
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francesco Mistretta | -- | 2194 | 2023-05-09 20:32:53 | | | |
| 2 | Peter Tang | Meta information modification | 2194 | 2023-05-10 03:21:55 | | |
Video Upload Options
Spontaneous intracranial hypotension (SIH) occurs due to a leakage of the cerebrospinal fluid (CSF) lowering the pressure of subarachnoid space, mostly caused by a dural breach or discogenic microspur. As a result of less support provided by CSF pressure, intracranial structures are stretched downward, leading to a constellation of more or less typical MRI findings, including venous congestion, subdural effusions, brainstem sagging and low-lying cerebellar tonsils.
1. Introduction
2. Pathophysiology
3. Classification of SIH
|
Morphological Type of Leak |
Location of Leak |
|
|---|---|---|
|
Type 1 (60% of cases) - 1a - 1b |
Dural tear Dural tear |
Ventral dura Posterolateral dura |
|
Type 2 (20% of cases) |
||
|
- 2a |
Simple single or multiple meningeal diverticula |
Lateral dura |
|
- 2b |
Complex meningeal diverticula or dural ectasia |
Lateral dura |
|
Type 3 (20% of cases) |
Direct CSF-venous fistula |
Distal nerve root sleeve |
|
Type 4 |
Indeterminate origin |
4. Clinical Presentation of SIH
5. SIH: Diagnostic Workup and Imaging Strategy
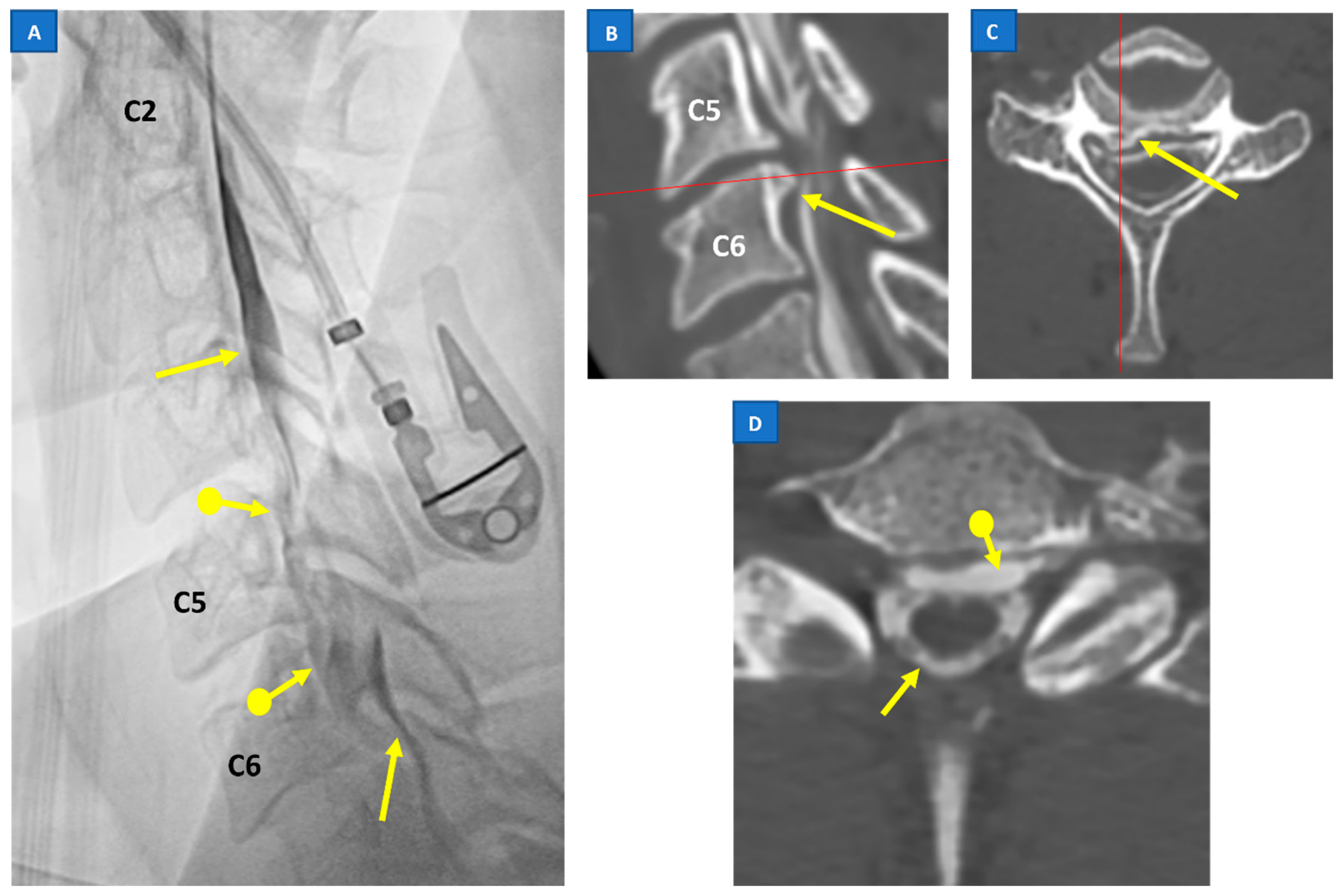
6. Treatment and Prognosis of SIH
References
- Schaltenbrand, G. Normal and pathological physiology of the cerebrospinal fluid circulation. Lancet 1953, 261, 805–808.
- Schievink, W.I.; Maya, M.; Moser, F.; Tourje, J.; Torbati, S. Frequency of spontaneous intracranial hypotension in the emergency department. J. Headache Pain 2007, 8, 325–328.
- Schievink, W.I. Misdiagnosis of spontaneous intracranial hypotension. Arch. Neurol. 2003, 60, 1713–1718.
- Schievink, W.I.; Maya, M.M.; Louy, C.; Moser, F.G.; Sloninsky, L. Spontaneous intracranial hypotension in childhood and adolescence. J. Pediatr. 2013, 163, 504–510.e3.
- Schievink, W.I.; Meyer, F.B.; Atkinson, J.L.; Mokri, B. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. J. Neurosurg. 1996, 84, 598–605.
- D’Antona, L.; Merchan, M.A.J.; Vassiliou, A.; Watkins, L.D.; Davagnanam, I.; Toma, A.K.; Matharu, M.S. Clinical presentation, investigation findings, and treatment outcomes of spontaneous intracranial hypotension syndrome: A systematic review and meta-analysis. JAMA Neurol. 2021, 78, 329–337.
- Schievink, W.I. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA 2006, 295, 2286–2296.
- Reinstein, E.; Pariani, M.; Bannykh, S.; Rimoin, D.L.; Schievink, W.I. Connective tissue spectrum abnormalities associated with spontaneous cerebrospinal fluid leaks: A prospective study. Eur. J. Hum. Genet. 2013, 21, 386–390.
- Olesen, J.; Dodick, D.; Ducros, A.; Evers, S.; First, M.; Goadsby, P. The International Classification of Headache Disorders, (ICHD-3). Cephalalgia 2018, 38, 1–211.
- Kranz, P.G.; Gray, L.; Amrhein, T.J. Spontaneous intracranial hypotension: 10 myths and misperceptions. Headache J. Head Face Pain 2018, 58, 948–959.
- Schievink, W.; Maya, M.; Louy, C.; Moser, F.; Tourje, J. Diagnostic criteria for spontaneous spinal CSF leaks and intracranial hypotension. Am. J. Neuroradiol. 2008, 29, 853–856.
- Schievink, W.I.; Schwartz, M.S.; Maya, M.M.; Moser, F.G.; Rozen, T.D. Lack of causal association between spontaneous intracranial hypotension and cranial cerebrospinal fluid leaks. J. Neurosurg. 2012, 116, 749–754.
- Mokri, B. Spontaneous intracranial hypotension. Contin. Lifelong Learn. Neurol. 2015, 21, 1086–1108.
- Schievink, W.I.; Jacques, L. Recurrent spontaneous spinal cerebrospinal fluid leak associated with “nude nerve root” syndrome: Case report. Neurosurgery 2003, 53, 1216–1219.
- Kranz, P.G.; Luetmer, P.H.; Diehn, F.E.; Amrhein, T.J.; Tanpitukpongse, T.P.; Gray, L. Myelographic techniques for the detection of spinal CSF leaks in spontaneous intracranial hypotension. Am. J. Roentgenol. 2016, 206, 8–19.
- Schievink, W.I.; Moser, F.G.; Maya, M.M. CSF–venous fistula in spontaneous intracranial hypotension. Neurology 2014, 83, 472–473.
- Luetmer, P.H.; Schwartz, K.; Eckel, L.; Hunt, C.; Carter, R.; Diehn, F. When should I do dynamic CT myelography? Predicting fast spinal CSF leaks in patients with spontaneous intracranial hypotension. Am. J. Neuroradiol. 2012, 33, 690–694.
- Yao, L.-L.; Hu, X.-Y. Factors affecting cerebrospinal fluid opening pressure in patients with spontaneous intracranial hypotension. J. Zhejiang Univ. Sci. B 2017, 18, 577–585.
- Kranz, P.G.; Tanpitukpongse, T.P.; Choudhury, K.R.; Amrhein, T.J.; Gray, L. How common is normal cerebrospinal fluid pressure in spontaneous intracranial hypotension? Cephalalgia 2016, 36, 1209–1217.
- Mokri, B. Spontaneous cerebrospinal fluid leaks: From intracranial hypotension to cerebrospinal fluid hypovolemia—Evolution of a concept. Mayo Clin. Proc. 1999, 74, 1113–1123.
- Alperin, N.; Lee, S.H.; Sivaramakrishnan, A.; Hushek, S.G. Quantifying the effect of posture on intracranial physiology in humans by MRI flow studies. J. Magn. Reson. Imaging 2005, 22, 591–596.
- Tain, R.W.; Bagci, A.M.; Lam, B.L.; Sklar, E.M.; Ertl-Wagner, B.; Alperin, N. Determination of cranio-spinal canal compliance distribution by MRI: Methodology and early application in idiopathic intracranial hypertension. J. Magn. Reson. Imaging 2011, 34, 1397–1404.
- Medina, J.H.; Abrams, K.; Falcone, S.; Bhatia, R.G. Spinal imaging findings in spontaneous intracranial hypotension. Am. J. Roentgenol. 2010, 195, 459–464.
- Alperin, N.; Bagci, A.; Lee, S.; Lam, B. Automated quantitation of spinal CSF volume and measurement of craniospinal CSF redistribution following lumbar withdrawal in idiopathic intracranial hypertension. Am. J. Neuroradiol. 2016, 37, 1957–1963.
- Ciaramitaro, P.; Massimi, L.; Bertuccio, A.; Solari, A.; Farinotti, M.; Peretta, P.; Saletti, V.; Chiapparini, L.; Barbanera, A.; Garbossa, D.; et al. Diagnosis and treatment of Chiari malformation and syringomyelia in adults: International consensus document. Neurol. Sci. 2022, 43, 1327–1342.
- Stovner, L.J.; Bergan, U.; Nilsen, G.; Sjaastad, O. Posterior cranial fossa dimensions in the Chiari I malformation: Relation to pathogenesis and clinical presentation. Neuroradiology 1993, 35, 113–118.
- Buell, T.J.; Heiss, J.D.; Oldfield, E.H. Pathogenesis and Cerebrospinal Fluid Hydrodynamics of the Chiari I Malformation. Neurosurg. Clin. N. Am. 2015, 26, 495–499.
- Tachibana, S.; Harada, K.; Abe, T.; Yamada, H.; Yokota, A. Syringomyelia secondary to tonsillar herniation caused by posterior fossa tumors. Surg. Neurol. 1995, 43, 470–475, discussion: 475–477.
- Schievink, W.I.; Maya, M.M.; Jean-Pierre, S.; Nuño, M.; Prasad, R.S.; Moser, F.G. A classification system of spontaneous spinal CSF leaks. Neurology 2016, 87, 673–679.
- Farb, R.I.; Forghani, R.; Lee, S.; Mikulis, D.; Agid, R. The venous distension sign: A diagnostic sign of intracranial hypotension at MR imaging of the brain. Am. J. Neuroradiol. 2007, 28, 1489–1493.
- Mokri, B. The Monro–Kellie hypothesis: Applications in CSF volume depletion. Neurology 2001, 56, 1746–1748.
- Cushing, H. The third circulation. In Studies in Intracranial Physiology and Surgery; Oxford University Press: London, UK, 1926; pp. 1–51.
- Luetzen, N.; Dovi-Akue, P.; Fung, C.; Beck, J.; Urbach, H. Spontaneous intracranial hypotension: Diagnostic and therapeutic workup. Neuroradiology 2021, 63, 1765–1772.
- Mokri, B. Headache associated with abnormalities in intracranial structure or function: Low cerebrospinal-fluid-pressure headache. In Wolff’s Headache and Other Head Pain; Oxford University Press: Oxford, UK, 2007; pp. 513–531.
- Mokri, B. Spontaneous CSF leaks: Low CSF volume syndromes. Neurol. Clin. 2014, 32, 397–422.
- Yamamoto, M.; Suehiro, T.; Nakata, H.; Nishioka, T.; Itoh, H.; Nakamura, T.; Hashimoto, K. Primary low cerebrospinal fluid pressure syndrome associated with galactorrhea. Intern. Med. 1993, 32, 228–231.
- Schievink, W.I.; Nuño, M.; Rozen, T.D.; Maya, M.M.; Mamelak, A.N.; Carmichael, J.; Bonert, V.S. Hyperprolactinemia due to spontaneous intracranial hypotension. J. Neurosurg. 2015, 122, 1020–1025.
- Albayram, S.; Wasserman, B.A.; Yousem, D.M.; Wityk, R. Intracranial hypotension as a cause of radiculopathy from cervical epidural venous engorgement: Case report. Am. J. Neuroradiol. 2002, 23, 618–621.
- Farb, R.; Nicholson, P.; Peng, P.; Massicotte, E.; Lay, C.; Krings, T. Spontaneous intracranial hypotension: A systematic imaging approach for CSF leak localization and management based on MRI and digital subtraction myelography. Am. J. Neuroradiol. 2019, 40, 745–753.
- Dobrocky, T.; Grunder, L.; Breiding, P.S.; Branca, M.; Limacher, A.; Mosimann, P.J.; Mordasini, P.; Zibold, F.; Haeni, L.; Jesse, C.M.; et al. Assessing spinal cerebrospinal fluid leaks in spontaneous intracranial hypotension with a scoring system based on brain magnetic resonance imaging findings. JAMA Neurol. 2019, 76, 580–587.
- Schievink, W.I. Spontaneous intracranial hypotension. N. Engl. J. Med. 2021, 385, 2173–2178.
- Brinjikji, W.; Savastano, L.; Atkinson, J.; Garza, I.; Farb, R.; Cutsforth-Gregory, J. A novel endovascular therapy for CSF hypotension secondary to CSF-venous fistulas. Am. J. Neuroradiol. 2021, 42, 882–887.
- Mamlouk, M.D.; Shen, P.Y.; Sedrak, M.F.; Dillon, W.P. CT-guided fibrin glue occlusion of cerebrospinal fluid–venous fistulas. Radiology 2021, 299, 409–418.
- Pagani-Estévez, G.L.; Cutsforth-Gregory, J.K.; Morris, J.M.; Mokri, B.; Piepgras, D.G.; Mauck, W.D.; Eldrige, J.S.; Watson, J.C. Procedural predictors of epidural blood patch efficacy in spontaneous intracranial hypotension. Reg. Anesth. Pain Med. 2019, 44, 212–220.
- Bergui, M.; Mistretta, F.; Bosco, G.; Cester, G.; Chioffi, F.; Gambino, A.; Molinaro, S.; Russo, R.; Sorarù, G.; Causin, F. CSF-venous leak responsible for spontaneous intracranial hypotension treated by endovascular venous route: First cases in Italy. Interv. Neuroradiol. 2022.




