
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Océane Pécheux | -- | 2626 | 2023-05-08 07:37:54 | | | |
| 2 | Peter Tang | + 1 word(s) | 2627 | 2023-05-08 09:29:27 | | |
Video Upload Options
The apelinergic system is a highly conserved pleiotropic system. It comprises the apelin receptor apelin peptide jejunum (APJ) and its two peptide ligands, Elabela/Toddler (ELA) and apelin, which have different spatiotemporal localizations. This system has been implicated in the regulation of the adipoinsular axis, in cardiovascular and central nervous systems, in carcinogenesis, and in pregnancy in humans. During pregnancy, the apelinergic system is essential for embryo cardiogenesis and vasculogenesis and for placental development and function. It may also play a role in the initiation of labor. The apelinergic system seems to be involved in the development of placenta-related pregnancy complications, such as preeclampsia (PE) and intrauterine growth restriction, but an improvement in PE-like symptoms and birth weight has been described in murine models after the exogenous administration of apelin or ELA.
1. Overview of the Apelinergic System
2. The Apelinergic System in the Reproductive System—Pregnancy and Postpartum
2.1. Reproductive System
2.2. Development of the Embryo
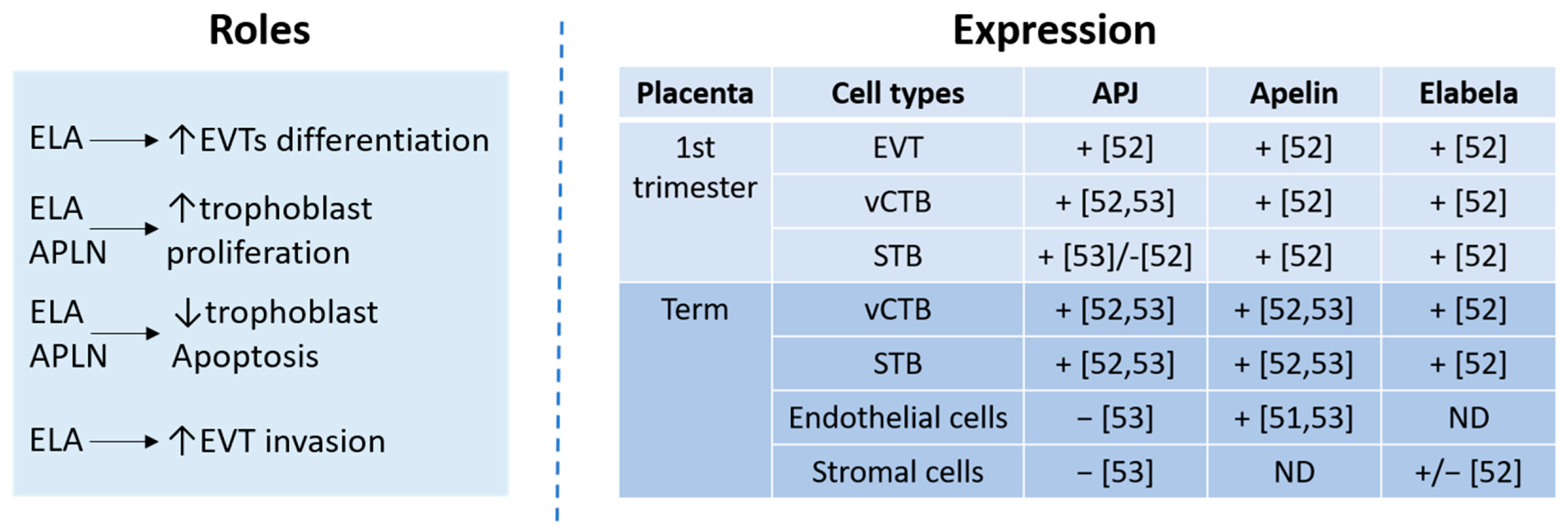
2.3. The Apelinergic System in Placenta
2.4. Labor
2.5. The Apelinergic System and Postpartum/Breastfeeding
3. Placenta-Related Complications
3.1. Preeclampsia (PE)
3.2. Intrauterine Growth Restriction (IUGR)
3.3. Gestational Diabetes Mellitus (GDM)
3.4. Miscarriage
References
- O’Carroll, A.-M.; Lolait, S.J.; Harris, L.E.; Pope, G.R. The apelin receptor APJ: Journey from an orphan to a multifaceted regulator of homeostasis. J. Endocrinol. 2013, 219, R13–R35.
- O’Dowd, B.F.; Heiber, M.; Chan, A.; Heng, H.H.Q.; Tsui, L.-C.; Kennedy, J.L.; Shi, X.; Petronis, A.; George, S.R.; Nguyen, T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 1993, 136, 355–360.
- Tatemoto, K.; Hosoya, M.; Habata, Y.; Fujii, R.; Kakegawa, T.; Zou, M.-X.; Kawamata, Y.; Fukusumi, S.; Hinuma, S.; Kitada, C.; et al. Isolation and Characterization of a Novel Endogenous Peptide Ligand for the Human APJ Receptor. Biochem. Biophys. Res. Commun. 1998, 251, 471–476.
- Charo, D.N.; Ho, M.; Fajardo, G.; Kawana, M.; Kundu, R.K.; Sheikh, A.Y.; Finsterbach, T.P.; Leeper, N.J.; Ernst, K.V.; Chen, M.M.; et al. Endogenous regulation of cardiovascular function by apelin-APJ. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H1904–H1913.
- Kang, Y.; Kim, J.; Anderson, J.P.; Wu, J.; Gleim, S.R.; Kundu, R.K.; McLean, D.L.; Kim, J.; Park, H.; Jin, S.; et al. Apelin-APJ Signaling Is a Critical Regulator of Endothelial MEF2 Activation in Cardiovascular Development. Circ. Res. 2013, 113, 22–31.
- Kasai, A.; Shintani, N.; Kato, H.; Matsuda, S.; Gomi, F.; Haba, R.; Hashimoto, H.; Kakuda, M.; Tano, Y.; Baba, A. Retardation of retinal vascular development in apelin-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1717–1722.
- Mayeur, S.; Wattez, J.-S.; Lukaszewski, M.-A.; Lecoutre, S.; Butruille, L.; Drougard, A.; Eberlé, D.; Bastide, B.; Laborie, C.; Storme, L.; et al. Apelin Controls Fetal and Neonatal Glucose Homeostasis and Is Altered by Maternal Undernutrition. Diabetes 2015, 65, 554–560.
- Hosoya, M.; Kawamata, Y.; Fukusumi, S.; Fujii, R.; Habata, Y.; Hinuma, S.; Kitada, C.; Honda, S.; Kurokawa, T.; Onda, H.; et al. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J. Biol. Chem. 2000, 275, 21061–21067.
- Kleinz, M.J.; Davenport, A.P. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul. Pept. 2004, 118, 119–125.
- Kleinz, M.J.; Skepper, J.N.; Davenport, A.P. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul. Pept. 2005, 126, 233–240.
- O’Carroll, A.M.; Selby, T.L.; Palkovits, M.; Lolait, S.J. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim. Et Biophys. Acta (BBA)-Gene Struct. Expr. 2000, 1492, 72–80.
- Medhurst, A.D.; Jennings, C.A.; Robbins, M.J.; Davis, R.P.; Ellis, C.; Winborn, K.Y.; Lawrie, K.W.M.; Hervieu, G.; Riley, G.; Bolaky, J.E.; et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J. Neurochem. 2003, 84, 1162–1172.
- Dray, C.; Debard, C.; Jager, J.; Disse, E.; Daviaud, D.; Martin, P.; Attané, C.; Wanecq, E.; Guigné, C.; Bost, F.; et al. Apelin and APJ regulation in adipose tissue and skeletal muscle of type 2 diabetic mice and humans. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1161–E1169.
- He, L.; Xu, J.; Chen, L.; Li, L. Apelin/APJ signaling in hypoxia-related diseases. Clin. Chim. Acta 2015, 451 Pt B, 191–198.
- Chen, M.M.; Ashley, E.A.; Deng, D.X.F.; Tsalenko, A.; Deng, A.; Tabibiazar, R.; Ben-Dor, A.; Fenster, B.; Yang, E.; King, J.Y.; et al. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation 2003, 108, 1432–1439.
- Kawamata, Y.; Habata, Y.; Fukusumi, S.; Hosoya, M.; Fujii, R.; Hinuma, S.; Nishizawa, N.; Kitada, C.; Onda, H.; Nishimura, O.; et al. Molecular properties of apelin: Tissue distribution and receptor binding. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2001, 1538, 162–171.
- Nyimanu, D.; Kay, R.G.; Sulentic, P.; Kuc, R.E.; Ambery, P.; Jermutus, L.; Reimann, F.; Gribble, F.M.; Cheriyan, J.; Maguire, J.J.; et al. Development and validation of an LC-MS/MS method for detection and quantification of in vivo derived metabolites of apelin-13 in humans. Sci. Rep. 2019, 9, 19934.
- Shin, K.; Landsman, M.; Pelletier, S.; Alamri, B.N.; Anini, Y.; Rainey, J.K. Proapelin is processed extracellularly in a cell line-dependent manner with clear modulation by proprotein convertases. Amino Acids 2019, 51, 395–405.
- Maguire, J.J.; Kleinz, M.J.; Pitkin, S.L.; Davenport, A.P. apelin-13 identified as the predominant apelin isoform in the human heart: Vasoactive mechanisms and inotropic action in disease. Hypertens. Dallas Tex 1979 2009, 54, 598–604.
- Chng, S.C.; Ho, L.; Tian, J.; Reversade, B. ELABELA: A hormone essential for heart development signals via the apelin receptor. Dev. Cell 2013, 27, 672–680.
- Pauli, A.; Norris, M.L.; Valen, E.; Chew, G.-L.; Gagnon, J.A.; Zimmerman, S.; Mitchell, A.; Ma, J.; Dubrulle, J.; Reyon, D.; et al. Toddler: An embryonic signal that promotes cell movement via Apelin receptors. Science 2014, 343, 1248636.
- Couvineau, P.; Llorens-Cortes, C.; Iturrioz, X. Elabela/Toddler and apelin bind differently to the apelin receptor. FASEB J. 2020, 34, 7989–8000.
- Wang, Z.; Yu, D.; Wang, M.; Wang, Q.; Kouznetsova, J.; Yang, R.; Qian, K.; Wu, W.; Shuldiner, A.; Sztalryd, C.; et al. Elabela-apelin receptor signaling pathway is functional in mammalian systems. Sci. Rep. 2015, 5, 8170.
- Ho, L.; van Dijk, M.; Chye, S.T.J.; Messerschmidt, D.M.; Chng, S.C.; Ong, S.; Yi, L.K.; Boussata, S.; Goh, G.H.-Y.; Afink, G.B.; et al. ELABELA deficiency promotes preeclampsia and cardiovascular malformations in mice. Science 2017, 357, 707–713.
- Yang, P.; Read, C.; Kuc, R.E.; Buonincontri, G.; Southwood, M.; Torella, R.; Upton, P.D.; Crosby, A.; Sawiak, S.J.; Carpenter, T.A.; et al. Elabela/Toddler Is an Endogenous Agonist of the Apelin APJ Receptor in the Adult Cardiovascular System, and Exogenous Administration of the Peptide Compensates for the Downregulation of Its Expression in Pulmonary Arterial Hypertension. Circulation 2017, 135, 1160–1173.
- Flahault, A.; Couvineau, P.; Alvear-Perez, R.; Iturrioz, X.; Llorens-Cortes, C. Role of the Vasopressin/Apelin Balance and Potential Use of Metabolically Stable Apelin Analogs in Water Metabolism Disorders. Front. Endocrinol. 2017, 8, 120.
- Ma, Y.; Yue, Y.; Ma, Y.; Zhang, Q.; Zhou, Q.; Song, Y.; Shen, Y.; Li, X.; Ma, X.; Li, C.; et al. Structural Basis for Apelin Control of the Human Apelin Receptor. Structure 1993 2017, 25, 858–866.e4.
- Murza, A.; Sainsily, X.; Coquerel, D.; Côté, J.; Marx, P.; Besserer-Offroy, É.; Longpré, J.-M.; Lainé, J.; Reversade, B.; Salvail, D.; et al. Discovery and Structure-Activity Relationship of a Bioactive Fragment of ELABELA that Modulates Vascular and Cardiac Functions. J. Med. Chem. 2016, 59, 2962–2972.
- Pitkin, S.L.; Maguire, J.J.; Bonner, T.I.; Davenport, A.P. International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function. Pharmacol. Rev. 2010, 62, 331–342.
- Reaux, A.; Gallatz, K.; Palkovits, M.; Llorens-Cortes, C. Distribution of apelin-synthesizing neurons in the adult rat brain. Neuroscience 2002, 113, 653–662.
- Newson, M.J.F.; Roberts, E.M.; Pope, G.R.; Lolait, S.J.; O’Carroll, A.-M. The effects of apelin on hypothalamic-pituitary-adrenal axis neuroendocrine function are mediated through corticotrophin-releasing factor- and vasopressin-dependent mechanisms. J. Endocrinol. 2009, 202, 123–129.
- Kurowska, P.; Barbe, A.; Różycka, M.; Chmielińska, J.; Dupont, J.; Rak, A. Apelin in Reproductive Physiology and Pathology of Different Species: A Critical Review. Int. J. Endocrinol. 2018, 2018, 9170480.
- Pope, G.R.; Roberts, E.M.; Lolait, S.J.; O’Carroll, A.-M. Central and peripheral apelin receptor distribution in the mouse: Species differences with rat. Peptides 2012, 33, 139–148.
- Roche, J.; Ramé, C.; Reverchon, M.; Mellouk, N.; Rak, A.; Froment, P.; Dupont, J. Apelin (APLN) regulates progesterone secretion and oocyte maturation in bovine ovarian cells. Reprod. Camb. Engl. 2017, 153, 589–603.
- Rak, A.; Drwal, E.; Rame, C.; Knapczyk-Stwora, K.; Słomczyńska, M.; Dupont, J.; Gregoraszczuk, E.L. Expression of apelin and apelin receptor (APJ) in porcine ovarian follicles and in vitro effect of apelin on steroidogenesis and proliferation through APJ activation and different signaling pathways. Theriogenology 2017, 96, 126–135.
- Roche, J.; Ramé, C.; Reverchon, M.; Mellouk, N.; Cornuau, M.; Guerif, F.; Froment, P.; Dupont, J. Apelin (APLN) and Apelin Receptor (APLNR) in Human Ovary: Expression, Signaling, and Regulation of Steroidogenesis in Primary Human Luteinized Granulosa Cells. Biol. Reprod. 2016, 95, 104.
- Wang, X.; Liu, X.; Song, Z.; Shen, X.; Lu, S.; Ling, Y.; Kuang, H. Emerging roles of APLN and APELA in the physiology and pathology of the female reproductive system. PeerJ 2020, 8, e10245.
- Ho, L.; Tan, S.Y.X.; Wee, S.; Wu, Y.; Tan, S.J.C.; Ramakrishna, N.B.; Chng, S.C.; Nama, S.; Szczerbinska, I.; Sczerbinska, I.; et al. ELABELA Is an Endogenous Growth Factor that Sustains hESC Self-Renewal via the PI3K/AKT Pathway. Cell Stem Cell 2015, 17, 435–447.
- Wang, C.; Liu, X.; Kong, D.; Qin, X.; Li, Y.; Teng, X.; Huang, X. Apelin as a novel drug for treating preeclampsia. Exp. Ther. Med. 2017, 14, 5917–5923.
- Helker, C.S.M.; Schuermann, A.; Pollmann, C.; Chng, S.C.; Kiefer, F.; Reversade, B.; Herzog, W. The hormonal peptide Elabela guides angioblasts to the midline during vasculogenesis. Elife 2015, 4, e06726.
- Lu, L.; Cao, J.; Li, L.; Chen, L. Elabela, a new endogenous ligand of APJ, functions in embryos and adults organisms. Acta Biochim. Biophys. Sin. 2017, 49, 378–381.
- Scott, I.C.; Masri, B.; D’Amico, L.A.; Jin, S.-W.; Jungblut, B.; Wehman, A.M.; Baier, H.; Audigier, Y.; Stainier, D.Y.R. The G Protein-Coupled Receptor Agtrl1b Regulates Early Development of Myocardial Progenitors. Dev. Cell 2007, 12, 403–413.
- Zeng, X.-X.I.; Wilm, T.P.; Sepich, D.S.; Solnica-Krezel, L. Apelin and Its Receptor Control Heart Field Formation during Zebrafish Gastrulation. Dev. Cell 2007, 12, 391–402.
- Paskaradevan, S.; Scott, I.C. The Aplnr GPCR regulates myocardial progenitor development via a novel cell-non-autonomous, Gαi/o protein-independent pathway. Biol. Open 2012, 1, 275–285.
- Piairo, P.; Moura, R.S.; Nogueira-Silva, C.; Correia-Pinto, J. The apelinergic system in the developing lung: Expression and signaling. Peptides 2011, 32, 2474–2483.
- Tatemoto, K.; Takayama, K.; Zou, M.X.; Kumaki, I.; Zhang, W.; Kumano, K.; Fujimiya, M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 2001, 99, 87–92.
- Zhang, Q.; Yao, F.; Raizada, M.K.; O’Rourke, S.T.; Sun, C. Apelin gene transfer into the rostral ventrolateral medulla induces chronic blood pressure elevation in normotensive rats. Circ. Res. 2009, 104, 1421–1428.
- Kidoya, H.; Ueno, M.; Yamada, Y.; Mochizuki, N.; Nakata, M.; Yano, T.; Fujii, R.; Takakura, N. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 2008, 27, 522–534.
- Antushevich, H.; Wójcik, M. Review: Apelin in disease. Clin. Chim. Acta Int. J. Clin. Chem. 2018, 483, 241–248.
- Mlyczyńska, E.; Kurowska, P.; Drwal, E.; Opydo-Chanek, M.; Tworzydło, W.; Kotula-Balak, M.; Rak, A. Apelin and apelin receptor in human placenta: Expression, signalling pathway and regulation of trophoblast JEG-3 and BeWo cells proliferation and cell cycle. Int. J. Mol. Med. 2020, 45, 691–702.
- Hehir, M.P.; Morrison, J.J. The adipokine apelin and human uterine contractility. Am. J. Obstet. Gynecol. 2012, 206, 359.e1–359.e5.
- Kacar, E.; Ercan, Z.; Serhatlioglu, I.; Sumer, A.; Kelestimur, H.; Kutlu, S. The effects of apelin on myometrium contractions in pregnant rats. Cell. Mol. Biol. Noisy-Gd. Fr. 2018, 64, 74–79.
- Carvajal, J.A.; Oporto, J.I. The Myometrium in Pregnant Women with Obesity. Curr. Vasc. Pharmacol. 2021, 19, 193–200.
- Habata, Y.; Fujii, R.; Hosoya, M.; Fukusumi, S.; Kawamata, Y.; Hinuma, S.; Kitada, C.; Nishizawa, N.; Murosaki, S.; Kurokawa, T.; et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 1999, 1452, 25–35.
- Aydin, S. The presence of the peptides apelin, ghrelin and nesfatin-1 in the human breast milk, and the lowering of their levels in patients with gestational diabetes mellitus. Peptides 2010, 31, 2236–2240.
- Marousez, L.; Hanssens, S.; Butruille, L.; Petit, C.; Pourpe, C.; Besengez, C.; Rakza, T.; Storme, L.; Deruelle, P.; Lesage, J.; et al. Breast milk apelin level increases with maternal obesity and high-fat feeding during lactation. Int. J. Obes. 2005 2021, 45, 1052–1060.
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; Van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074.
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333.
- Binder, J.; Kalafat, E.; Palmrich, P.; Pateisky, P.; Khalil, A. Should angiogenic markers be included in diagnostic criteria of superimposed pre-eclampsia in women with chronic hypertension? Ultrasound Obstet. Gynecol. 2022, 59, 192–201.
- Liu, Y.; Wang, L.; Shi, H. The biological function of ELABELA and APJ signaling in the cardiovascular system and pre-eclampsia. Hypertens. Res. 2019, 42, 928–934.
- Ma, J.; Hu, H.; Lin, M.; Chen, L.; Liu, M.; Li, H.; Quan, S. ELABELA alleviates syncytiotrophoblast hypoxia/reoxygenation injury and preeclampsia-like symptoms in mice by reducing apoptosis. Placenta 2021, 106, 30–39.
- Yallampalli, C.; Garfield, R.E. Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am. J. Obstet. Gynecol. 1993, 169, 1316–1320.
- Khalil, R.A.; Crews, J.K.; Novak, J.; Kassab, S.; Granger, J.P. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension 1979 1998, 31, 1065–1069.
- Osol, G.; Barron, C.; Gokina, N.; Mandala, M. Inhibition of nitric oxide synthases abrogates pregnancy-induced uterine vascular expansive remodeling. J. Vasc. Res. 2009, 46, 478–486.
- Zuo, J.; Jiang, Z. Melatonin attenuates hypertension and oxidative stress in a rat model of L-NAME-induced gestational hypertension. Vasc. Med. 2020, 25, 295–301.
- Motta, C.; Grosso, C.; Zanuzzi, C.; Molinero, D.; Picco, N.; Bellingeri, R.; Alustiza, F.; Barbeito, C.; Vivas, A.; Romanini, M. Effect of Sildenafil on Pre-Eclampsia-Like Mouse Model Induced By L-Name. Reprod. Domest. Anim. 2015, 50, 611–616.
- de Souza, C.O.; Peraçoli, M.T.S.; Weel, I.C.; Bannwart, C.F.; Romão, M.; Nakaira-Takahagi, E.; de Medeiros, L.T.L.; Silva, M.G.d.; Peraçoli, J.C. Hepatoprotective and anti-inflammatory effects of silibinin on experimental preeclampsia induced by L-NAME in rats. Life Sci. 2012, 91, 159–165.
- Deniz, R.; Baykus, Y.; Ustebay, S.; Ugur, K.; Yavuzkir, Ş.; Aydin, S. Evaluation of elabela, apelin and nitric oxide findings in maternal blood of normal pregnant women, pregnant women with pre-eclampsia, severe pre-eclampsia and umbilical arteries and venules of newborns. J. Obstet. Gynaecol. 2019, 39, 907–912.
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761.
- Resnik, R. Intrauterine growth restriction. Obstet. Gynecol. 2002, 99, 490–496.
- Gordijn, S.J.; Beune, I.M.; Ganzevoort, W. Building consensus and standards in fetal growth restriction studies. Best Pract. Res. Clin. Obs. Gynaecol. 2018, 49, 117–126.
- Malamitsi-Puchner, A.; Gourgiotis, D.; Boutsikou, M.; Baka, S.; Hassiakos, D.; Briana, D.D. Circulating apelin concentrations in mother/infant pairs at term. Acta Paediatr. 2007, 96, 1751–1754.
- Van Mieghem, T.; Doherty, A.; Baczyk, D.; Drewlo, S.; Baud, D.; Carvalho, J.; Kingdom, J. Apelin in Normal Pregnancy and Pregnancies Complicated by Placental Insufficiency. Reprod. Sci. 2016, 23, 1037–1043.
- Tang, S.-Y.; Xie, H.; Yuan, L.-Q.; Luo, X.-H.; Huang, J.; Cui, R.-R.; Zhou, H.-D.; Wu, X.-P.; Liao, E.-Y. Apelin stimulates proliferation and suppresses apoptosis of mouse osteoblastic cell line MC3T3-E1 via JNK and PI3-K/Akt signaling pathways. Peptides 2007, 28, 708–718.
- Behram, M.; Oğlak, S.C.; Dağ, İ. Circulating levels of Elabela in pregnant women complicated with intrauterine growth restriction. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102127.
- Yener, G.; Kavak, S.B.; Gul, Y.; Celik Kavak, E.; Gulcu Bulmus, F.; Sanli, C.; Batmaz, I.; Bulu, G. Elabela levels in pregnancies with intrauterine growth retardation. Ginekol. Pol. 2022, 94, 113–118.
- Guo, Y.-Y.; Li, T.; Liu, H.; Tang, L.; Li, Y.-C.; Hu, H.-T.; Su, Y.-F.; Lin, Y.; Wang, Y.-Y.; Li, C.; et al. Circulating levels of Elabela and Apelin in the second and third trimesters of pregnancies with gestational diabetes mellitus. Gynecol. Endocrinol. 2020, 36, 890–894.
- Aslan, M.; Celik, O.; Celik, N.; Turkcuoglu, I.; Yilmaz, E.; Karaer, A.; Simsek, Y.; Celik, E.; Aydin, S. Cord blood nesfatin-1 and apelin-36 levels in gestational diabetes mellitus. Endocrine 2012, 41, 424–429.
- Akinci, B.; Celtik, A.; Tunali, S.; Genc, S.; Yuksel, F.; Secil, M.; Ozcan, M.A.; Bayraktar, F. Circulating apelin levels are associated with cardiometabolic risk factors in women with previous gestational diabetes. Arch. Gynecol. Obstet. 2014, 289, 787–793.
- Boyadzhieva, M.; Atanasova, I.; Zacharieva, S.; Kedikova, S. Adipocytokines during pregnancy and postpartum in women with gestational diabetes and healthy controls. J. Endocrinol. Invest. 2013, 36, 944–949.
- Mierzyński, R.; Poniedziałek-Czajkowska, E.; Dłuski, D.; Kamiński, M.; Mierzyńska, A.; Leszczyńska-Gorzelak, B. The Potential Role of Chemerin, Lipocalin 2, and Apelin in the Diagnosis and Pathophysiology of Gestational Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 5547228.
- Oncul, M.; Tuten, A.; Erman, H.; Gelisgen, R.; Benian, A.; Uzun, H. Maternal and cord blood apelin, resistin and visfatin levels in gestational diabetes mellitus. Minerva Med. 2013, 104, 527–535.
- Telejko, B.; Kuzmicki, M.; Zonenberg, A.; Modzelewska, A.; Niedziolko-Bagniuk, K.; Ponurkiewicz, A.; Wawrusiewicz-Kurylonek, N.; Nikolajuk, A.; Szamatowicz, J.; Laudanski, P.; et al. Ghrelin in gestational diabetes: Serum level and mRNA expression in fat and placental tissue. Exp. Clin. Endocrinol. Diabetes 2010, 118, 87–92.
- Sun, J.; Ren, J.; Zuo, C.; Deng, D.; Pan, F.; Chen, R.; Zhu, J.; Chen, C.; Ye, S. Circulating apelin, chemerin and omentin levels in patients with gestational diabetes mellitus: A systematic review and meta-analysis. Lipids Health Dis. 2020, 19, 26.
- Dasgupta, S.; Banerjee, U.; Mukhopadhyay, P.; Maity, P.; Saha, S.; Das, B. Clinicopathological study and immunohistochemical analysis of expression of annexin A5 and apelin in human placentae of gestational diabetes mellitus. Diabetes Metab. Syndr. 2022, 16, 102435.
- Zheng, X.-D.; Huang, Y.; Li, H. Regulatory role of Apelin-13-mediated PI3K/AKT signaling pathway in the glucose and lipid metabolism of mouse with gestational diabetes mellitus. Immunobiology 2021, 226, 152135.
- Romero, R.; Kusanovic, J.P.; Chaiworapongsa, T.; Hassan, S.S. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 313–327.
- Manolea, M.M.; Dijmărescu, A.L.; Popescu, F.C.; Novac, M.B.; DiŢescu, D. Evaluation of the implantation site morphology in spontaneous abortion. Romanian J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2015, 56, 125–131.
- Tug, E.; Yirmibes Karaoguz, M.; Nas, T. Expression of the syncytin-1 and syncytin-2 genes in the trophoblastic tissue of the early pregnancy losses with normal and abnormal karyotypes. Gene 2020, 741, 144533.
- Freyer, L.; Hsu, C.-W.; Nowotschin, S.; Pauli, A.; Ishida, J.; Kuba, K.; Fukamizu, A.; Schier, A.F.; Hoodless, P.A.; Dickinson, M.E.; et al. Loss of Apela Peptide in Mice Causes Low Penetrance Embryonic Lethality and Defects in Early Mesodermal Derivatives. Cell Rep. 2017, 20, 2116–2130.
- Qi, Y.; Hou, Y.; Ma, M.; Li, X.; Wu, J. Circulating levels of Elabela in pregnant women with missed abortion. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2022, 38, 693–696.




