][218]. Notably, aromatase inhibitors-treated chickens exhibit varying degrees of sex reversal (
Figure 2)
[45][184]. The gonadal cortex is reduced in highly sex-reversed individuals, and germ cells are relocated into developed medullary structures
[45][184]. During the development of sex-reversed female embryos,
DMRT1,
SOX9, and
AMH expression is significantly upregulated in gonadal medullae, whereas
FOXL2 and
CPY19A1 are downregulated in gonadal cortexes with fewer residues in juxtacortical medullae
[44][46][47][91,218,219]. In addition, the primary AMH Receptor, AMH receptor type-II (
AMHR2), is upregulated in fadrozole-treated females and co-locates with
DMRT1 in Sertoli cells
[48][220]. Moreover, gonadal morphology and inner structures of sex-reversed chickens continue to change after hatching. In 1-day-old sex-reversed chickens (D1), both follicles and tubular structures are visible in the gonads
[49][221]. These tubular structures differentiate into abnormal seminiferous tubules at D11 and form atypical tubules with areas of loose connective tissue at D21
[49][221]. The left and right gonads grow into small ovotestes in D42 chickens, containing greatly enlarged atypical seminiferous tubules and fewer normal appearing seminiferous tubules
[49][221]. This consecutive alteration is due to the permanent effect of aromatase inhibitors on chicken sex differentiation, which is likely achieved by cell reprogramming during the embryonic period. Research has emphasized that fadrozole-mediated female-to-male sex reversal includes a crucial event, namely, pre-granulosa cells trans-differentiate into undifferentiated
PAX2+ supporting cells before forming Sertoli cells, which is lost in estrogens-mediated male-to-female sex reversal
[50][222]. However, current evidences are still unable to clarify the potential mechanism of this phenomenon, which needs to be studied in detail.
The permanent effect of aromatase inhibitors on avian sex differentiation can significantly influence the development of reproductive tissues and the appearance of birds. Adult sex-reversed chickens form almost symmetrical gonads and small testes with regressed oviducts on the left side
[17][51][52][17,107,223]. In addition, injecting aromatase inhibitors before sex differentiation during the embryonic period or after sexual maturity in the adult period can induce testosterone production while inhibiting estradiol synthesis in the blood
[52][53][223,224]. The sex-revered females progressively form masculinized hackles, saddle feathers, combs, and wattles. In contrast, their body weights, especially muscle mass and fat pad weight, remain in line with normal females, suggesting that growth performance, unlike secondary sexual characteristics, may be controlled directly by genetic factors and independent of hormones
[40][49][52][53][54][214,221,223,224,225]. Notably, under the influence of an aromatase inhibitor, Wolffian ducts in sex-reversed chickens develop into vas deferens, and gonads can produce sperms carrying the W chromosome
[55][226]. Further investigation has shown that W-carrying sperms have oocyte-activating potency and can induce the formation of male and female pronuclei
[56][57][227,228]. In summary, aromatase inhibitors can significantly disrupt estrogen synthesis and permanently influence the process of sex differentiation, causing the formation of sperm-carrying small testes and masculinized secondary sexual characteristics in adult sex-reversed birds.
7. Avian Sex Reversal and Cell Autonomous Sex Identity
Sex reversal can be induced in birds, but neither does the male bird has the ability to form well-functional ovaries, nor do female birds show normal masculinized courtship behavior and successfully produce fertile sperm after sexual maturity
[17][58][17,229]. This incomplete sex reversal may be regulated by the CASI of avian somatic cells, which is a cellular inherent sex identity that can help individuals maintain their genetic sex independently of the impact of hormones
[21][59][21,74]. Current research points out that the CASI is determined by the genetic information, especially that carried by sex chromosomes, and epigenetic factors, such as DNA methylation and histone lysine methylation
[21][38][21,177]. At early developmental stages when primary gonads have not been induced to differentiation, sexually dimorphic gene expression can be detected in several tissues between male and female birds, indicating that the CASI is widely involved in organogenesis programs
[60][61][230,231]. Studies of the CASI mainly focus on gynandromorphic birds, which display a significant bilateral asymmetry; one side of the body appears to be male, but the other appears to be female
[59][74]. In gynandromorphic chickens, most cells on the side with a female appearance are of the ZW genotype, and most cells on the other side with a male appearance are of the ZZ genotype
[21]. Notably, the gonadal morphologies of these gynandromorphic chickens conform to their cellular compositions, testicular and ovarian appearances are mainly composed of ZZ and ZW genotypic cells, whereas ovotestis is composed of a mixture of both cells
[21]. Consistently, male-determining genes, such as
DMRT1 and
SOX9, are highly expressed in Sertoli cells of the seminiferous cords on the masculinized side, where aromatase is not detectable
[62][232]. However, the gonads of the feminized side show areas of peripheral aromatase expression, together with areas of
SOX9-positive and
DMRT1-positive seminiferous tubules
[62][232]. Moreover, the concept of CASI has been confirmed in mixed-sex chicken chimeras. Cells from donor females are restricted to the interstitial tissue between the sex cords and are not recruited into functional Sertoli cells in testes. Similarly, donor male cells cannot be recruited to form granulosa cells in ovaries
[21]. That is, there is no interaction between
GFP+ donor female cells and
AMH+ host male cells in the testes, and
GFP+ donor male cells and
CYP19A1+ host female cells in the ovaries, suggesting that the differentiation of these cells is confirmed by their genetic fates
[21]. Notably, epigenetic factors are likely to contribute to maintaining gonadal fate. The chromatin accessibility of
DMRT1 in embryonic female-to-male sex-reversed chickens cannot be increased to the normal male level, corresponding to its transcriptional activity
[63][163]. In addition, the pattern of DNA methylation around
CYP19A1 in estrogen-mediated sex-reversed chicken gonads fails to be induced to form the same as that in control females, suggesting that epigenetic elements are involved in the establishment of avian gonadal CASI
[38][39][177,178].
In addition, studies have found that the CASI also affects the growth of avian secondary sexual characteristics
[64][65][233,234]. In a gynandromorphic finch, although both halves of the brain are exposed to the same hormone environment, the neural song circuit of the male side has a more masculine phenotype than that of the female side
[66][76]. Strikingly, recent research found that
DMRT1-knockout male chicken, which had ovaries instead of testes, can form the normal masculinized appearance, featuring large combs and wattles, hackle feathers, and long leg spurs in adult periods
[14]. Similarly, in gynandromorphic chickens, the female side forms sex-linked feathering patterns, smaller spurs, and wattles than those on the male side after sexual maturity
[62][232]. These results suggest that avian CASI may be critical in differentiating sex phenotypes.