Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Simona Concilio | -- | 1627 | 2023-05-15 16:27:57 | | | |
| 2 | Fanny Huang | -1 word(s) | 1626 | 2023-05-16 04:45:32 | | | | |
| 3 | Fanny Huang | Meta information modification | 1626 | 2023-05-16 04:46:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Di Martino, M.; Sessa, L.; Diana, R.; Piotto, S.; Concilio, S. Photoresponsive Biomaterials. Encyclopedia. Available online: https://encyclopedia.pub/entry/44322 (accessed on 08 February 2026).
Di Martino M, Sessa L, Diana R, Piotto S, Concilio S. Photoresponsive Biomaterials. Encyclopedia. Available at: https://encyclopedia.pub/entry/44322. Accessed February 08, 2026.
Di Martino, Miriam, Lucia Sessa, Rosita Diana, Stefano Piotto, Simona Concilio. "Photoresponsive Biomaterials" Encyclopedia, https://encyclopedia.pub/entry/44322 (accessed February 08, 2026).
Di Martino, M., Sessa, L., Diana, R., Piotto, S., & Concilio, S. (2023, May 15). Photoresponsive Biomaterials. In Encyclopedia. https://encyclopedia.pub/entry/44322
Di Martino, Miriam, et al. "Photoresponsive Biomaterials." Encyclopedia. Web. 15 May, 2023.
Copy Citation
Photoresponsive biomaterials have garnered increasing attention due to their ability to dynamically regulate biological interactions and cellular behaviors in response to light. Photoresponsive biomaterials are created by integrating photoresponsive molecules, such as spiropyrans, azobenzenes, hydrazones, and diarylethenes, into biomaterials like hydrogels, nanoparticles, or scaffolds.
photoresponsive
biomaterials
azobenzene
hydrazones
diarylethene
spiropyran
1. Introduction
Stimuli-responsive molecules, which an external stimulus can manipulate, have recently received considerable interest. The use of light as a stimulus is fascinating for its precise spatiotemporal control and non-destructive nature, as the intensity and wavelength can be easily regulated [1][2][3]. Light-responsive molecules, also known as photoresponsive molecules, are a class of compounds that undergo reversible or irreversible structural changes upon exposure to light. The changes in their structure can alter their physical or chemical properties, such as solubility, polarity, or reactivity. This ability to respond to light has made light-responsive molecules an attractive area of research in many fields, including chemistry, materials science, and biology [4]. When chromophores are irradiated with light of a specific wavelength, they can undergo a reversible or irreversible conversion between isomeric forms or ring-opening/closure. One of the most studied types of light-responsive molecules is azobenzene. Azobenzene has a unique structure consisting of two benzene rings connected by a central nitrogen–nitrogen bond. When exposed to UV light, azobenzene undergoes a reversible trans-cis isomerization, changing from a planar to a bent conformation. The cis form reverts to the trans form upon exposure to visible light or heat. This reversible photoisomerization has led to the development of many applications, such as molecular switches, sensors, and photoresponsive materials. Among photoswitches, azobenzenes, and their heteroaromatic analogs, like stilbenes and hydrazones, undergo double-bond isomerization. Diarylethenes undergo cyclization; spiropyrans follow a mixed mechanism, as they experience electrocyclization first and then trans-cis isomerization (Figure 1) [5][6][7]. Spiropyran has a unique structure consisting of two rings connected by a single carbon atom. The two rings are typically a benzene ring and a pyran ring, giving the molecule a bicyclic structure. The spiropyran group is sensitive to UV light; it can be converted from its closed, non-polar form (spiropyran, SP) to its open, polar form (merocyanine, MC) when exposed to the light of a certain wavelength (Figure 1). This ring-opening reaction is reversible and can be triggered by visible light or heat, leading to the development of photochromic materials and sensors. Diarylethene molecules are compounds that have been discovered relatively recently, and they possess a distinctive characteristic of transitioning from colorless to red when exposed to light. Diarylethene compounds provide several advantages, such as excellent thermal stability and high quantum yield for both their isomers. Due to these properties, they are well-suited for application in photoresponsive materials, exhibiting robust performance.
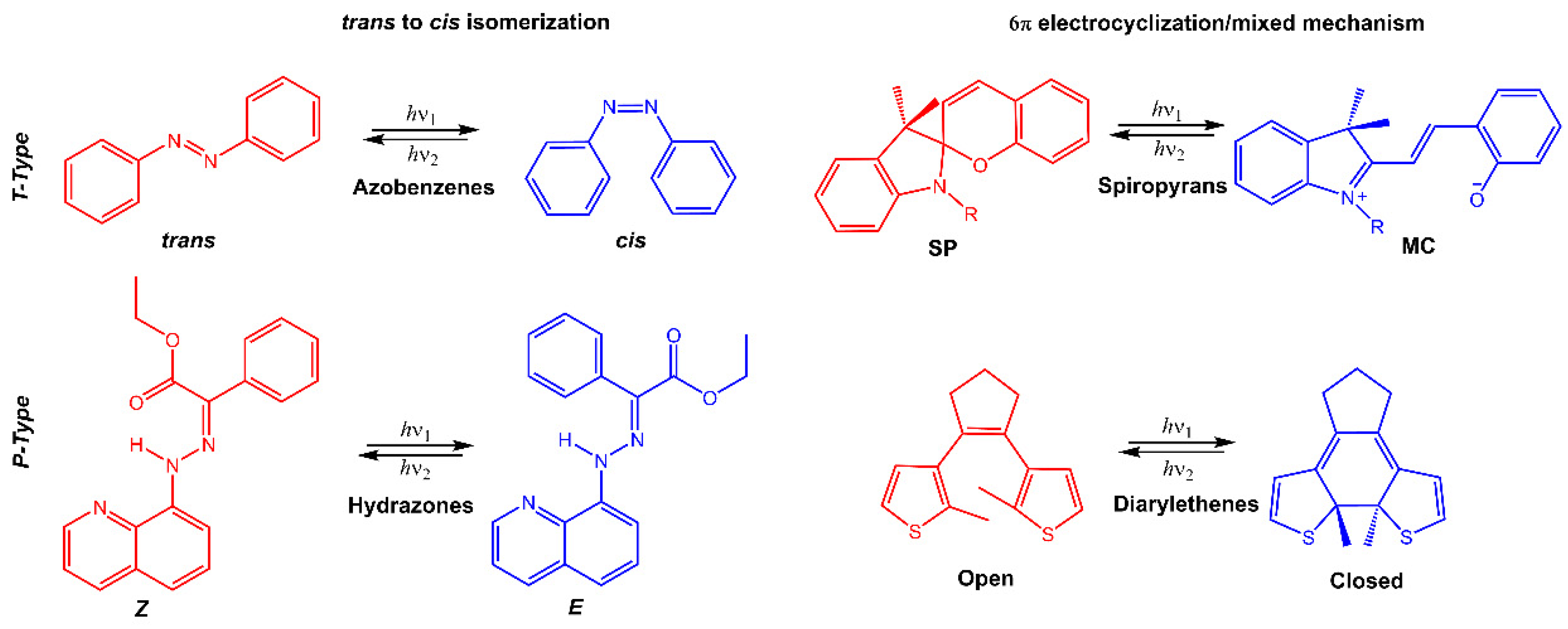
Figure 1. Chemical structures of the most used photoresponsive molecules.
Many of these molecules exhibit positive photochromism (P-type), whereby the photochemically induced species has an absorption maximum λmax (metastable) that is shifted to longer wavelengths (bathochromic shift) compared to λmax (stable). However, if the initial photoisomer undergoes a decolorization (bleaching) during photoisomerization, and λmax (stable) is greater than λmax (metastable), then the molecule displays negative or inverse photochromism (T-type) [8]. When inserted into a polymeric matrix, the conversion of photochromic molecules modifies the properties of the polymer, such as polarity, hydrophilicity, solubility, or electrical and optical properties.
Photoresponsive biomaterials are created by integrating photoresponsive molecules, such as spiropyrans, azobenzenes, hydrazones, and diarylethenes, into biomaterials like hydrogels, nanoparticles, or scaffolds. This development has the potential to revolutionize biomedicine by enabling new approaches to drug delivery, tissue engineering, and imaging. These materials can be controlled and manipulated using light, making them an increasingly prominent part of biomedical research in fields like tissue engineering, dynamic hydrogels for cell encapsulation, controlled drug delivery, biosensors, and microdevices [9][10][11]. Additionally, photoresponsive biomaterials are versatile platforms for various biomedical applications because they can be engineered and designed with specific properties and functionalities.
2. Azobenzene Photoswitches
Thanks to its simple molecular structure and unique characteristics, azobenzene (AB) has probably been the most studied photoswitches since its reversible isomerization was first reported about eight decades ago [12]. When the trans isomer, which is thermodynamically stable, is exposed to UV light (λ ≈ 365 nm), it isomerizes into the cis form. The latter isomer is metastable, i.e., spontaneously returning to the trans form once the UV source is removed. The return to the trans form can be significantly accelerated by heating or exposure to visible light (λ ≈ 430 nm) [13]. Moreover, they proceed with high quantum yields and are completely reversible. The most important aspect is that the two isomers differ significantly in their geometries and electronic properties, making AB an attractive component for the design of photoswitchable materials [14][15]. Some materials containing AB can convert light into mechanical energy due to the change in molecular geometry that occurs during AB isomerization. Additionally, the molecule’s polarity undergoes a significant change as lone electron pairs on the nitrogen atoms take on opposite orientations in the trans configuration, resulting in a non-polar molecule. On the other hand, in the cis isomer, the lone electron pairs arrange themselves in a way that increases the molecule’s dipole moment, typically ranging from 3.1–4.4 D compared to 0–1.2 D in the trans configuration (Figure 2) [14][16].
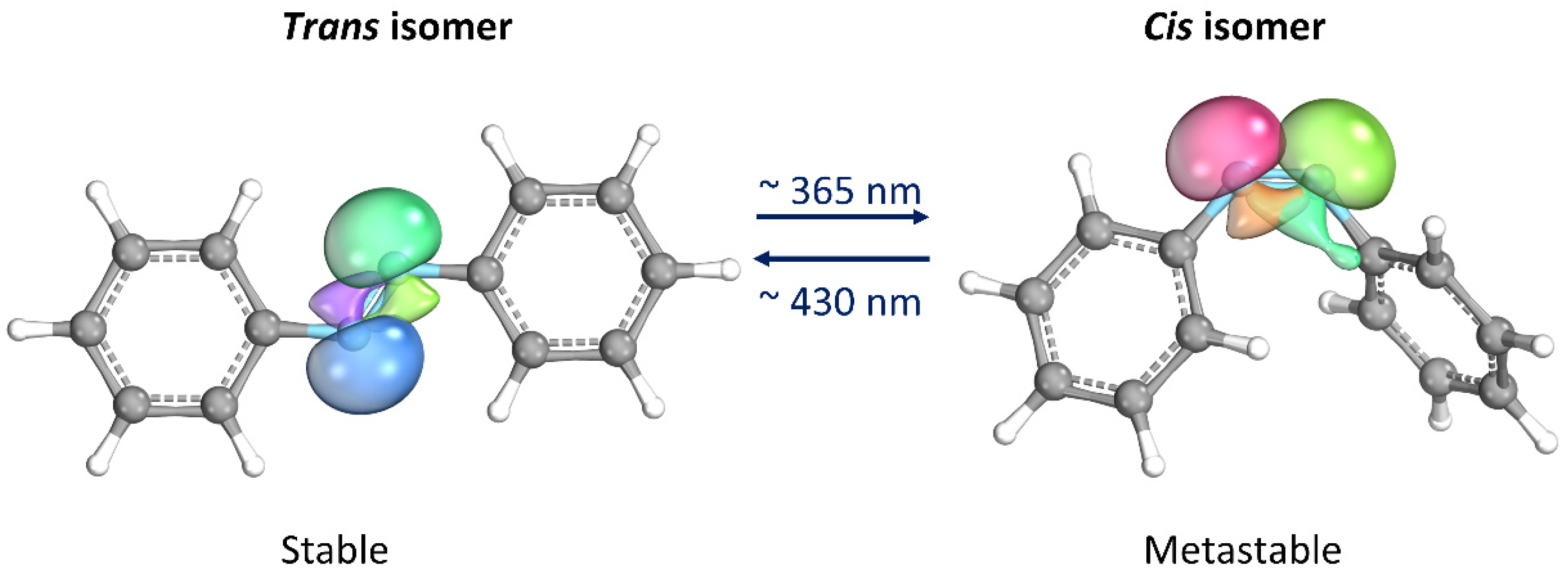
Figure 2. Photoisomerization process of azobenzene. Lone electron pairs on the nitrogen atoms are evidenced. This image has been realized with the IboView program [17].
Several studies have reported the synthesis of azobenzenes and their derivatives for potential use in biomedical applications due to their various biological properties, ranging from antioxidant, antiviral, and antimicrobial to antitumor and antidiabetic [13][18][19][20][21]. Introducing these molecules into polymer matrices has resulted in the development of intelligent biomaterials, known as ‘smart’ materials. These materials can respond to light energy and cause large-scale changes or can utilize isomerization to provide intrinsic activities, such as antibacterial properties [22].
3. Hydrazone Photoswitches
Hydrazones are organic compounds that contain a carbon–nitrogen double bond (C=N) that links a hydrazine functional group (-NHNH2). They can be considered derivatives of hydrazine (H2NNH2) in which one of the hydrogen atoms has been replaced by a carbonyl group (C=O). Hydrazones can be synthesized by the condensation reaction of a hydrazine derivative with a carbonyl compound such as an aldehyde or a ketone. This reaction is typically catalyzed by an acid catalyst, such as hydrochloric acid or sulfuric acid [23]. Hydrazones have a wide range of applications, including as intermediates in the synthesis of pharmaceuticals, as ligands in coordination chemistry, and as chromogenic and fluorogenic sensors for various analytes [24]. They also exhibit biological activities, such as antitumor, antimicrobial, and antiviral properties. Hydrazones can undergo trans-to-cis isomerization of the C=N bond upon light exposure (Figure 3).
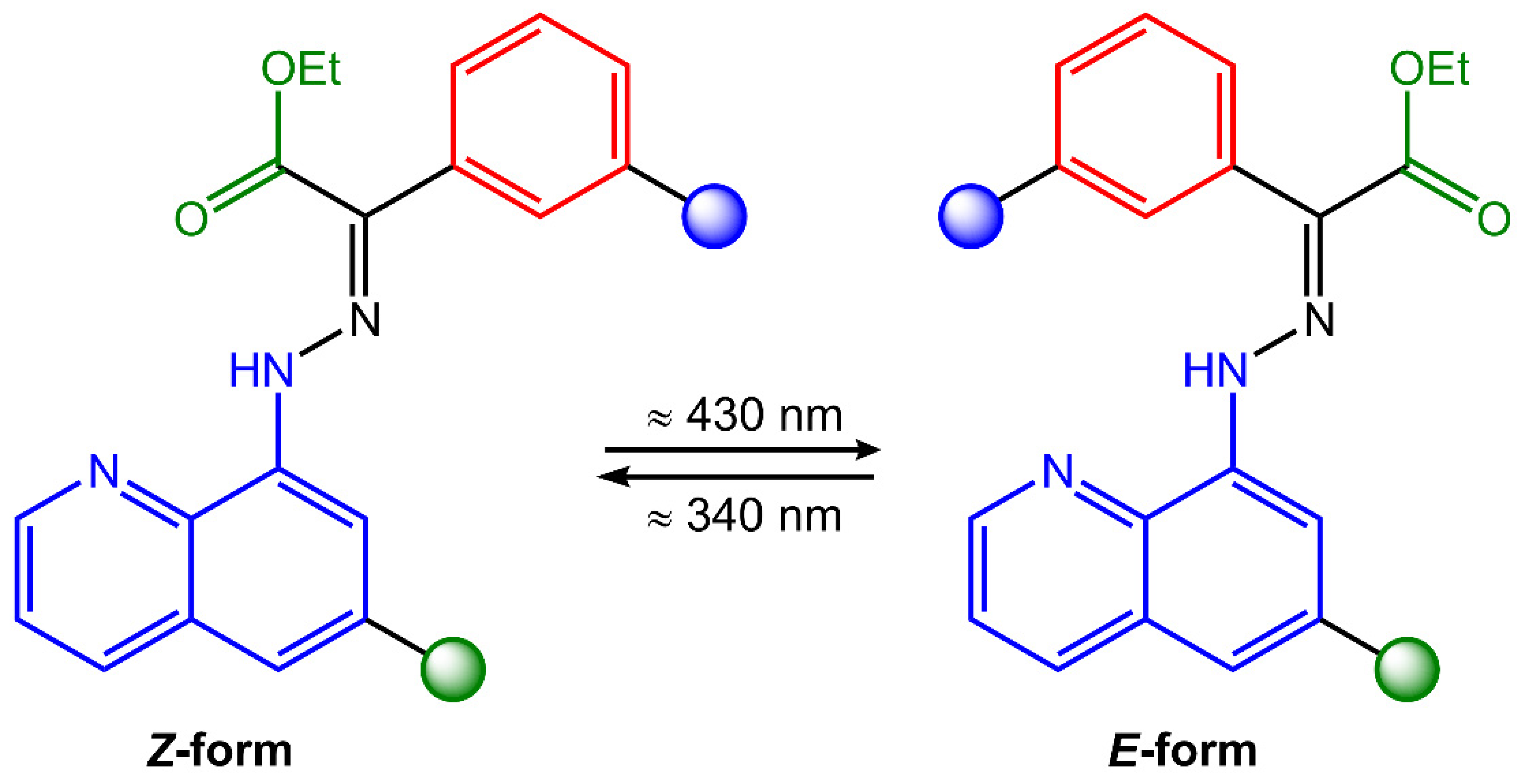
Figure 3. Chemical structure and photoisomerization process of hydrazone molecule.
Hydrazones also undergo photochromism, the reversible change of color or absorbance upon irradiation with light. The photochromic behavior of hydrazones is typically attributed to the formation of a tautomeric form upon photoisomerization, which has a different absorption spectrum than the original form. The tautomeric form can then revert to the original state upon removal of the light source. As a result, hydrazone photoswitches have been adopted in various adaptive materials, such as liquid crystals, hydrogels, actuating polymers, and nanoparticles for drug release [25].
4. Diarylethene Photoswitches
Diarylethene molecules (DAE) are a relatively recent class of compounds with the unique property of changing color from colorless to red. They belong to the P-type photochromic molecules, which are photochemically reversible but thermally irreversible [26]. This means that once the photogenerated right-side isomers are formed, they are highly stable and do not easily revert to the left-side isomers in the dark at room temperature (Figure 4). Diarylethenes are derived from stilbene, featuring five-membered heterocyclic rings, such as thiophene or furan rings, in place of the phenyl rings found in stilbene. These modifications increase thermal stability for both the open and closed-ring isomers, making diarylethenes highly suitable for repeated cycles of coloration and discoloration [27][28].
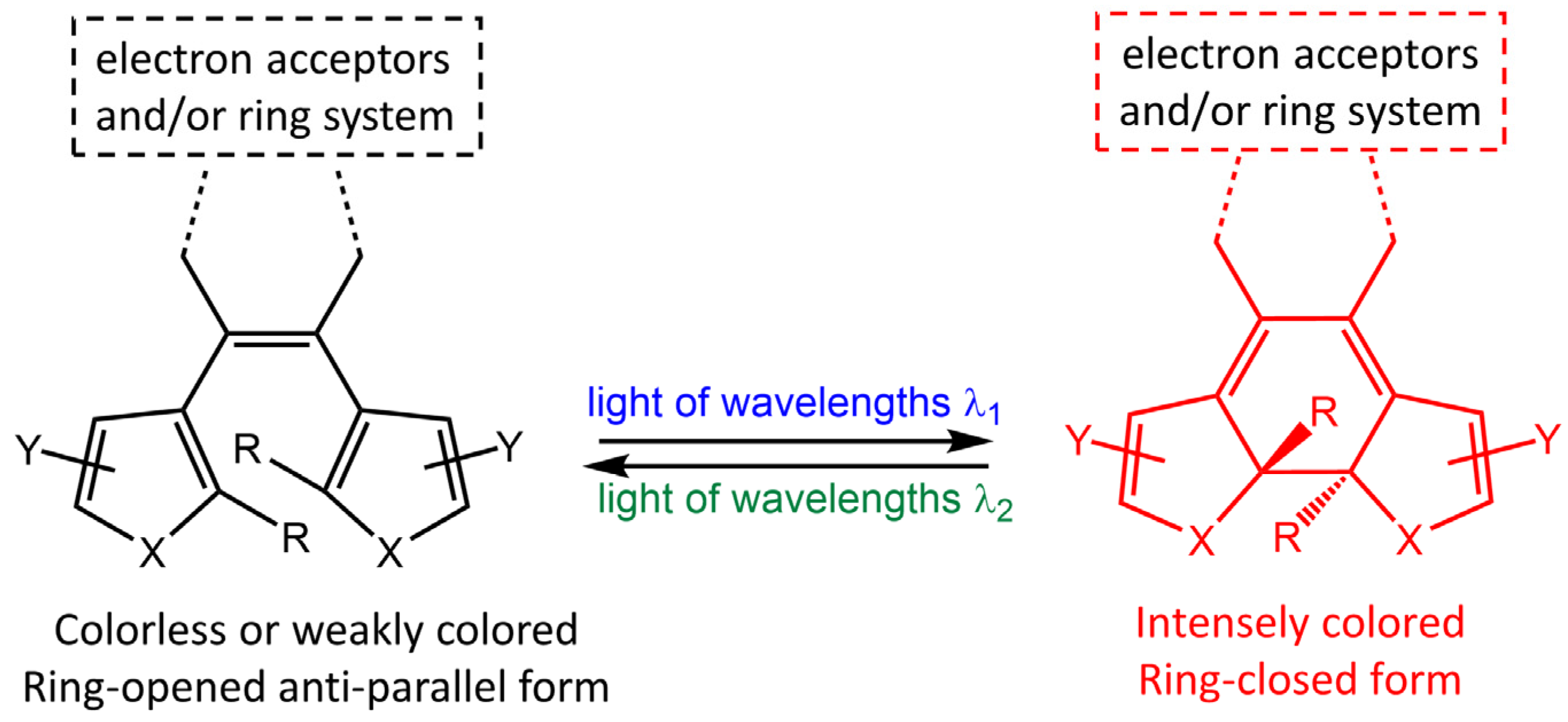
Figure 4. Chemical structures of open- and closed-ring isomers of diarylethene molecule and their properties.
Diarylethenes offer several benefits, including a high quantum yield and excellent thermal stability for both isomers. These qualities make them ideal for photoresponsive materials, as they exhibit strong performance. One area where they have been particularly useful is in supramolecular systems, where they can regulate photoresponsive assembly, induce morphological alterations, and trigger the gel-to-sol transition [24].
5. Spiropyran Photoswitches
Spiropyran is a photochromic molecule that exhibits reversible changes in color or optical properties upon exposure to light of specific wavelengths. Spiropyran molecules typically exist in two forms: a colorless, non-planar, closed-ring form and a colored, planar, open-ring form. When exposed to ultraviolet (UV) light or visible light of a specific wavelength, spiropyran molecules undergo a photoisomerization reaction, transforming the non-planar, colorless form into the planar, colored form (Figure 5) [29]. The photoisomerization process involves breaking a carbon-oxygen bond in the spiro ring and forming a new carbon-carbon bond. This leads to a significant change in molecular geometry and electronic structure. The colored form of spiropyran (SP) is called merocyanine (MC), which absorbs light in the visible region and has a distinct color depending on the substituents on the molecule. Upon exposure to light of a different wavelength, the merocyanine form can undergo a reverse reaction, or thermal relaxation, to convert back to the spiropyran form [2][8].
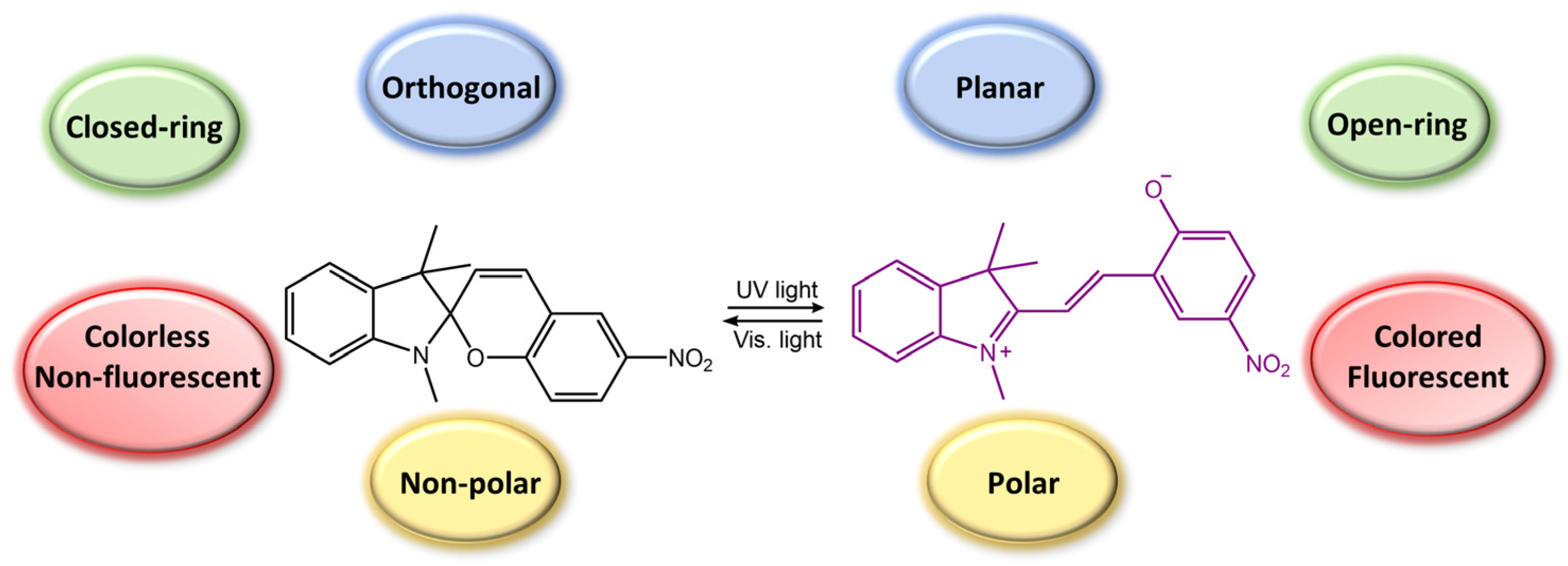
Figure 5. Spiropyran photoisomerization process and isomers characteristics.
The photoresponsive effect of spiropyran has numerous potential applications in areas such as photochromic materials, optical storage, photonic devices, sensors, and actuators. For example, spiropyran-based materials can be used as reversible optical switches or sensors, as the color change can be used to indicate the presence or absence of a particular stimulus. In addition, spiropyran can be incorporated into polymers, films, or surfaces to create photoresponsive coatings that can be tuned to respond to specific wavelengths of light [30]. Spiropyran-based compounds have been studied extensively for their potential use in biomaterials due to their reversible photochromic properties, biocompatibility, and ease of functionalization [31][32].
References
- Chang, V.Y.; Fedele, C.; Priimagi, A.; Shishido, A.; Barrett, C.J. Photoreversible soft azo dye materials: Toward optical control of bio-interfaces. Adv. Opt. Mater. 2019, 7, 1900091.
- Keyvan Rad, J.; Balzade, Z.; Mahdavian, A.R. Spiropyran-based advanced photoswitchable materials: A fascinating pathway to the future stimuli-responsive devices. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100487.
- Quan, J.; Guo, Y.; Ma, J.; Long, D.; Wang, J.; Zhang, L.; Sun, Y.; Dhinakaran, M.K.; Li, H. Light-responsive nanochannels based on the supramolecular host–guest system. Front. Chem. 2022, 10, 986908.
- Di Martino, M.; Marrafino, F.; Diana, R.; Iannelli, P.; Concilio, S. Fluorescent Probes for Applications in Bioimaging. In Advances in Bionanomaterials II: Selected Papers, Proceedings of the 3rd International Conference on Bio and Nanomaterials, BIONAM 2019, Mediterranean Sea, Italy, 29 September–3 October 2019; Springer: Berlin/Heidelberg, Germany, 2020; pp. 243–258.
- Welleman, I.M.; Hoorens, M.W.H.; Feringa, B.L.; Boersma, H.H.; Szymański, W. Photoresponsive molecular tools for emerging applications of light in medicine. Chem. Sci. 2020, 11, 11672–11691.
- Yang, Z.; Liu, Z.; Yuan, L. Recent advances of photoresponsive supramolecular switches. Asian J. Org. Chem. 2021, 10, 74–90.
- Wang, G.; Zhang, J. Photoresponsive molecular switches for biotechnology. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 299–309.
- Volarić, J.; Szymanski, W.; Simeth, N.A.; Feringa, B.L. Molecular photoswitches in aqueous environments. Chem. Soc. Rev. 2021, 50, 12377–12449.
- Pearson, S.; Feng, J.; del Campo, A. Lighting the path: Light delivery strategies to activate photoresponsive biomaterials in vivo. Adv. Funct. Mater. 2021, 31, 2105989.
- Xiong, X.; del Campo, A.; Cui, J. Chapter 4—Photoresponsive Polymers. In Smart Polymers and their Applications, 2nd ed.; Aguilar, M.R., San Román, J., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 87–153.
- Diana, R.; Panunzi, B.; Shikler, R.; Nabha, S.; Caruso, U. Highly efficient dicyano-phenylenevinylene fluorophore as polymer dopant or zinc-driven self-assembling building block. Inorg. Chem. Commun. 2019, 104, 145–149.
- Hartley, G.S. The cis-form of azobenzene. Nature 1937, 140, 281.
- Di Martino, M.; Sessa, L.; Di Matteo, M.; Panunzi, B.; Piotto, S.; Concilio, S. Azobenzene as Antimicrobial Molecules. Molecules 2022, 27, 5643.
- Diana, R.; Caruso, U.; Piotto, S.; Concilio, S.; Shikler, R.; Panunzi, B. Spectroscopic behaviour of two novel azobenzene fluorescent dyes and their polymeric blends. Molecules 2020, 25, 1368.
- Diana, R.; Panunzi, B.; Concilio, S.; Marrafino, F.; Shikler, R.; Caruso, T.; Caruso, U. The Effect of Bulky Substituents on Two π-Conjugated Mesogenic Fluorophores. Their Organic Polymers and Zinc-Bridged Luminescent Networks. Polymers 2019, 11, 1379.
- Jerca, F.A.; Jerca, V.V.; Hoogenboom, R. Advances and opportunities in the exciting world of azobenzenes. Nat. Rev. Chem. 2022, 6, 51–69.
- Knizia, G. Intrinsic Atomic Orbitals: An Unbiased Bridge between Quantum Theory and Chemical Concepts. J. Chem. Theory Comput. 2013, 9, 4834–4843.
- Piotto, S.; Concilio, S.; Sessa, L.; Diana, R.; Torrens, G.; Juan, C.; Caruso, U.; Iannelli, P. Synthesis and Antimicrobial Studies of New Antibacterial Azo-Compounds Active against Staphylococcus aureus and Listeria monocytogenes. Molecules 2017, 22, 1372.
- Piotto, S.; Concilio, S.; Sessa, L.; Porta, A.; Calabrese, E.C.; Zanfardino, A.; Varcamonti, M.; Iannelli, P. Small azobenzene derivatives active against bacteria and fungi. Eur. J. Med. Chem. 2013, 68, 178–184.
- Sessa, L.; Concilio, S.; Iannelli, P.; De Santis, F.; Porta, A.; Piotto, S. Antimicrobial Azobenzene Compounds and Their Potential Use in Biomaterials. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; p. 020018.
- Sessa, L.; Concilio, S.; Walde, P.; Robinson, T.; Dittrich, P.S.; Porta, A.; Panunzi, B.; Caruso, U.; Piotto, S. Study of the Interaction of a Novel Semi-Synthetic Peptide with Model Lipid Membranes. Membranes 2020, 10, 294.
- Concilio, S.; Sessa, L.; Petrone, A.M.; Porta, A.; Diana, R.; Iannelli, P.; Piotto, S. Structure Modification of an Active Azo-Compound as a Route to New Antimicrobial Compounds. Molecules 2017, 22, 875.
- Pichon, R.; Le Saint, J.; Courtot, P. Photoisomerisation d’arylhydrazones-2 de dicetones-1,2 substituees en 2.: Mecanisme d’isomerisation thermique de la double liaison C N. Tetrahedron 1981, 37, 1517–1524.
- Xu, F.; Feringa, B.L. Photoresponsive Supramolecular Polymers: From Light-controlled Small Molecules to Smart Materials. Adv. Mater. 2022, 35, 2204413.
- Jeong, M.; Park, J.; Seo, Y.; Lee, K.; Pramanik, S.; Ahn, S.; Kwon, S. Hydrazone Photoswitches for Structural Modulation of Short Peptides. Chem.-A Eur. J. 2022, 28, e202103972.
- Irie, M. Diarylethenes for Memories and Switches. Chem. Rev. 2000, 100, 1685–1716.
- Irie, M.; Fukaminato, T.; Matsuda, K.; Kobatake, S. Photochromism of Diarylethene Molecules and Crystals: Memories, Switches, and Actuators. Chem. Rev. 2014, 114, 12174–12277.
- Cheng, H.B.; Zhang, S.; Bai, E.; Cao, X.; Wang, J.; Qi, J.; Liu, J.; Zhao, J.; Zhang, L.; Yoon, J. Future-Oriented Advanced Diarylethene Photoswitches: From Molecular Design to Spontaneous Assembly Systems. Adv. Mater. 2022, 34, 2108289.
- Fischer, E.; Hirshberg, Y. Formation of Coloured Forms of Spirans by Low-Temperature Irradiation; Royal Soc Chemistry Thomas Graham House: Cambridge, UK, 1952; pp. 4522–4524.
- Fagan, A.; Bartkowski, M.; Giordani, S. Spiropyran-Based Drug Delivery Systems. Front. Chem. 2021, 9, 720087.
- Cardano, F.; Del Canto, E.; Giordani, S. Spiropyrans for light-controlled drug delivery. Dalton Trans. 2019, 48, 15537–15544.
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
3 times
(View History)
Update Date:
16 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




