Diabetes mellitus (DM) is a metabolic disorder with an alarming incidence rate and a considerable burden on the patient’s life and health care providers. An increase in blood glucose level and insulin resistance characterizes it. Internal and external factors such as urbanization, obesity, and genetic mutations could increase the risk of DM. Microbes in the gut influence overall health through immunity and nutrition. Recently, more studies have been conducted to evaluate and estimate the role of the gut microbiome in diabetes development, progression, and management. This review summarizes the current knowledge addressing three main bacterial species: Bifidobacterium adolescentis, Bifidobacterium bifidum, and Lactobacillus rhamnosus and their influence on diabetes and its underlying molecular mechanisms. Most studies illustrate that using those bacterial species positively reduces blood glucose levels and activates inflammatory markers. Additionally, we reported the relationship between those bacterial species and metformin, one of the commonly used antidiabetic drugs. Overall, more research is needed to understand the influence of the gut microbiome on the development of diabetes. Furthermore, more efforts are required to standardize the model used, concentration ranges, and interpretation tools to advance the field further.
- Bifidobacterium adolescentis
- Bifidobacterium bifidum
- diabetes
- gut
- Lactobacillus rhamnosus
Diabetes Management Using Microbial Species
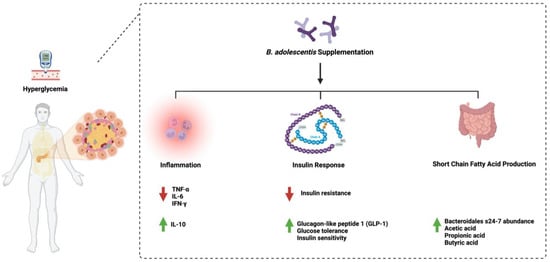
1. Bifidobacterium adolescentis
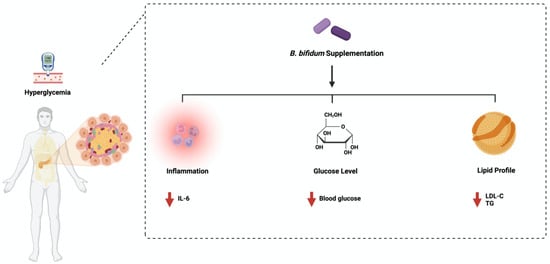
2. Bifidobacterium bifidum
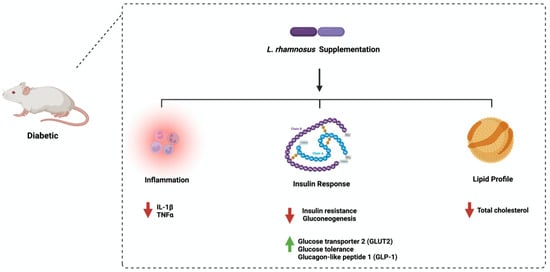
3. Lactobacillus rhamnosus
Discussion
This entry is adapted from the peer-reviewed paper 10.3390/ijms24098118
References
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521.
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767.
- Shah, N.; Swami, O. Role of probiotics in diabetes: A review of their rationale and efficacy. EMJ Diabetes 2017, 5, 104–110.
- Zhang, Q. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina 2016, 52, 28–34.
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543.
- Mykhal’chyshyn, H.P. Effect of probiotics on proinflammatory cytokines level in patients with type 2 diabetes and nonalcoholic fatty liver disease. Likars’ ka Sprava 2013, 56–62.
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2021, 14, 166.
- Li, Y.; Wu, Y.; Wu, L.; Qin, L.; Liu, T. The effects of probiotic administration on patients with prediabetes: A meta-analysis and systematic review. J. Transl. Med. 2022, 20, 498.
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: Molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011, 3, 559–572.
- Yun, S.; Park, H.; Kang, J. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J. Appl. Microbiol. 2009, 107, 1681–1686.
- Turroni, F.; Peano, C.; Pass, D.A.; Foroni, E.; Severgnini, M.; Claesson, M.J.; Ventura, M. Diversity of bifidobacteria within the infant gut microbiota. PLoS ONE 2012, 7, e36957.
- Picard, C.; Fioramonti, J.; Francois, A.; Robinson, T.; Neant, F.; Matuchansky, C. Review article: Bifidobacteria as probiotic agents—Physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 2005, 22, 495–512.
- Turroni, F.; van Sinderen, D.; Ventura, M. Genomics and ecological overview of the genus Bifidobacterium. Int. J. Food Microbiol. 2011, 149, 37–44.
- Jung, D.H.; Chung, W.H.; Seo, D.H.; Kim, Y.J.; Nam, Y.D.; Park, C.S. Complete genome sequence of Bifidobacterium adolescentis P2P3, a human gut bacterium possessing strong resistant starch-degrading activity. 3 Biotech 2020, 10, 31.
- Sedighi, M.; Razavi, S.; Navab-Moghadam, F.; Khamseh, M.E.; Alaei-Shahmiri, F.; Mehrtash, A.; Amirmozafari, N. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb. Pathog. 2017, 111, 362–369.
- Moroti, C.; Souza Magri, L.F.; de Rezende Costa, M.; Cavallini, D.C.; Sivieri, K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis. 2012, 11, 29.
- Qian, X.; Si, Q.; Lin, G.; Zhu, M.; Lu, J.; Zhang, H.; Chen, W. Bifidobacterium adolescentis Is Effective in Relieving Type 2 Diabetes and May Be Related to Its Dominant Core Genome and Gut Microbiota Modulation Capacity. Nutrients 2022, 14, 2479.
- Chen, J.; Wang, R.; Li, X.F.; Wang, R.L. Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br. J. Nutr. 2012, 107, 1429–1434.
- Ku, S.; Park, M.S.; Ji, G.E.; You, H.J. Review on Bifidobacterium bifidum BGN4: Functionality and Nutraceutical Applications as a Probiotic Microorganism. Int. J. Mol. Sci. 2016, 17, 1544.
- Tannock, G.W.; Lawley, B.; Munro, K.; Gowri Pathmanathan, S.; Zhou, S.J.; Makrides, M.; Hodgkinson, A.J. Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk-based formula, or breast milk. Appl. Environ. Microbiol. 2013, 79, 3040–3048.
- Turroni, F.; Duranti, S.; Milani, C.; Lugli, G.A.; van Sinderen, D.; Ventura, M. Bifidobacterium bifidum: A Key Member of the Early Human Gut Microbiota. Microorganisms 2019, 7, 544.
- Turroni, F.; Bottacini, F.; Foroni, E.; Mulder, I.; Kim, J.H.; Zomer, A.; Ventura, M. Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proc. Natl. Acad. Sci. USA 2010, 107, 19514–19519.
- Sharma, P.; Bhardwaj, P.; Singh, R. Administration of Lactobacillus casei and Bifidobacterium bifidum Ameliorated Hyperglycemia, Dyslipidemia, and Oxidative Stress in Diabetic Rats. Int. J. Prev. Med. 2016, 7, 102.
- Raygan, F.; Rezavandi, Z.; Bahmani, F.; Ostadmohammadi, V.; Mansournia, M.A.; Tajabadi-Ebrahimi, M.; Asemi, Z. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol. Metab. Syndr. 2018, 10, 51.
- Le, T.K.; Hosaka, T.; Le, T.T.; Nguyen, T.G.; Tran, Q.B.; Le, T.H.; Pham, X.D. Oral administration of Bifidobacterium spp. improves insulin resistance, induces adiponectin, and prevents inflammatory adipokine expressions. Biomed. Res. 2014, 35, 303–310.
- Capurso, L. Thirty Years of Lactobacillus rhamnosus GG: A Review. J. Clin. Gastroenterol. 2019, 53 (Suppl. 1), S1–S41.
- Park, K.Y.; Kim, B.; Hyun, C.K. Lactobacillus rhamnosus GG improves glucose tolerance through alleviating ER stress and suppressing macrophage activation in db/db mice. J. Clin. Biochem. Nutr. 2015, 56, 240–246.
- Han, M.; Liao, W.; Dong, Y.; Bai, C.; Gai, Z. Lacticaseibacillus rhamnosus Hao9 exerts antidiabetic effects by regulating gut microbiome, glucagon metabolism, and insulin levels in type 2 diabetic mice. Front. Nutr. 2022, 9, 1081778.
- Farida, E.; Nuraida, L.; Giriwono, P.E.; Jenie, B.S.L. Lactobacillus rhamnosus Reduces Blood Glucose Level through Downregulation of Gluconeogenesis Gene Expression in Streptozotocin-Induced Diabetic Rats. Int. J. Food Sci. 2020, 2020, 6108575.
- Yan, F.; Li, N.; Yue, Y.; Wang, C.; Zhao, L.; Evivie, S.E.; Huo, G. Screening for Potential Novel Probiotics with Dipeptidyl Peptidase IV-Inhibiting Activity for Type 2 Diabetes Attenuation in vitro and in vivo. Front. Microbiol. 2019, 10, 2855.
- Bootorabi, F.; Saadat, F.; Falak, R.; Manouchehri, H.; Changizi, R.; Mohammadi, H.; Khorramizadeh, M.R. Gut micobiota alteration by Lactobacillus rhamnosus reduces proinflammatory cytokines and glucose level in the adult model of Zebrafish. BMC Res. Notes 2021, 14, 302.
