Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Raghad AL-Ishaq | -- | 1514 | 2023-05-12 10:02:50 | | | |
| 2 | Catherine Yang | -149 word(s) | 1365 | 2023-05-15 05:39:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Al-Ishaq, R.K.; Samuel, S.M.; Büsselberg, D. The Influence of Specific Microbial Species on Diabetes. Encyclopedia. Available online: https://encyclopedia.pub/entry/44193 (accessed on 04 March 2026).
Al-Ishaq RK, Samuel SM, Büsselberg D. The Influence of Specific Microbial Species on Diabetes. Encyclopedia. Available at: https://encyclopedia.pub/entry/44193. Accessed March 04, 2026.
Al-Ishaq, Raghad Khalid, Samson Mathews Samuel, Dietrich Büsselberg. "The Influence of Specific Microbial Species on Diabetes" Encyclopedia, https://encyclopedia.pub/entry/44193 (accessed March 04, 2026).
Al-Ishaq, R.K., Samuel, S.M., & Büsselberg, D. (2023, May 12). The Influence of Specific Microbial Species on Diabetes. In Encyclopedia. https://encyclopedia.pub/entry/44193
Al-Ishaq, Raghad Khalid, et al. "The Influence of Specific Microbial Species on Diabetes." Encyclopedia. Web. 12 May, 2023.
Copy Citation
Diabetes mellitus (DM) is a metabolic disorder with an alarming incidence rate and a considerable burden on the patient’s life and health care providers. An increase in blood glucose level and insulin resistance characterizes it. Internal and external factors such as urbanization, obesity, and genetic mutations could increase the risk of DM. Microbes in the gut influence overall health through immunity and nutrition. More studies have been conducted to evaluate and estimate the role of the gut microbiome in diabetes development, progression, and management.
Bifidobacterium adolescentis
Bifidobacterium bifidum
diabetes
gut
Lactobacillus rhamnosus
1. Diabetes Management Using Microbial Species
The development of diabetes is associated with profound gut dysbiosis [1]. Restoring the balance of gut microbiome composition by administering probiotics (live non-pathogenic microorganisms) in an adequate concentration has been reported to improve diabetes [2]. Several human and non-human studies reported the influence of using probiotics for diabetes. For example, in diabetic patients given yogurt containing L. acidophilus La5 and B. lactis Bb12 as probiotics, fasting blood glucose, insulin, insulin resistance, and glycosylated hemoglobin levels were reduced [3]. A meta-analysis of 520 type 2 diabetic patients reported that probiotic administration improved glycemic control and lipid metabolism [4]. Probiotic administration also influences oxidative status and inflammatory parameters in diabetic patients [5]. Seventy participants with diabetes were given probiotics for a month which significantly reduced the levels of IL-6, IL-1, IL-8, and TNF-a compared to the control group [6]. The data showed how probiotic administration influenced the inflammatory response in participants with diabetes. The administration of Lactobacillus for two months reduces uric nitrogen in the blood [7]. Additionally, probiotic administration influenced and regulated the level of glycated hemoglobin, total cholesterol, triglycerides, and low-density lipoprotein cholesterol in pre-clinical diabetes [8]. On the other hand, similar results were observed when the probiotic was given to animal models. The administration of Bifidobacterium for one month and Lactobacillus for three months in a mouse model with type 2 diabetes was reported to normalize glucose metabolism and insulin sensitivity [9][10]. One of the concerns with probiotic treatment is safety and tolerability. All the mentioned studies reported no adverse effects and probiotic usage was safe. Despite that, more efforts are required to standardize the protocol and estimate the proper dosage.
2. Bifidobacterium adolescentis
Bifidobacteria are Gram-positive, non-spore-forming, and non-motile bacteria known to be the first colonizer of the infant gut [11]. Their presence in the gut has been linked to several beneficial effects on the host as they prevent intestinal inflammation, colonic adenomas, and cancer [12]. Bifidobacterium adolescentis is a vital gut flora in adults [13][14]. In patients with type 2 diabetes, the abundance of B. adolescentis in the intestine is significantly reduced [15]. Using B. adolescentis (1 × 108 cfu/mL) daily on twenty volunteers aged 50 to 60 for thirty days as a supplementation alleviates gut microbiome disorder and reduces blood glucose [16].
Additionally, administering eight strains of B. adolescentis (2 × 108 cfu/mL) for 12 weeks in diabetic mice restored gut microbiome homeostasis, alleviated inflammation, and increased the abundance of short-chain fatty acid-producing microorganisms [17]. Moreover, supplementing B. adolescentis (5 × 108 cfu/mL) in mice fed a high-fat diet daily for twelve weeks improved insulin sensitivity and reduced visceral fat accumulation [18]. Unfortunately, the literature lacks more data that support or challenge the observed beneficial effects of B. adolescentis administration in diabetes. Furthermore, protocol standardization is required to ensure the safety and efficacy of using such an approach. Figure 1 highlights the main pathways affected by B. adolescentis administration in diabetes.
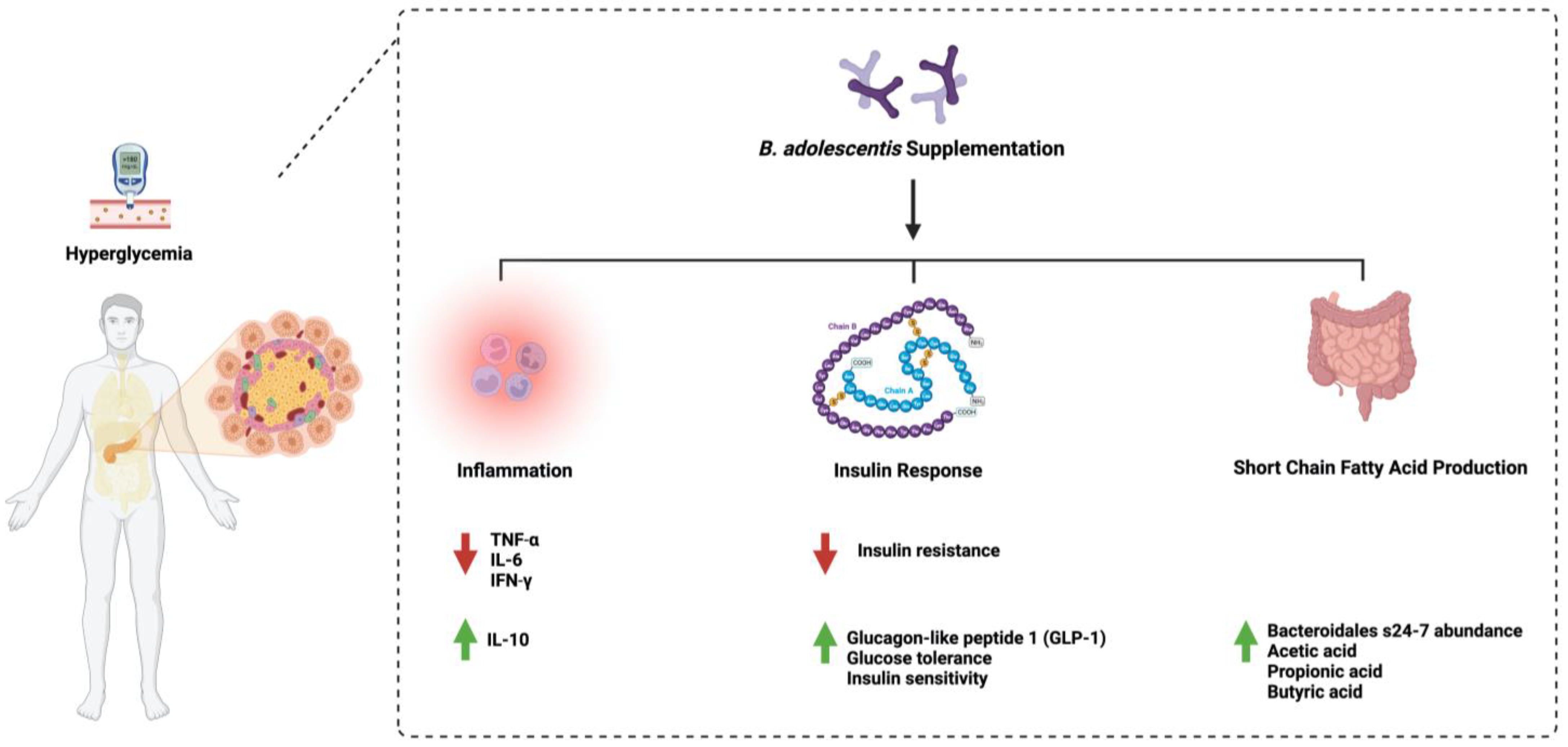
Figure 1. Overview illustration of Bifidobacterium adolescentis on diabetes. The figure shows the three main pathways: inflammation, insulin response, and the production of short-chain fatty acids by microorganisms. Created with BioRender.com.
3. Bifidobacterium bifidum
Bifidobacterium bifidum is one species of naturally occurring microbiota detected in breastfed infants [19]. It is considered a dominant resident of the gut population [20]. B. bifidum consists of 3000 genes that encode carbohydrate enzymes such as glycosyl transferases (GTs), glycosyl hydrolases (GHs), and carbohydrate esterases (CEs) [21]. This showed the ability of B. bifidum to metabolize host-derived glycans such as human milk oligosaccharides and mucin [22]. Using B. bifidum in diabetes management has started to gain more scientific attention recently. A single administration dosage of 1 × 107 cfu/mL daily for 28 days reduced fasting blood glucose, glycosylated hemoglobin, triglycerides (TG), and total cholesterol in Wistar rats [23]. Additionally, diabetic patients treated with a collection of probiotics, including B. bifidum (2 × 109 cfu/mL) daily for 12 weeks, significantly decreased insulin resistance, fasting blood glucose, and increased insulin sensitivity and HDL cholesterol level. It also improved the total antioxidant capacity and reduced the C-reactive protein level [24]. The combination treatment of different Bifidobacterium spp., including B. bifidum and excluding B. adolescentis, ameliorated insulin resistance and reduced blood glucose levels in mice [25]. More studies are needed to evaluate how B. bifidum manages diabetes. Additionally, studies that address the influence of B. bifidum and B. adolescentis may be essential for better treatment outcomes. Figure 2 highlights the main pathways affected by B. bifidum administration in diabetes.
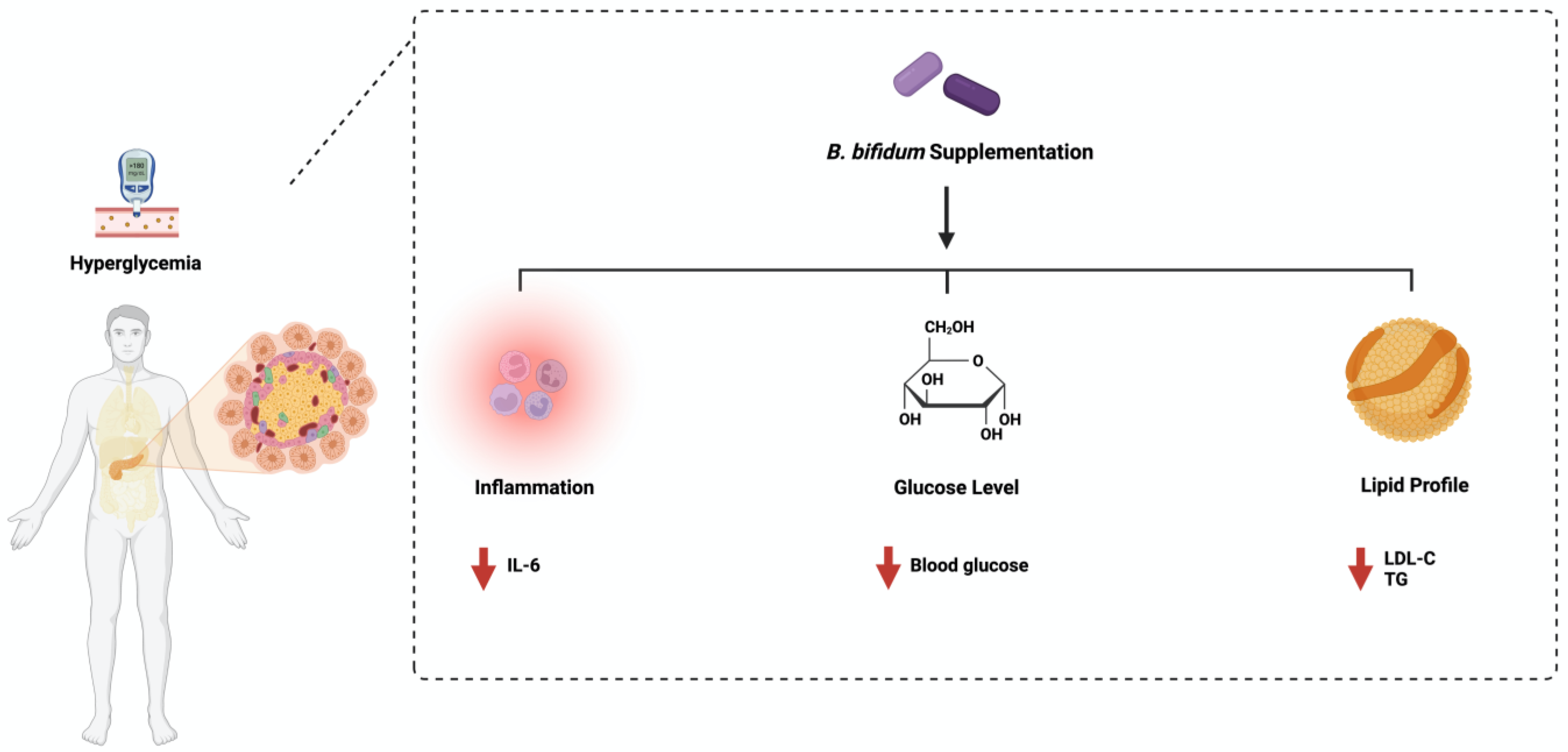
Figure 2. Overview illustration of Bifidobacterium bifidum on diabetes. The figure shows the three main pathways: inflammation, insulin response, and lipid profile. Created with BioRender.com.
4. Lactobacillus rhamnosus
Lactobacillus rhamnosus was first isolated in 1983 and is known for its ability to resist stomach acidity and strong avidity for intestinal cells. It has been widely used in targeting multiple pathological conditions, such as cancer, as an effective probiotic [26]. Administering L. rhamnosus daily (1 × 108 cfu/mL) in rodents for four weeks improved glucose tolerance by reducing endoplasmic reticulum stress [27]. Additionally, in mice fed a high-fat diet, treating 109 cfu/mL of L. rhamnosus daily significantly reduced the insulin level and fasting blood glucose. It also reduced proinflammatory cytokines such as IL-6 and TNF-a [28].
Furthermore, oral administration of L. rhamnosus improved glucose tolerance in diabetic rats by downregulating the expression of glucose 6 phosphatase [29]. The administration of L. rhamnosus to diabetic mice reduced insulin, glycosylated hemoglobin, and fasting blood glucose levels and increased glucagon-like peptide 1 levels in serum [30]. Similar results were obtained when 3 month old male Zebrafish were used [31]. These observations show the urgent need for protocol standardization and model specification to estimate the beneficial effect of L. rhamnosus in diabetes. Figure 3 highlights the main pathways affected by L. rhamnosus administration in diabetes.
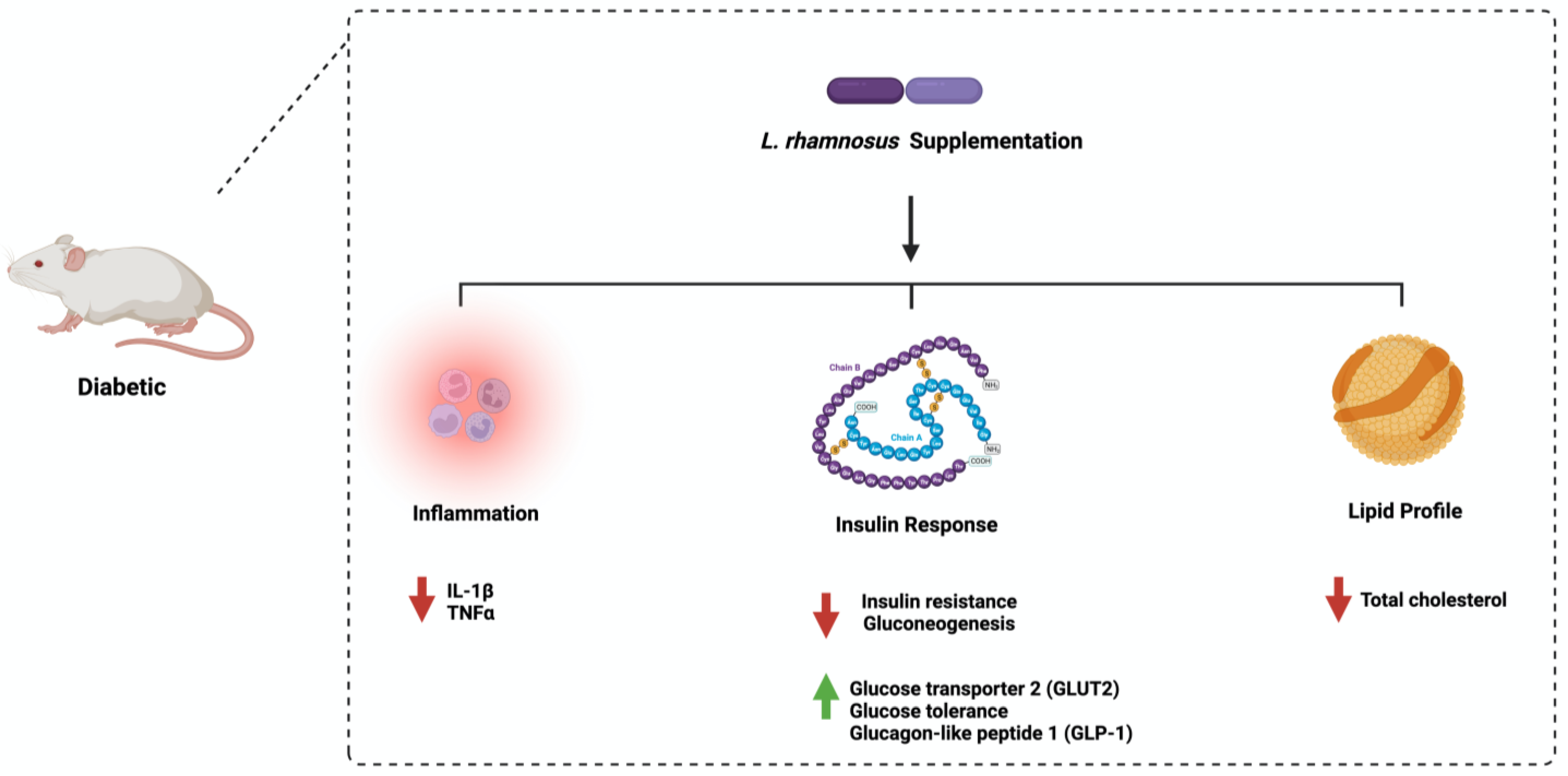
Figure 4. Overview illustration of Lactobacillus rhamnosus on diabetes. The figure shows the three main pathways: inflammation, insulin response, and lipid profile. Created with BioRender.com.
5. The Influence of Gut Microbial Species on Diabetes Mellitus
Diabetes is a global metabolic condition with a high incidence rate worldwide. Developing new and improved therapeutic approaches to target the disease and its complications is necessary. The gut microbiota has been linked recently to diabetes. Here, the researchers searched the literature and reported the role played by the three commonly addressed microbial species on diabetes: Bifidobacterium adolescentis, Bifidobacterium bifidum, and Lactobacillus rhamnosus. Both animal and human studies reported the influence of Bifidobacterium adolescentis administration on blood glucose level, an abundance of short-chain fatty acids, and inflammatory response in a dose dependent manner that ranges from 1 × 108 to 5 × 108 CFU/mL. Moreover, administering Bifidobacterium bifidum (1 × 107–2 × 109 CFU/mL) reduced fasting blood glucose, insulin resistance, and improved sensitivity in human participants with diabetes and animal models. Unfortunately, this is not the case with Lactobacillus rhamnosus as most of the available studies reported the role of this species on diabetes in animal models only. Despite that, the data support the positive influence of this species on insulin resistance and lipid profile.
Throughout the literature, the researchers observed the lack of standardization regarding the protocol followed, the model used, the diet used to induce diabetes in animal models, and the mode of administration, as most studies followed oral or intraperitoneal administration. Establishing standardized protocols that specify specific guidelines will help further advance the field. Additionally, the literature shows that many studies investigate a single microbial species. The gut microbiome is a community of microorganisms interacting with each other and the host. Isolating and investigating a single organism only might not be of great interest. As a starting point, a study may investigate the influence of the three bacterial species mentioned here on diabetes in human and animal models and report the challenges and limitations. By doing so, the researchers can then, step by step, look at the gut microbiome as a community in the context of health and diseases. The following sections highlight some essential topics that need further discussion and research for better treatment outcomes.
References
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521.
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767.
- Shah, N.; Swami, O. Role of probiotics in diabetes: A review of their rationale and efficacy. EMJ Diabetes 2017, 5, 104–110.
- Zhang, Q. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina 2016, 52, 28–34.
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543.
- Mykhal’chyshyn, H.P. Effect of probiotics on proinflammatory cytokines level in patients with type 2 diabetes and nonalcoholic fatty liver disease. Likars’ ka Sprava 2013, 56–62.
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2021, 14, 166.
- Li, Y.; Wu, Y.; Wu, L.; Qin, L.; Liu, T. The effects of probiotic administration on patients with prediabetes: A meta-analysis and systematic review. J. Transl. Med. 2022, 20, 498.
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011, 3, 559–572.
- Yun, S.; Park, H.; Kang, J. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J. Appl. Microbiol. 2009, 107, 1681–1686.
- Turroni, F.; Peano, C.; Pass, D.A.; Foroni, E.; Severgnini, M.; Claesson, M.J.; Ventura, M. Diversity of bifidobacteria within the infant gut microbiota. PLoS ONE 2012, 7, e36957.
- Picard, C.; Fioramonti, J.; Francois, A.; Robinson, T.; Neant, F.; Matuchansky, C. Review article: Bifidobacteria as probiotic agents—Physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 2005, 22, 495–512.
- Turroni, F.; van Sinderen, D.; Ventura, M. Genomics and ecological overview of the genus Bifidobacterium. Int. J. Food Microbiol. 2011, 149, 37–44.
- Jung, D.H.; Chung, W.H.; Seo, D.H.; Kim, Y.J.; Nam, Y.D.; Park, C.S. Complete genome sequence of Bifidobacterium adolescentis P2P3, a human gut bacterium possessing strong resistant starch-degrading activity. 3 Biotech 2020, 10, 31.
- Sedighi, M.; Razavi, S.; Navab-Moghadam, F.; Khamseh, M.E.; Alaei-Shahmiri, F.; Mehrtash, A.; Amirmozafari, N. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb. Pathog. 2017, 111, 362–369.
- Moroti, C.; Souza Magri, L.F.; de Rezende Costa, M.; Cavallini, D.C.; Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012, 11, 29.
- Qian, X.; Si, Q.; Lin, G.; Zhu, M.; Lu, J.; Zhang, H.; Chen, W. Bifidobacterium adolescentis Is Effective in Relieving Type 2 Diabetes and May Be Related to Its Dominant Core Genome and Gut Microbiota Modulation Capacity. Nutrients 2022, 14, 2479.
- Chen, J.; Wang, R.; Li, X.F.; Wang, R.L. Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br. J. Nutr. 2012, 107, 1429–1434.
- Ku, S.; Park, M.S.; Ji, G.E.; You, H.J. Review on Bifidobacterium bifidum BGN4: Functionality and Nutraceutical Applications as a Probiotic Microorganism. Int. J. Mol. Sci. 2016, 17, 1544.
- Tannock, G.W.; Lawley, B.; Munro, K.; Gowri Pathmanathan, S.; Zhou, S.J.; Makrides, M.; Hodgkinson, A.J. Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk-based formula, or breast milk. Appl. Environ. Microbiol. 2013, 79, 3040–3048.
- Turroni, F.; Duranti, S.; Milani, C.; Lugli, G.A.; van Sinderen, D.; Ventura, M. Bifidobacterium bifidum: A Key Member of the Early Human Gut Microbiota. Microorganisms 2019, 7, 544.
- Turroni, F.; Bottacini, F.; Foroni, E.; Mulder, I.; Kim, J.H.; Zomer, A.; Ventura, M. Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proc. Natl. Acad. Sci. USA 2010, 107, 19514–19519.
- Sharma, P.; Bhardwaj, P.; Singh, R. Administration of Lactobacillus casei and Bifidobacterium bifidum Ameliorated Hyperglycemia, Dyslipidemia, and Oxidative Stress in Diabetic Rats. Int. J. Prev. Med. 2016, 7, 102.
- Raygan, F.; Rezavandi, Z.; Bahmani, F.; Ostadmohammadi, V.; Mansournia, M.A.; Tajabadi-Ebrahimi, M.; Asemi, Z. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol. Metab. Syndr. 2018, 10, 51.
- Le, T.K.; Hosaka, T.; Le, T.T.; Nguyen, T.G.; Tran, Q.B.; Le, T.H.; Pham, X.D. Oral administration of Bifidobacterium spp. improves insulin resistance, induces adiponectin, and prevents inflammatory adipokine expressions. Biomed. Res. 2014, 35, 303–310.
- Capurso, L. Thirty Years of Lactobacillus rhamnosus GG: A Review. J. Clin. Gastroenterol. 2019, 53 (Suppl. 1), S1–S41.
- Park, K.Y.; Kim, B.; Hyun, C.K. Lactobacillus rhamnosus GG improves glucose tolerance through alleviating ER stress and suppressing macrophage activation in db/db mice. J. Clin. Biochem. Nutr. 2015, 56, 240–246.
- Han, M.; Liao, W.; Dong, Y.; Bai, C.; Gai, Z. Lacticaseibacillus rhamnosus Hao9 exerts antidiabetic effects by regulating gut microbiome, glucagon metabolism, and insulin levels in type 2 diabetic mice. Front. Nutr. 2022, 9, 1081778.
- Farida, E.; Nuraida, L.; Giriwono, P.E.; Jenie, B.S.L. Lactobacillus rhamnosus Reduces Blood Glucose Level through Downregulation of Gluconeogenesis Gene Expression in Streptozotocin-Induced Diabetic Rats. Int. J. Food Sci. 2020, 2020, 6108575.
- Yan, F.; Li, N.; Yue, Y.; Wang, C.; Zhao, L.; Evivie, S.E.; Huo, G. Screening for Potential Novel Probiotics with Dipeptidyl Peptidase IV-Inhibiting Activity for Type 2 Diabetes Attenuation in vitro and in vivo. Front. Microbiol. 2019, 10, 2855.
- Bootorabi, F.; Saadat, F.; Falak, R.; Manouchehri, H.; Changizi, R.; Mohammadi, H.; Khorramizadeh, M.R. Gut micobiota alteration by Lactobacillus rhamnosus reduces proinflammatory cytokines and glucose level in the adult model of Zebrafish. BMC Res. Notes 2021, 14, 302.
More
Information
Subjects:
Microbiology; Endocrinology & Metabolism
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
15 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





