
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Brahim EL HEJJIOUI | -- | 2818 | 2023-05-09 13:58:24 | | | |
| 2 | Wendy Huang | Meta information modification | 2818 | 2023-05-10 05:47:17 | | |
Video Upload Options
Breast cancer (BC) is a disease characterized by various clinical behaviors and biological characteristics. Breast cancer (BC) is heterogenous, showing variable morphologic and biological features; thus, it has different clinical behaviors and responses to treatment. Based on molecular and histological evidence, BC could be categorized into three groups: (1) BC expressing hormone receptor (estrogen receptor (ER+) or progesterone receptor (PR+)) commonly noted as luminal tumors and are responsive to endocrine therapy, (2) BC expressing human epidermal receptor 2 (HER2+) which is characterized by the overexpression of HER2 oncogene and is treated with trastuzumab, (3) Triple-negative breast cancer (TNBC) (ER−, PR−, HER2−) subtype, which is associated with high mortality rates and is not responsive to some drug treatment approaches.
1. Introduction
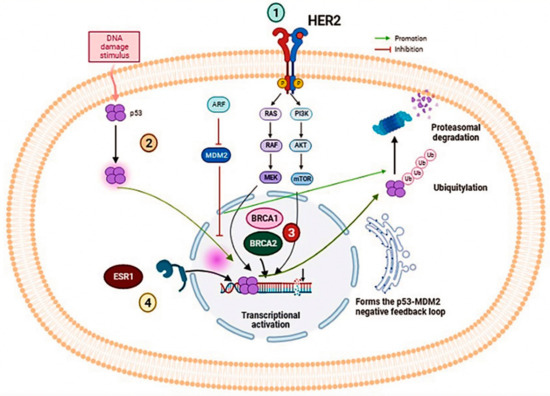
Several genes present a potential target for studying breast cancer, The genes studied have been listed in Table 1, and the signaling pathways of these genes were mentioned in Figure 1.
| Gene | Patient Cohort | Molecule Testing Technique | Main Findings | Clinical Significance | References |
|---|---|---|---|---|---|
| ESR1 | 541 postmenopausal women with a diagnosis of MBC. | Analyzed ESR1 mutations (Y537S and D538G) on cell-free DNA (cfDNA) using droplet digital polymerase chain reaction (ddPCR). | D538G (21.1%) Y537S (13.3%) 30 had both mutations. |
These mutations were associated with shorter overall survival: - wild-type, 32.1 months - D538G, 25.99 months - Y537S, 19.98 months - Both mutations, 15.15 months. |
[1] |
| 86 estrogen receptor-positive BC patients.185 plasma samples (151 plasma samples from 69 MBC patients, and 34 plasma samples from 17 primary BC (PBC) patients). | Multiplex droplet digital PCR assays in a snapshot and serially. | cfDNA ESR1 and PIK3CA mutations were found in 28.9% and 24.6% of MBC patients, respectively. | All patients with ESR1 mutations had resistance to prior AI (aromatase inhibitor) therapy. 85% of patients with ESR1 mutations had resistance to prior SERM (Selective estrogen receptor modulators) therapy. |
[2] | |
| BRCA1/2 | 828 patients with advanced breast, ovarian, prostate, or pancreatic cancer. (the study was conducted in accordance with the Declaration of Helsinki). |
Plasma-based NGS assay. | Of 828 patients, 60 (7.2%) had at least one BRCA1/2 loss-of-function mutation, 42 patients with germline mutations and 18 (14 patients had breast cancer) with somatic mutations only. | NGS analysis of cfDNA identified high rates of therapeutically relevant mutations, including deleterious BRCA1/2 somatic mutations missed by germline testing. | [3] |
| 24 patients with proven BRCA1/2 germline mutations (19 ovarian cancer patients and 5 patients with MBC who received prior treatment with platinum-based chemotherapy and/or PARP inhibitors). | Targeted massively parallel sequencing of tumor DNA from ovarian cancer patients, cfDNA from ovarian and breast cancer patients, and their germline DNA. | Identification of BRCA1 or BRCA2 reversion mutations in the cfDNA of 4 ovarian cancer patients (21%) and 2 breast cancer patients (40%). | cfDNA sequencing can help identify putative BRCA1/2 reversion mutations which may facilitate patient selection for PARP inhibition therapy. | [4] | |
| PIK3CA | Thirty patients with advanced BC (ABC); | PIK3CA mutation analysis was performed using ddPCR. | The presence of a PI3K mutation in liquid biopsy correlates with worse PFS in patients with ABC receiving CDK4/6i. | Integration of PI3K status assessment with other molecular information could improve the management of patients with aggressive breast cancer and better suggest the best therapeutic strategy. | [5] |
| TP53 | 46 patients with nonmetastatic triple-negative breast cancer; | Characterization of TP53 gene mutations in tumor tissue through massively parallel sequencing (MPS). Monitoring of previously characterized mutations based on ctDNA analysis by ddPCR at four time points: pre-NCT, post-cycle, pre-surgery, and post-surgery. | Results show a marked decrease in ctDNA levels and positivity rate during chemotherapy cycles. | The high prevalence of TP53 mutations in TNBC is a potential biomarker for ctDNA monitoring during NCT, and therefore is a tool for TNBC management. | [6] |
| 113 lung and 18 breast cancer patients | NGS analysis of ctDNA: Panel for hot spot regions in 11 genes for lung cancer and 10 genes for breast cancer. | Variations in the TP53 gene were detected at a high frequency in both tumor types, followed by the PIK3CA gene in breast cancer. | Based on NGS and ddPCR techniques, liquid biopsy could be a very effective method for managing terminal cancer cases and monitoring treatment responses. | [7] | |
| 68 patients with metastatic breast cancer (MBC). | cfDNA and gDNA (Genomic DNA) analysis by next-generation sequencing (NGS) | TP53 mutations occurred in 10 (45.45%) TNBC patients, 9 (36.00%) HER2+ patients, and 7 (22.22%) HR+ patients. TP53 represents the gene with the highest number of somatic mutations. |
Mutations in TP53 cDNA and PIK3CA genes likely limit survival and promote disease progression. | [8] | |
| ERBB2 | 636 women with HER2 nonamplified MBC. | ctDNA analysis by NGS. | Results of this study indicate the efficacy of neratinib for HER2-mutated nonamplified breast cancer. | This study supports the potential use of ctDNA to identify patients with HER2-mutated breast cancer to establish a new standard of care. | [9] |
| Multicohort, phase 2a, platform trial of ctDNA testing in 18 UK hospitals.1051 patients were registered in the study. | ddPCR and NGS are used to detect ctDNA mutations. Patients were recruited into four parallel treatment cohorts corresponding to the mutations identified in the ctDNA (ESR1; HER2; AKT1 and PTEN). |
The findings of this study demonstrate the clinically relevant activity of targeted therapies against rare HER2 and AKT1 mutations. | The results of this research show that ctDNA analysis, with the technologies used in this study, is accurate enough to be routinely adopted into clinical practice. | [10] |
2. BRCA1/2 Genes
3. ESR1 Gene
4. PIK3CA Gene
5. TP53 Gene
6. ERBB2 Gene
References
- Chandarlapaty, S.; Chen, D.; He, W.; Sung, P.; Samoila, A.; You, D.; Bhatt, T.; Patel, P.; Voi, M.; Gnant, M.; et al. Prevalence of ESR1 Mutations in Cell-Free DNA and Outcomes in Metastatic Breast Cancer: A Secondary Analysis of the BOLERO-2 Clinical Trial. JAMA Oncol. 2016, 2, 1310–1315.
- Takeshita, T.; Yamamoto, Y.; Yamamoto-Ibusuki, M.; Tomiguchi, M.; Sueta, A.; Murakami, K.; Omoto, Y.; Iwase, H. Analysis of ESR1 and PIK3CA mutations in plasma cell-free DNA from ER-positive breast cancer patients. Oncotarget 2017, 8, 52142–52155.
- Vidula, N.; Rich, T.A.; Sartor, O.; Yen, J.; Hardin, A.; Nance, T.; Lilly, M.B.; Nezami, M.A.; Patel, S.P.; Carneiro, B.A.; et al. Routine Plasma-Based Genotyping to Comprehensively Detect Germline, Somatic, and Reversion BRCA Mutations among Patients with Advanced Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 2546–2555.
- Weigelt, B.; Comino-Méndez, I.; de Bruijn, I.; Tian, L.; Meisel, J.L.; García-Murillas, I.; Fribbens, C.; Cutts, R.; Martelotto, L.G.; Ng, C.K.; et al. Diverse BRCA1 and BRCA2 Reversion Mutations in Circulating Cell-Free DNA of Therapy-Resistant Breast or Ovarian Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 6708–6720.
- Del Re, M.; Crucitta, S.; Lorenzini, G.; De Angelis, C.; Diodati, L.; Cavallero, D.; Bargagna, I.; Cinacchi, P.; Fratini, B.; Salvadori, B.; et al. PI3K mutations detected in liquid biopsy are associated to reduced sensitivity to CDK4/6 inhibitors in metastatic breast cancer patients. Pharmacol. Res. 2021, 163, 105241.
- Riva, F.; Bidard, F.-C.; Houy, A.; Saliou, A.; Madic, J.; Rampanou, A.; Hego, C.; Milder, M.; Cottu, P.; Sablin, M.-P.; et al. Patient-Specific Circulating Tumor DNA Detection during Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Clin. Chem. 2017, 63, 691–699.
- Savli, H.; Sertdemir, N.; Aydin, D.; Dursun, B.; Kurtas, O.; Reka, S.; Sunnetci-Akkoyunlu, D.; Eren-Keskin, S.; Uygun, K.; Ozden, E.; et al. TP53, EGFR and PIK3CA gene variations observed as prominent biomarkers in breast and lung cancer by plasma cell-free DNA genomic testing. J. Biotechnol. 2019, 300, 87–93.
- Hu, Z.-Y.; Xie, N.; Tian, C.; Yang, X.; Liu, L.; Li, J.; Xiao, H.; Wu, H.; Lu, J.; Gao, J.; et al. Identifying Circulating Tumor DNA Mutation Profiles in Metastatic Breast Cancer Patients with Multiline Resistance. EBioMedicine 2018, 32, 111–118.
- Ma, C.X.; Bose, R.; Gao, F.; Freedman, R.A.; Telli, M.L.; Kimmick, G.; Winer, E.; Naughton, M.; Goetz, M.P.; Russell, C.; et al. Neratinib Efficacy and Circulating Tumor DNA Detection of HER2 Mutations in HER2 Nonamplified Metastatic Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 5687–5695.
- Turner, N.C.; Kingston, B.; Kilburn, L.S.; Kernaghan, S.; Wardley, A.M.; Macpherson, I.R.; Baird, R.D.; Roylance, R.; Stephens, P.; Oikonomidou, O.; et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): A multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020, 21, 1296–1308.
- Baretta, Z.; Mocellin, S.; Goldin, E.; Olopade, O.I.; Huo, D. Effect of BRCA germline mutations on breast cancer prognosis. Medicine (Baltimore) 2016, 95, e4975.
- Pascual, T.; Gonzalez-Farre, B.; Teixidó, C.; Oleaga, L.; Oses, G.; Ganau, S.; Chic, N.; Riu, G.; Adamo, B.; Galván, P.; et al. Significant Clinical Activity of Olaparib in a Somatic BRCA1-Mutated Triple-Negative Breast Cancer with Brain Metastasis. JCO Precis. Oncol. 2019, 3, 1–6.
- Claus, E.B.; Risch, N.; Thompson, W.D. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am. J. Hum. Genet. 1991, 48, 232–242.
- Wang, Y.; Cortez, D.; Yazdi, P.; Neff, N.; Elledge, S.J.; Qin, J. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 2000, 14, 927–939.
- Algebaly, A.S.; Suliman, R.S.; Al-Qahtani, W.S. Comprehensive study for BRCA1 and BRCA2 entire coding regions in breast cancer. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2021, 23, 74–81.
- Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70.
- Winter, C.; Nilsson, M.P.; Olsson, E.; George, A.M.; Chen, Y.; Kvist, A.; Törngren, T.; Vallon-Christersson, J.; Hegardt, C.; Häkkinen, J.; et al. Targeted sequencing of BRCA1 and BRCA2 across a large unselected breast cancer cohort suggests that one-third of mutations are somatic. Ann. Oncol. 2016, 27, 1532–1538.
- Diehl, F.; Schmidt, K.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A.; et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 2008, 14, 985–990.
- Clatot, F.; Perdrix, A.; Sefrioui, D.; Sarafan-Vasseur, N.; Di Fiore, F. Intérêt clinique de la détection des mutations circulantes d’ESR1 chez les patientes traitées pour un cancer du sein métastatique exprimant les récepteurs hormonaux. Bull. Cancer (Paris) 2018, 105, 46–54.
- Zundelevich, A.; Dadiani, M.; Kahana-Edwin, S.; Itay, A.; Sella, T.; Gadot, M.; Cesarkas, K.; Farage-Barhom, S.; Saar, E.G.; Eyal, E.; et al. ESR1 mutations are frequent in newly diagnosed metastatic and loco-regional recurrence of endocrine-treated breast cancer and carry worse prognosis. Breast Cancer Res. 2020, 22, 16.
- Leiser, M. ESR1 Mutations Predict Survival, Endocrine Therapy Failure in Early Breast Cancer, 28 Avril 2020. Consulté le: 5 décembre 2022. . Available online: https://www.healio.com/news/hematology-oncology/20200428/esr1-mutations-predict-survival-endocrine-therapy-failure-in-early-breast-cancer (accessed on 24 September 2022).
- Song, P.; Wu, L.R.; Yan, Y.H.; Zhang, J.X.; Chu, T.; Kwong, L.N.; Patel, A.A.; Zhang, D.Y. Limitations and opportunities of technologies for the analysis of cell-free DNA in cancer diagnostics. Nat. Biomed. Eng. 2022, 6, 232–245.
- Smyth, L.M.; Reichel, J.B.; Tang, J.; Patel, J.A.A.; Meng, F.; Selcuklu, D.S.; Houck-Loomis, B.; You, D.; Samoila, A.; Schiavon, G.; et al. Utility of Serial cfDNA NGS for Prospective Genomic Analysis of Patients on a Phase I Basket Study. JCO Precis. Oncol. 2021, 5, 6–16.
- Zhu, W.; Ren, C.; Wang, Y.; Wen, L.; Zhang, G.; Liao, N. Prevalence of ESR1 Mutation in Chinese ER-Positive Breast Cancer. OncoTargets Ther. 2020, 13, 615–621.
- Lefebvre, C.; Bachelot, T.; Filleron, T.; Pedrero, M.; Campone, M.; Soria, J.-C.; Massard, C.; Lévy, C.; Arnedos, M.; Lacroix-Triki, M.; et al. Mutational Profile of Metastatic Breast Cancers: A Retrospective Analysis. PLoS Med. 2016, 13, e1002201.
- Shibayama, T.; Low, S.-K.; Ono, M.; Kobayashi, T.; Kobayashi, K.; Fukada, I.; Ito, Y.; Ueno, T.; Ohno, S.; Nakamura, Y.; et al. Clinical significance of gene mutation in ctDNA analysis for hormone receptor-positive metastatic breast cancer. Breast Cancer Res. Treat. 2020, 180, 331–341.
- Mosele, F.; Stefanovska, B.; Lusque, A.; Tran Dien, A.; Garberis, I.; Droin, N.; Le Tourneau, C.; Sablin, M.-P.; Lacroix, L.; Enrico, D.; et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 377–386.
- Saal, L.H.; Holm, K.; Maurer, M.; Memeo, L.; Su, T.; Wang, X.; Yu, J.S.; Malmström, P.-O.; Mansukhani, M.; Enoksson, J.; et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005, 65, 2554–2559.
- Zhao, J.J.; Cheng, H.; Jia, S.; Wang, L.; Gjoerup, O.V.; Mikami, A.; Roberts, T.M. The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc. Natl. Acad. Sci. USA 2006, 103, 16296–16300.
- Herrera-Abreu, M.T.; Palafox, M.; Asghar, U.; Rivas, M.A.; Cutts, R.J.; Garcia-Murillas, I.; Pearson, A.; Guzman, M.; Rodriguez, O.; Grueso, J.; et al. Early Adaptation and Acquired Resistance to CDK4/6 Inhibition in Estrogen Receptor-Positive Breast Cancer. Cancer Res. 2016, 76, 2301–2313.
- Li, H.; Zhang, J.; Tong, J.H.M.; Chan, A.W.H.; Yu, J.; Kang, W.; To, K.F. Targeting the Oncogenic p53 Mutants in Colorectal Cancer and Other Solid Tumors. Int. J. Mol. Sci. 2019, 20, 5999.
- Duffy, M.J.; Synnott, N.C.; Crown, J. Mutant p53 in breast cancer: Potential as a therapeutic target and biomarker. Breast Cancer Res. Treat. 2018, 170, 213–219.
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discov. 2019, 9, 176–198.
- Rodriguez, B.J.; Córdoba, G.D.; Aranda, A.G.; Álvarez, M.; Vicioso, L.; Pérez, C.L.; Hernando, C.; Bermejo, B.; Parreño, A.J.; Lluch, A.; et al. Detection of TP53 and PIK3CA Mutations in Circulating Tumor DNA Using Next-Generation Sequencing in the Screening Process for Early Breast Cancer Diagnosis. J. Clin. Med. 2019, 8, 1183.
- Madic, J.; Kiialainen, A.; Bidard, F.-C.; Birzele, F.; Ramey, G.; Leroy, Q.; Rio Frio, T.; Vaucher, I.; Raynal, V.; Bernard, V.; et al. Circulating tumor DNA and circulating tumor cells in metastatic triple negative breast cancer patients. Int. J. Cancer 2015, 136, 2158–2165.
- Ma, F.; Zhu, W.; Guan, Y.; Yang, L.; Xia, X.; Chen, S.; Li, Q.; Guan, X.; Yi, Z.; Qian, H.; et al. ctDNA dynamics: A novel indicator to track resistance in metastatic breast cancer treated with anti-HER2 therapy. Oncotarget 2016, 7, 66020–66031.
- Cooke, T.; Reeves, J.; Lanigan, A.; Stanton, P. HER2 as a prognostic and predictive marker for breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2001, 12 (Suppl. 1), S23–S28.
- Houssami, N.; Macaskill, P.; Balleine, R.L.; Bilous, M.; Pegram, M.D. HER2 discordance between primary breast cancer and its paired metastasis: Tumor biology or test artefact? Insights through meta-analysis. Breast Cancer Res. Treat. 2011, 129, 659–674.
- Abraham, J.; Montero, A.J.; Jankowitz, R.C.; Salkeni, M.A.; Beumer, J.H.; Kiesel, B.F.; Piette, F.; Adamson, L.M.; Nagy, R.J.; Lanman, R.B.; et al. Safety and Efficacy of T-DM1 Plus Neratinib in Patients With Metastatic HER2-Positive Breast Cancer: NSABP Foundation Trial FB-10. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2601–2609.
- Gevensleben, H.; Garcia-Murillas, I.; Graeser, M.K.; Schiavon, G.; Osin, P.; Parton, M.; Smith, I.E.; Ashworth, A.; Turner, N.C. Noninvasive detection of HER2 amplification with plasma DNA digital PCR. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 3276–3284.
- Kleftogiannis, D.; Ho, D.; Liew, J.X.; Poon, P.S.Y.; Gan, A.; Ng, R.C.-H.; Tan, B.K.-T.; Tay, K.H.; Lim, S.H.; Tan, G.S.; et al. Detection of genomic alterations in breast cancer with circulating tumour DNA sequencing. Sci. Rep. 2020, 10, 16774.




