
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tran Thi Ngoc Anh | -- | 2394 | 2023-05-10 13:59:29 | | | |
| 2 | Lindsay Dong | Meta information modification | 2394 | 2023-05-11 02:59:42 | | |
Video Upload Options
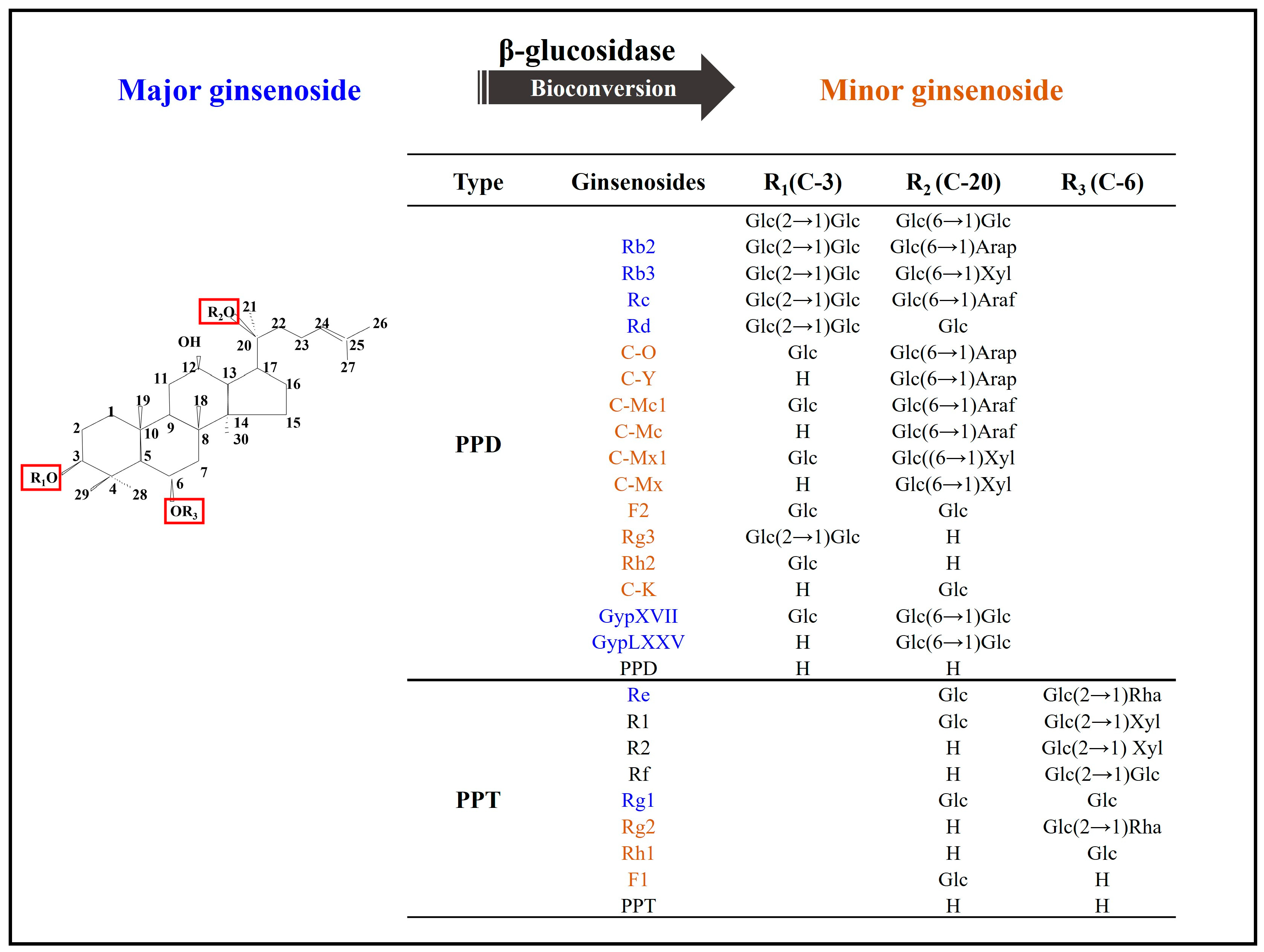
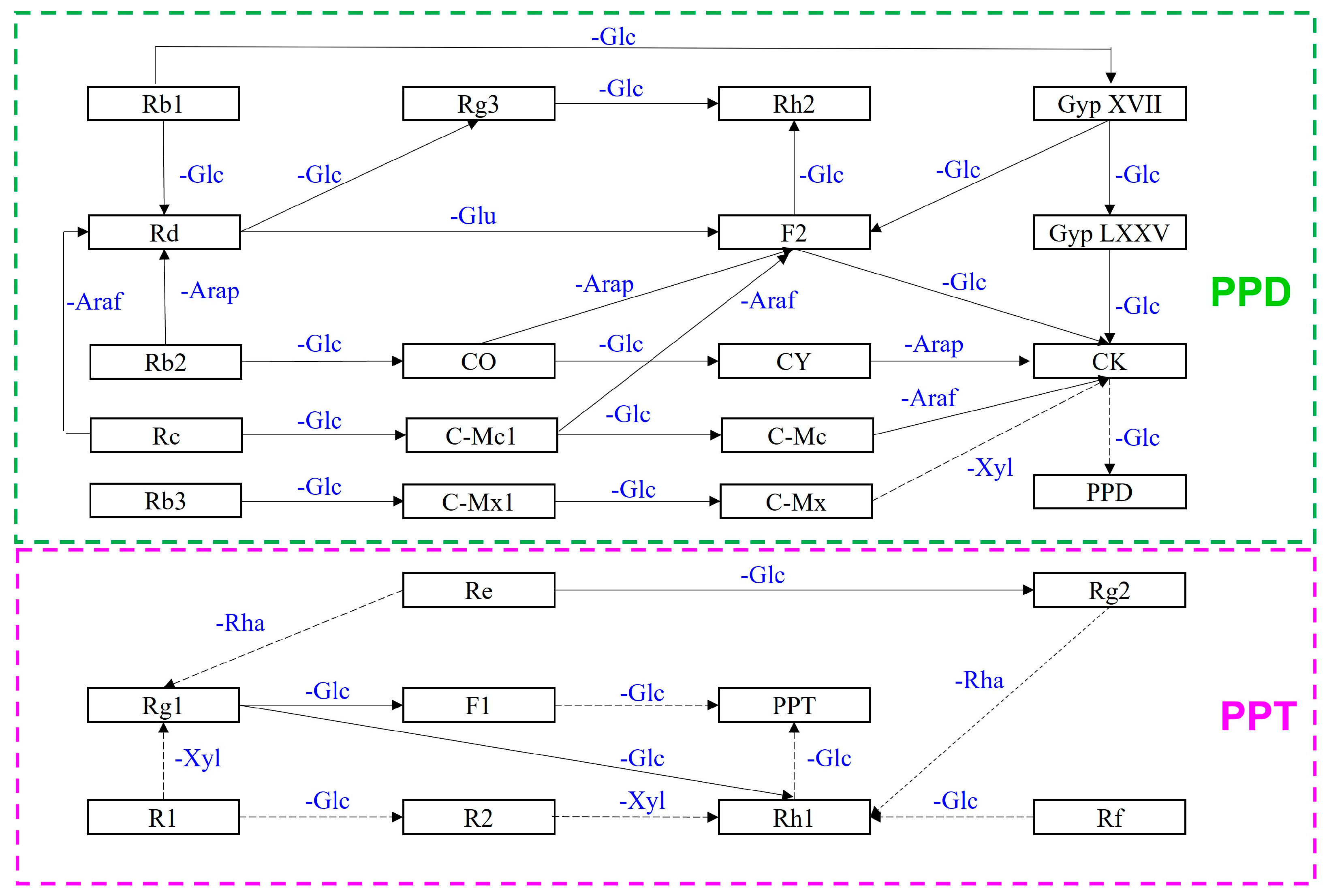
Ginsenosides are a group of bioactive compounds isolated from Panax ginseng. Conventional major ginsenosides have a long history of use in traditional medicine for both illness prevention and therapy. Bioconversion processes have the potential to create new and valuable products in pharmaceutical and biological activities, making them both critical for research and highly economic to implement. This has led to an increase in the number of studies that use major ginsenosides as a precursor to generate minor ones using β-glucosidase. Minor ginsenosides may also have useful properties but are difficult to isolate from raw ginseng because of their scarcity. Bioconversion processes have the potential to create novel minor ginsenosides from the more abundant major ginsenoside precursors in a cost-effective manner. While numerous bioconversion techniques have been developed, an increasing number of studies have reported that β-glucosidase can effectively and specifically generate minor ginsenosides.
1. Ginseng and Ginsenosides
2. β-Glucosidases and Their Functions
3. Bioconversion of Ginsenosides
4. β-Glucosidases Applications in Bioconversion of Ginsenosides
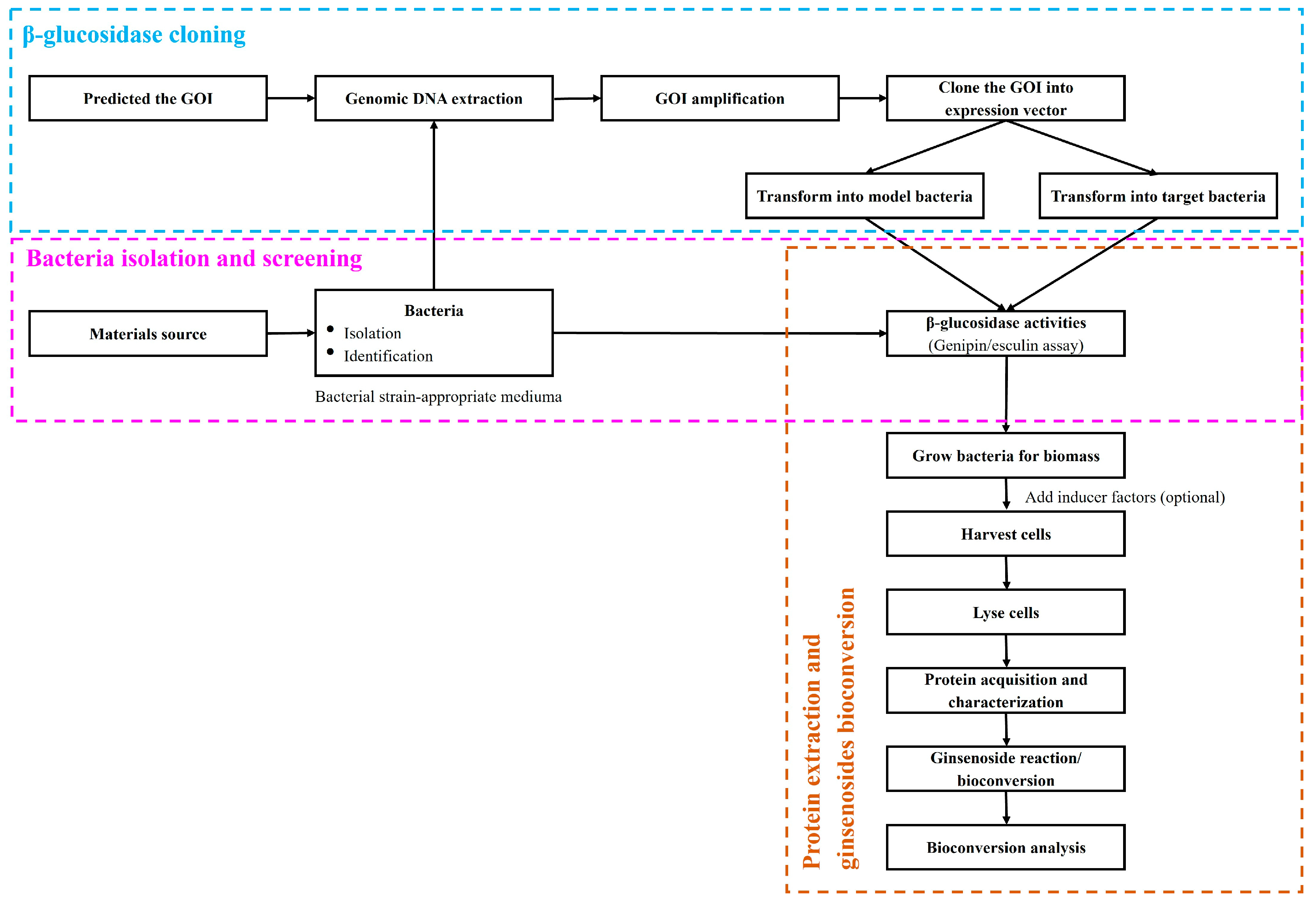
5. Bioconversion of Ginsenosides by β-Glucosidase Enzymes Obtained from Microorganism
6. Bioconversion of Ginsenoside by Recombinant β-Glucosidases
7. Other Methods for Ginsenoside Bioconversion
References
- Lin, Y.; Hao, B.; Lu, Y.C.; Dong, Y.; Li, Y.; Zhang, G.H.; Yang, Z.J.; Xiang, G.S.; Liu, G.Z.; Li, X.J.; et al. PanaxGDB: A Comprehensive Platform for Panax. Front. Plant Sci. 2022, 13, 883818.
- Eom, S.J.; Kim, K.T.; Paik, H.D. Microbial bioconversion of ginsenosides in Panax ginseng and their improved bioactivities. Food Rev. Int. 2018, 34, 698–712.
- Yun, T.K. Brief introduction of Panax ginseng C.A. Meyer. J. Korean Med. Sci. 2001, 16, 16–18.
- Piao, X.; Zhang, H.; Kang, J.P.; Yang, D.U.; Li, Y.; Pang, S.; Jin, Y.; Yang, D.C.; Wang, Y. Advances in saponin diversity of panax ginseng. Molecules 2020, 25, 3452.
- Shin, K.C.; Oh, D.K. Classification of glycosidases that hydrolyze the specific positions and types of sugar moieties in ginsenosides. Crit. Rev. Biotechnol. 2016, 36, 1036–1049.
- An, C.; Ma, S.; Shi, X.; Liu, C.; Ding, H.; Xue, W. Diversity and Ginsenoside Biotransformation Potential of Cultivable Endophytic Fungi Associated With Panax bipinnatifidus var. bipinnatifidus in Qinling Mountains, China. Front. Pharmacol. 2022, 13, 762862.
- Huq, A.; Siraj, F.M.; Kim, Y.-J.; Yang, D.-C. Enzymatic transformation of ginseng leaf saponin by recombinant β-glucosidase (bgp1) and its efficacy in an adipocyte cell line. Biotechnol. Appl. Biochem. 2015, 63, 532–538.
- Kim, Y.J.; Zhang, D.; Yang, D.C. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv. 2015, 33, 717–735.
- Chu, L.L.; Bae, H. Bacterial endophytes from ginseng and their biotechnological application. J. Ginseng Res. 2022, 46, 1–10.
- Cairns, J.R.K.; Esen, A. β-Glucosidases. Cell Mol. Life Sci. 2010, 67, 3389–3405.
- Kim, D.; Kim, M.; Raña, G.S.; Han, J. Seasonal variation and possible biosynthetic pathway of ginsenosides in korean ginseng Panax ginseng meyer. Molecules 2018, 23, 1824.
- Piao, X.M.; Huo, Y.; Kang, J.P.; Mathiyalagan, R.; Zhang, H. Diversity of Ginsenoside Profiles Produced by Various Processing Technologies. Molecules 2020, 25, 4390.
- Ying, Z.; Awais, M.; Akter, R.; Xu, F.; Baik, S.; Jung, D.; Yang, D.C.; Kwak, G.Y.; Wenying, Y. Discrimination of Panax ginseng from counterfeits using single nucleotide polymorphism: A focused review. Front. Plant Sci. 2022, 13, 903306.
- Berrnard, H.; Gideon, D. Structure and sequence-base classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997, 7, 637–644.
- Henrissat, B.; Vegetales, M.; Grenoble, F. A classification of glycosyl hydrolases based sequence similarities amino acid. Biochem. J. 1991, 280, 309–316.
- Singhania, R.R.; Patel, A.K.; Pandey, A.; Ganansounou, E. Genetic modification: A tool for enhancing beta-glucosidase production for biofuel application. Bioresour. Technol. 2017, 245, 1352–1361.
- Hu, Y.; Zhai, L.; Hong, H.; Shi, Z.; Zhao, J.; Liu, D. Study on the Biochemical Characterization and Selectivity of Three β-Glucosidases From Bifidobacterium adolescentis ATCC15703. Front. Microbiol. 2022, 13, 860014.
- Hati, S.; Vij, S.; Singh, B.P.; Mandal, S. β-Glucosidase activity and bioconversion of isoflavones during fermentation of soymilk. J. Sci. Food Agric. 2015, 95, 216–220.
- Costa, R.; Domínguez, A.; Choupina, A. Cloning and expression analysis of an endo-1,3-β-d-glucosidase from Phytophthora cinnamomi. Mol. Biol. Rep. 2020, 47, 935–942.
- Kim, Y.S.; Lee, C.J.; Ma, J.Y. Enhancement of active compound, genipin, from Gardeniae Fructus using immobilized glycosyl hydrolase family 3 β-glucosidase from Lactobacillus antri. AMB Express 2017, 7, 64.
- Kim, W.Y.; Kim, J.M.; Han, S.B.; Lee, S.K.; Kim, N.D.; Park, M.K.; Kim, C.K.; Park, J.H. Steaming of ginseng at high temperature enhances biological activity. J. Nat. Prod. 2000, 63, 1702–1704.
- Zhang, S.; Xie, J.; Zhao, L.; Pei, J.; Su, E.; Xiao, W.; Wang, Z. Cloning, overexpression and characterization of a thermostable β-xylosidase from Thermotoga petrophila and cooperated transformation of ginsenoside extract to ginsenoside 20(S)-Rg3 with a β-glucosidase. Bioorg. Chem. 2019, 85, 159–167.
- Wei, Y.; Zhao, W.; Zhang, Q.; Zhao, Y.; Zhang, Y. Purification and characterization of a novel and unique ginsenoside Rg 1-hydrolyzing β-D-Glucosidase from Penicillium sclerotiorum. Acta Biochim. Biophys. Sin. (Shanghai) 2011, 43, 226–231.
- Amin, K.; Tranchimand, S.; Benvegnu, T.; Abdel-Razzak, Z.; Chamieh, H. Glycoside hydrolases and glycosyltransferases from hyperthermophilic archaea: Insights on their characteristics and applications in biotechnology. Biomolecules 2021, 11, 1557.
- Wang, D.D.; Kim, Y.J.; Beak, N.I.; Mathiyalagan, R.; Wang, C.; Jin, Y.; Xu, X.Y.; Yang, D.C. Glycosyltransformation of ginsenoside Rh2 into two novel ginsenosides using recombinant glycosyltransferase from Lactobacillus rhamnosus and its in vitro applications. Ginseng Res. 2021, 45, 48–57.
- Zhang, Y.; Yao, L.; Tang, C.; Jiang, J.; Ye, Y.; Liu, J. Qualitatively and quantitatively investigating the metabolism of 20(S)-protopanaxadiol-type ginsenosides by gut microbiota of different species. Biomed. Chromatogr. 2021, 35, e5219.
- Kim, S.Y.; Lee, H.N.; Hong, S.J.; Kang, H.J.; Cho, J.Y.; Kim, D.; Ameer, K.; Kim, Y.M. Enhanced biotransformation of the minor ginsenosides in red ginseng extract by Penicillium decumbens β-glucosidase. Enzym. Microb. Technol. 2022, 153, 109941.
- Du, J.; Cui, C.H.; Park, S.C.; Kim, J.K.; Yu, H.S.; Jin, F.X.; Sun, C.; Kim, S.C.; Im, W.T. Identification and characterization of a ginsenoside-transforming β-glucosidase from Pseudonocardia sp. Gsoil 1536 and its application for enhanced production of minor ginsenoside Rg2(S). PLoS ONE 2014, 9, e96914.
- Zhong, F.L.; Ma, R.; Jiang, M.; Dong, W.W.; Jiang, J.; Wu, S.; Li, D.; Quan, L.H. Cloning and characterization of ginsenoside-hydrolyzing β-glucosidase from lactobacillus brevis that transforms ginsenosides Rb1 and F2 into ginsenoside Rd and compound K. J. Microbiol. Biotechnol. 2016, 26, 1661–1667.
- Siddiqi, M.Z.; Cui, C.H.; Park, S.K.; Han, N.S.; Kim, S.C.; Im, W.T. Comparative analysis of the expression level of recombinant ginsenoside-transforming β-glucosidase in GRAS hosts and mass production of theginsenoside Rh2-Mix. PLoS ONE 2017, 12, e0176098.
- Siddiqi, M.Z.; Medjebouri, S.; Liu, Q.; Park, H.Y.; Kim, G.R.; Im, W.T. Efficient Production of Various Minor Ginsenosides from PPD- and PPT-type Major Ginsenosides Using a Single Recombinant BglFc Isolated from Flavobacterium chilense. Biotechnol. Bioprocess Eng. 2021, 26, 232–246.
- Wang, D.D.; Jin, Y.; Wang, C.; Kim, Y.J.; Zuly, E.J.P.; Baek, N.I.; Mathiyalagan, R.; Josua, M.; Yang, D.C. Rare ginsenoside Ia synthesized from F1 by cloning and overexpression of the UDP-glycosyltransferase gene from Bacillus subtilis: Synthesis, characterization, and in vitro melanogenesis inhibition activity in BL6B16 cells. J. Ginseng Res. 2018, 42, 42–49.
- Li, L.; Lee, S.J.; Yuan, Q.P.; Im, W.T.; Kim, S.C.; Han, N.S. Production of bioactive ginsenoside Rg3(S) and compound K using recombinant Lactococcus lactis. J. Ginseng Res. 2018, 42, 412–418.
- Li, W.N.; Fan, D. Di Biocatalytic strategies for the production of ginsenosides using glycosidase: Current state and perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 3807–3823.
- Zhang, R.; Huang, X.M.; Yan, H.J.; Liu, X.Y.; Zhou, Q.; Luo, Z.Y.; Tan, X.N.; Zhang, B.L. Highly selective production of compound k from ginsenoside rd by hydrolyzing glucose at c-3 glycoside using β-glucosidase of bifidobacterium breve atcc 15700. J. Microbiol. Biotechnol. 2019, 29, 410–418.
- Cui, C.H.; Jeon, B.M.; Fu, Y.; Im, W.T.; Kim, S.C. High-density immobilization of a ginsenoside-transforming β-glucosidase for enhanced food-grade production of minor ginsenosides. Appl. Microbiol. Biotechnol. 2019, 103, 7003–7015.
- Yang, W.; Zhou, J.; Harindintwali, J.D.; Yu, X. Production of minor ginsenosides by combining Stereum hirsutum and cellulase. PLoS ONE 2021, 16, e0255899.
- Qi, G.; Ji, B.; Zhang, Y.; Huang, L.; Wang, J.; Gao, W. Microbiome-based screening and co-fermentation of rhizospheric microorganisms for highly ginsenoside Rg3 production. Microbiol. Res. 2022, 261, 127054.
- Shin, K.C.; Kim, T.H.; Choi, J.H.; Oh, D.K. Complete Biotransformation of Protopanaxadiol-Type Ginsenosides to 20-O-β-Glucopyranosyl-20(S)-protopanaxadiol Using a Novel and Thermostable β-Glucosidase. J. Agric. Food Chem. 2018, 66, 2822–2829.
- Han, X.; Li, W.; Ma, X.; Fan, D. Enzymatic hydrolysis and extraction of ginsenoside recovered from deep eutectic solvent-salt aqueous two-phase system. J. Biosci. Bioeng. 2020, 130, 390–396.
- An, D.S.; Cui, C.H.; Siddiqi, M.Z.; Yu, H.S.; Jin, F.X.; Kim, S.G.; Im, W.T. Gram-scale production of ginsenoside F1 using a recombinant bacterial β-glucosidase. J. Microbiol. Biotechnol. 2017, 27, 1559–1565.
- Kim, H.W.; Han, S.H.; Lee, S.W.; Choi, H.S.; Suh, H.J.; Hong, K.B. Enzymatic hydrolysis increases ginsenoside content in Korean red ginseng (Panax ginseng CA Meyer) and its biotransformation under hydrostatic pressure. J. Sci. Food Agric. 2019, 99, 6806–6813.
- Park, B.; Hwang, H.; Lee, J.; Sohn, S.O.; Lee, S.H.; Jung, M.Y.; Lim, H.I.; Park, H.W.; Lee, J.H. Evaluation of ginsenoside bioconversion of lactic acid bacteria isolated from kimchi. J. Ginseng Res. 2017, 41, 524–530.
- Cheng, L.Q.; Na, J.R.; Kim, M.K.; Bang, M.H.; Yang, D.C. Microbial Conversion of Ginsenoside Rb1 to Minor Ginsenoside F2 and Gypenoside XVII by Intrasporangium sp. GS603 Isolated from Soil. J. Microbiol. 2007, 17, 1937–1943.
- Quan, L.H.; Piao, J.Y.; Min, I.W.; Yang, D.U.; Lee, H.N.; Yang, D.C. Bioconversion Of Ginsenoside Rb1 Into Compound K By Leuconostoc Citreum Lh1 Isolated From Kimchi. Braz. J. Microbiol. 2011, 42, 1227–1237.
- Hong, H.; Cui, C.H.; Kim, J.K.; Jin, F.X.; Kim, S.C.; Im, W.T. Enzymatic biotransformation of ginsenoside Rb1 and gypenoside XVII into ginsenosides Rd and F2 by recombinant β-glucosidase from Flavobacterium johnsoniae. J. Ginseng Res. 2012, 36, 418–424.
- Huq, M.A.; Kim, Y.J.; Min, J.W.; Bae, K.S.; Yang, D.C. Use of Lactobacillus rossiae DC05 for Bioconversion of the Major Ginsenosides Rb1 and Re into the Pharmacologically Active Ginsenosides C-K and Rg2. Food Sci. Biotechnol. 2014, 23, 1561–1567.
- Huq, M.A.; Kim, Y.J.; Min, J.W.; Siraj, F.M.; Siddiqi, M.Z.; Yang, D.C. Enzymatic transformation of the major ginsenoside Rb1 to compound K by Weissella hellenica DC06. Indian J. Biotechnol. 2015, 14, 270–275.
- Cui, C.H.; Kim, D.J.; Jung, S.C.; Kim, S.C.; Im, W.T. Enhanced production of gypenoside LXXV using a novel ginsenoside-transforming β-glucosidase from ginseng-cultivating soil bacteria and its anti-cancer property. Molecules 2017, 22, 844.
- Zheng, Y.; Zheng, Z.; Ming, Y.; Bai, Y.; Chen, L.; Huang, W.; Lin, M.; Liu, S.; Xiao, J.; Lin, H. Compound K producing from the enzymatic conversion of gypenoside by naringinase. Food Chem. Toxicol. 2019, 130, 253–261.
- Siddiqi, M.Z.; Srinivasan, S.; Park, H.Y.; Im, W.T. Exploration and characterization of novel glycoside hydrolases from the whole genome of lactobacillus ginsenosidimutans and enriched production of minor ginsenoside Rg3(S) by a recombinant enzymatic process. Biomolecules 2020, 10, 288.
- Upadhyaya, J.; Yoon, M.S.; Kim, M.J.; Ryu, N.S.; Song, Y.E.; Kim, Y.H.; Kim, M.K. Purification and characterization of a novel ginsenoside Rc-hydrolyzing β-glucosidase from Armillaria mellea mycelia. AMB Express 2016, 6, 112.
- Chang, K.H.; Jo, M.N.; Kim, K.T.; Paik, H.D. Purification and characterization of a ginsenoside Rb1-hydrolyzing β-glucosidase from Aspergillus niger KCCM 11239. Int. J. Mol. Sci. 2012, 13, 12140–12152.
- Jeong, E.B.; Kim, S.A.; Shin, K.C.; Oh, D.K. Biotransformation of protopanaxadiol-type ginsenosides in Korean ginseng extract into food-available compound K by an extracellular enzyme from aspergillus niger. J. Microbiol. Biotechnol. 2020, 30, 1560–1567.
- Wu, X.; Qu, B.; Liu, Y.; Ren, X.; Wang, S.; Quan, Y. Highly enhanced activity and stability via affinity induced immobilization β-glucosidase from Aspergillus niger onto amino-based silica for the biotransformation of ginsenoside Rb1. J. Chromatogr. A 2021, 1653, 462388.
- Jiang, Y.; Li, W.; Fan, D. Biotransformation of Ginsenoside Rb1 to Ginsenoside CK by Strain XD101: A Safe Bioconversion Strategy. Appl. Biochem. Biotechnol. 2021, 193, 2110–2127.
- Jung, J.; Paik, N.L.H. Bioconversion, health benefits, and application of ginseng and red ginseng in dairy products. Food Sci. Biotechnol. 2017, 26, 1155–1168.
- Quan, L.H.; Min, J.W.; Jin, Y.; Wang, C.; Kim, Y.J.; Yang, D.C. Enzymatic Biotransformation of Ginsenoside Rb1 to Compound K by Recombinant β-Glucosidase from Microbacterium esteraromaticum. Agric. Food Chem. 2012, 60, 3776–3781.
- Jung, I.H.; Lee, J.H.; Hyun, Y.J.; Kim, D.H. Metabolism of ginsenoside Rb1 by human intestinal microflora and cloning of its metabolizing β-D-glucosidase from Bifidobacterium longum H-1. Biol. Pharm. Bull. 2012, 35, 573–581.
- Cui, L.; Yan, H.; Wang, D.; Chen, Q. An efficient isolation and purification of broad partition coefficient range ginsenosides from roots of Panax quinquefolium L. by linear gradient counter-current chromatography coupled with preparative high-performance liquid chromatography. J. Sep. Sci. 2023, 2300046.
- Pérez, G.; Fariña, L.; Barquet, M.; Boido, E.; Gaggero, C.; Dellacassa, E.; Carrau, F. A quick screening method to identify β-glucosidase activity in native wine yeast strains: Application of Esculin Glycerol Agar (EGA) medium. World J. Microbiol. Biotechnol. 2011, 27, 47–55.
- Fu, Y.; Yin, Z.; Wu, L.; Yin, C. Diversity of cultivable b -glycosidase-producing micro-organisms isolated from the soil of a ginseng field and their ginsenosides-hydrolysing activity. Lett. Appl. Microbiol. 2013, 58, 138–144.
- Yan, S.; Wei, P.C.; Chen, Q.; Chen, X.; Wang, S.C.; Li, J.R.; Gao, C. Functional and structural characterization of a β-glucosidase involved in saponin metabolism from intestinal bacteria. Biochem. Biophys. Res. Commun. 2018, 496, 1349–1356.
- Keum, D.H.; Yeon, J.M.; Yun, C.S.; Lee, S.Y.; Im, W.T. Chryseobacterium panacisoli sp. Nov., isolated from ginseng-cultivation soil with ginsenoside-converting activity. Int. J. Syst. Evol. Microbiol. 2021, 71, 005086.
- Cui, C.H.; Kim, S.C.; Im, W.T. Characterization of the ginsenoside-transforming recombinant β-glucosidase from Actinosynnema mirum and bioconversion of major ginsenosides into minor ginsenosides. Appl. Microbiol. Biotechnol. 2013, 97, 649–659.
- Siddiqi, M.Z.; Hashmi, M.S.; Oh, J.M.; Chun, S.; Im, W.T. Identification of novel glycoside hydrolases via whole genome sequencing of Niabella ginsenosidivorans for production of various minor ginsenosides. 3 Biotech. 2019, 9, 258.
- Quan, L.H.; Min, J.W.; Subramaniyam, S.; Yang, D.U.; Kim, Y.J.; Yang, D.C. Biotransformation of ginsenosides Re and Rg1 into ginsenosides Rg2 and Rh1 by recombinant b-glucosidase. Biotechnol. Lett. 2012, 34, 913–917.
- Xie, J.; Zhao, D.; Zhao, L.; Pei, J.; Xiao, W.; Ding, G.; Wang, Z. Overexpression and characterization of a Ca2+ activated thermostable β-glucosidase with high ginsenoside Rb1 to ginsenoside 20(S)-Rg3 bioconversion productivity. J. Ind. Microbiol. Biotechnol. 2015, 42, 839–850.
- Renchinkhand, G.; Magsar, U.; Bae, H.C.; Choi, S.H.; Nam, M.S. Identification of β-Glucosidase Activity of Lentilactobacillus buchneri URN103L and Its Potential to Convert Ginsenoside Rb1 from Panax ginseng. Foods 2022, 11, 529.
- Park, J.K.; Yang, D.U.; Arunkumar, L.; Han, Y.; Lee, S.J.; Arif, M.H.; Li, J.F.; Huo, Y.; Kang, J.P.; Hoang, V.A.; et al. Cumulative production of bioactive RG3, RG5, RK1, and CK from fermented black ginseng using novel aspergillus niger KHNT-1 strain isolated from Korean traditional food. Processes 2021, 9, 227.
- Kim, S.A.; Shin, K.C.; Oh, D.K. Complete biotransformation of protopanaxadiol-type ginsenosides into 20-O-β-glucopyranosyl-20(S)-protopanaxadiol by permeabilized recombinant Escherichia coli cells coexpressing β-glucosidase and chaperone genes. J. Agric. Food Chem. 2019, 67, 8393–8401.




