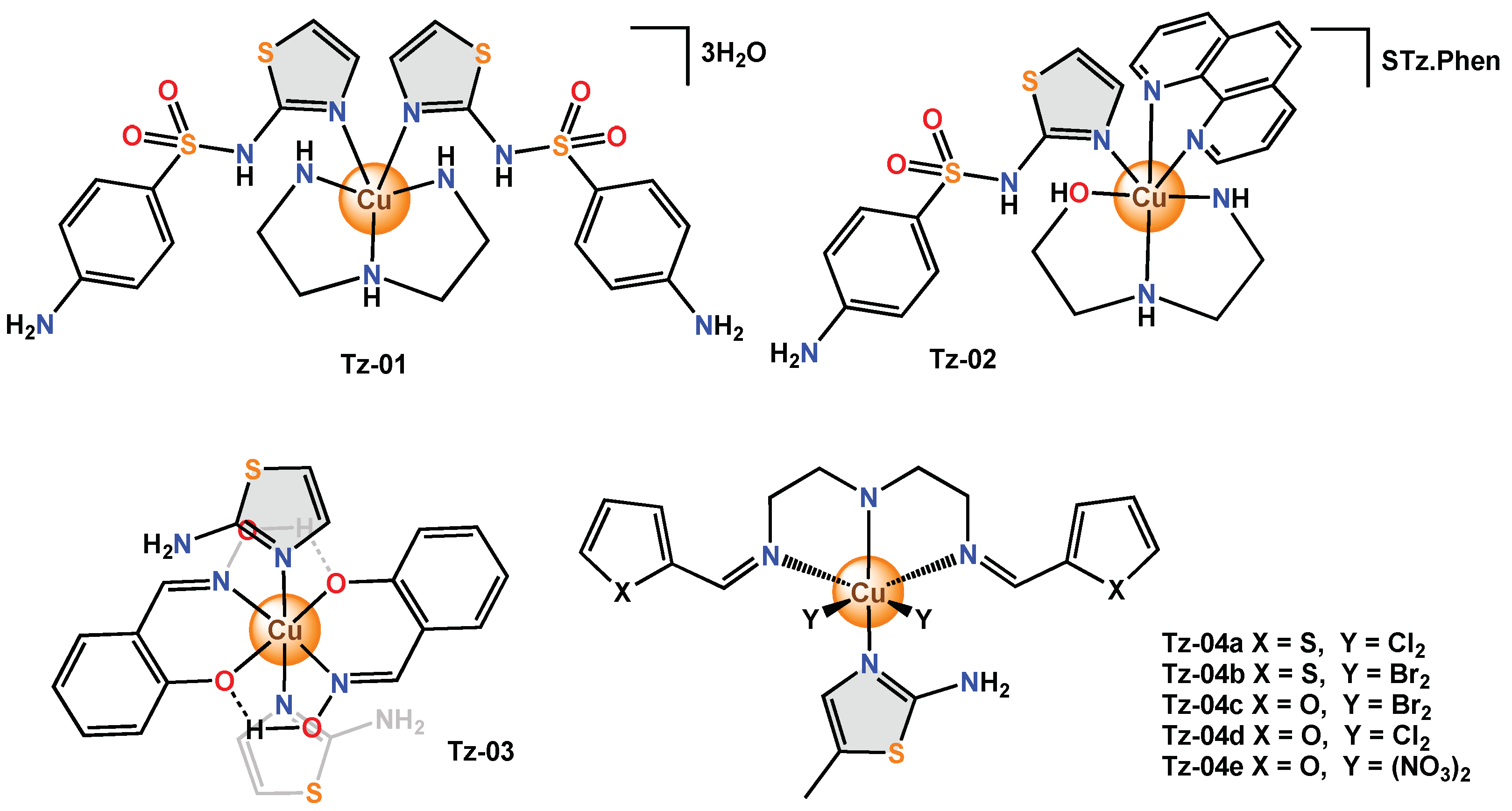
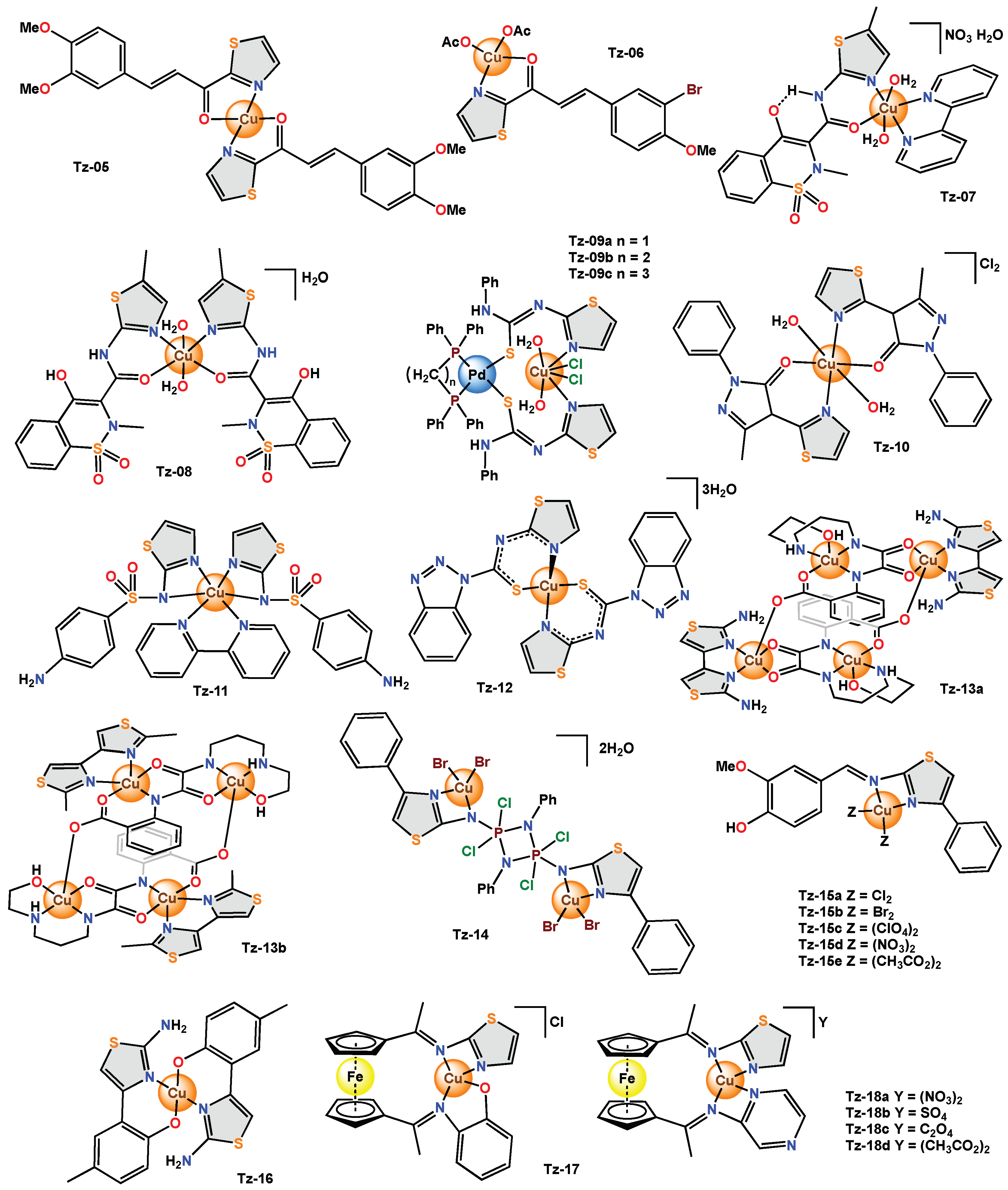Figure 2. Copper complexes with monodentate thiazole ligands.
2.2. Copper Complexes with Bidentate Thiazole Ligands
On the other hand, coordination complexes that use bidentate ligands that contain different thiazole derivatives in their structure have been reported; such is the case with Tz-05, which presented a square planar geometry around the metal centre. In the study, the antimicrobial activity was investigated using the disk diffusion method against the bacteria (E. coli, S. aureus, P. aeruginosa, B. subtilis), and the fungi (C. albicans and A. flavus).
The antimicrobial activity of Tz-06 was described using a new chalconoid ligand. In this compound, there are keto groups and π-olefin bonds that act as the preferred binding sites for metal ions. In Tz-06, the nitrogen atom of the thiazole and the oxygen of the carbonyl group act as effective donors on the metal bond to obtain a complex with a square planar geometry.
Meloxicam, a non-steroidal anti-inflammatory drug (NSAID), has been used as an organic ligand in coordination complexes to produce better pharmacokinetic and pharmacodynamic properties and to study their chemical characteristics. In Tz-07, meloxicam acts in a monobasic bidentate manner and coordinates through the oxygen of the amide and the nitrogen of the thiazole rings, forming a complex with a slightly distorted octahedral geometry. Tz-07 was tested on several species of bacteria (E. coli, S. aureus, S. typhi, Citrobacter and Listeria) and fungi (A. niger and P. expansum) using the disk diffusion method and calculating the value of MIC.
On the other hand,
Tz-08 was obtained with two bidentate ligands coordinated to the metal centre and was evaluated in
S. aureus by the agar diffusion method.
Tz-08 did not show relevant antibacterial activity in
S. aureus; however, the scholars mention that it would be important to test this hexacoordinated copper(II) complex using other microorganism species to prove its potential
[13]. The heterobimetallic mixed complexes (
Tz-09a–TZ-09c) presented an octahedral geometry and were evaluated in the
S. aureus and
E. coli strains by the agar disk diffusion method.
Tz-09a and
Tz-09c were highly sensitive against
S. aureus, while the same complexes exhibited resistance against
E. coli.
Tz-09b presented resistance against both bacteria.
Tz-10 was reported to have a distorted octahedral geometry and was tested for its antibacterial activity on
S. aureus,
B. subtilis,
E. coli, and
Streptococcus and for its antifungal activity on
C. albicans and
A. niger by the agar well diffusion method.
Tz-10 showed an antibacterial inhibition zone of 12–15 mm and an antifungal inhibition zone of 8–10 mm, respectively. In addition, the MIC values of
Tz-10 were determined, and ranges of 17–22 μg/mL for antibacterial activity and 13–22 μg/mL for antifungal activity were found, respectively
[14]. Sulfathiazole was used again, but this time as a bidentate ligand, and the hexacoordinated copper(II) complex (
Tz-11) was obtained, which presented a distorted octahedral geometry. The complex was tested in bacteria such as
S. aureus,
E. coli and
P. aeruginosa by determining the MIC value.
Tz-12 was prepared from N-(2-thiazolyl)-1H-benzotriazole-1-carbothioamide, a polydentate ligand with several coordination sites (benzotriazole, carbothioamide, and thiazole). The thiazole donor sites (N and C-S-) of two ligand molecules were coordinated with a copper(II) ion, obtaining a structure with a square planar geometry. Preliminary biological screening was carried out at 20 mg/mL with S. aureus and E. coli.
Two new tetracopper(II) (
Tz-13a–
Tz-13b) complexes with a square-pyramidal geometry were reported. The in vitro antibacterial activities of
Tz-13a-b were qualitatively determined by the paper disc method against
S. aureus,
B. subtilis,
E. coli,
P. vulgaris and
P. aeruginosa. Furthermore, the complexes were evaluated by calculating the MIC value using the microdilution broth method. Both complexes showed good antibacterial activity (10–16 mm for
Tz-13a and 12–20 mm for
Tz-13b) against the selected microorganisms. In addition,
Tz-13a presented MIC values in the range of 50 to 180 μM, while
Tz-13b was in the range of 25 to 140 μM against the selected microorganisms. The scholars deduced that the higher activity of
Tz-13b may be due to the better permeability of the
trans-oxamido structure of the bacterial cells
[15].
In Tz-14, the organic ligand was coordinated through the nitrogen atom of the thiazole ring and the amino group linked to C2, and it acted in a bidentate manner. According to the spectroscopic results, the geometry of Tz-14 was square planar. The disk diffusion method was used to measure the antimicrobial activity of Tz-14 against different bacteria (S. pyogenes, P. aeruginosa and E. coli) and a fungus (Candida).
In the
Tz-15a–
Tz-15e complexes, the scholars did not report the structure; however, spectral studies revealed that the ligand is attached to the metal ion through the nitrogen atoms of the azomethine group and the thiazole ring. The effect of the anions on the antibacterial activity of the ligand and its metal complexes against the strains
P. aeruginosa,
K. pneumoniae,
S. typhi,
V. cholera and
B. megaterium was examined. The free ligand presented a low zone of inhibition for
K. pneumoniae and
P. aeruginosa (2 mm) and a slightly higher one for the other three bacteria (6–8 mm).
Tz-15e and
Tz-15b produced a very high inhibition halo for
P. aeruginosa (28–22 mm) and
K. pneumoniae (18–12 mm), and in other bacteria, the inhibition halo was low (8–10 mm).
Tz-15d showed moderate inhibition (6–18 mm) for all bacteria tested. The least effective bactericides were
Tz-15a (4–8 mm) and
Tz-15c (2–10 mm). The difference in the antibacterial activity of the
Tz-15a–
Tz-15e was related to the anions present in the complexes
[16].
For Tz-16, the scholars did not report the structure; however, the data from the elemental analyses and the determined molecular weight suggest a 1:2 stoichiometry (metal-ligand) and a mononuclear nature of the complex ML2.
The mixed heterobimetallic complex Tz-17 possesses a square planar structure conferred by the Schiff base ligand. Its antibacterial activity against various pathogenic bacteria (E. coli, S. aureus, P. aeruginosa and K. pneumoniae) was determined using the paper disk diffusion method.
The heterobimetallic complexes (
Tz-18a–Tz-18d) with the square planar structure were proposed, and the effect of anions (sulphate, nitrate, acetate or oxalate) on antibacterial activity was studied. They were screened against various pathogenic bacteria (
E. coli,
S. aureus,
P. aeruginosa and
K. pneumoniae) using the paper disk diffusion method. The complex with the nitrate anion (
Tz-18a) presented the highest percentage of inhibition (82–100%) in
E. coli., while in
S. aureus, there was no difference in the percentage of inhibition with respect to the anions used (64–82%). On the other hand, in
P. aeruginosa, the complex with the acetate anion (
Tz-18d) presented the highest percentage of inhibition (82–100%). In
K. pneumoniae, the complexes with nitrate, acetate and oxalate anions presented a higher percentage of inhibition (64–82%). With these evaluations, it was concluded that anions play an important role in the biological behaviour of copper(II) and iron(II) complexes. Some factors, such as solubility, conductivity, dipole moment, and cell permeability mechanism, are influenced by the presence of these anions
[17] (
Figure 3).
Figure 3. Copper complexes with bidentate thiazole ligands.
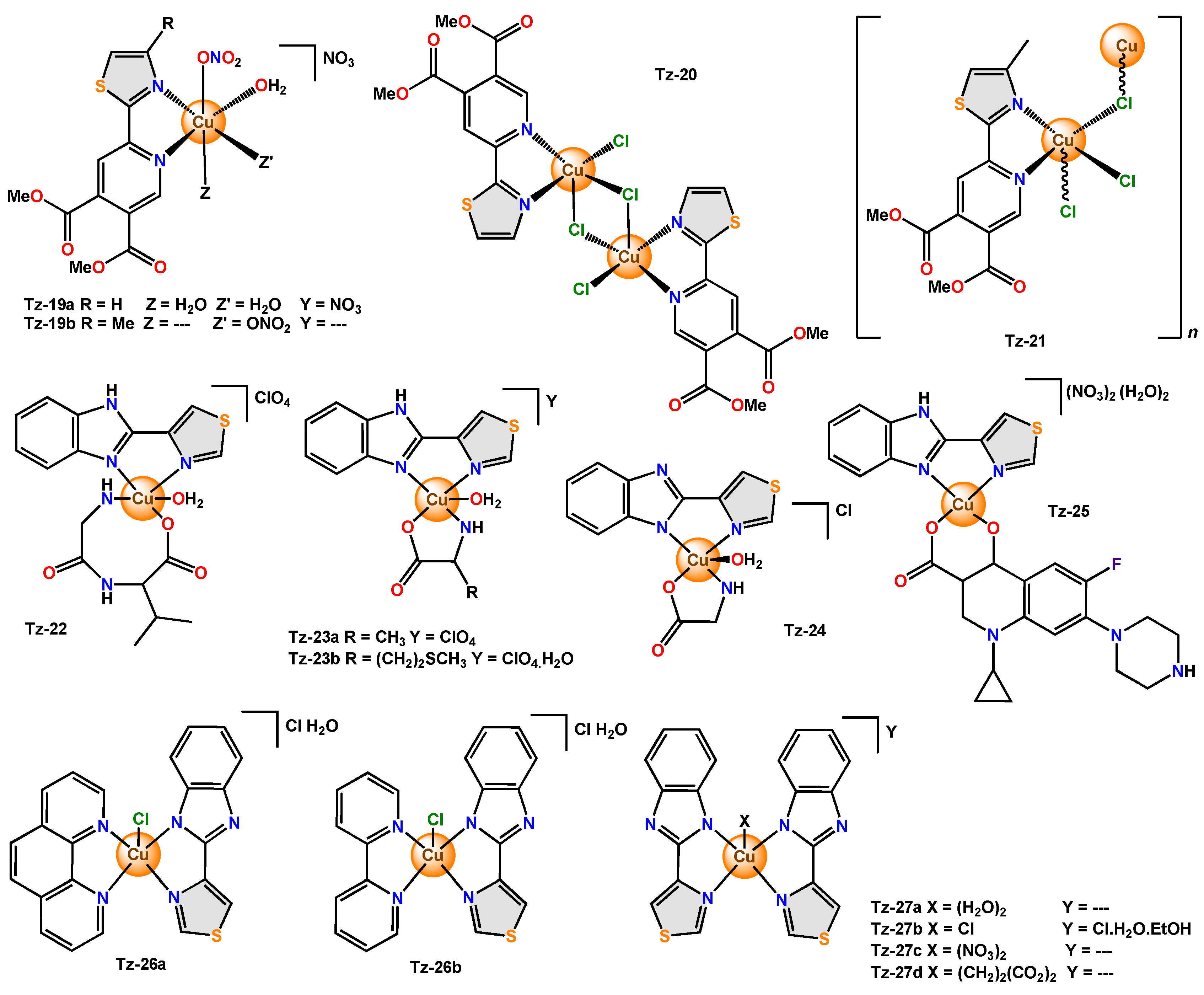
In the exploration of novel compounds with antimicrobial activity, complexes with bidentate ligands derived from thiazole have been reported, for example,
Tz-19a with elongated octahedral geometry and
Tz-19b,
Tz-20, and
Tz-21 with distorted square-pyramidal geometry.
Tz-20 is a dinuclear complex, and
Tz-21 is a polymeric complex. Both complexes did not affect the bacterial growth of
P. aeruginosa and
S. aureus. However, moderate antifungal activity was observed, especially in the case of
Tz-19b and
Tz-21, with MIC values of 31.25 µg/mL for both compounds against
C. albicans and no significant activity against
C. parapsilosis. Activity in
C. albicans was related to the ability to inhibit the hyphal formation of this strain
[18].
Furthermore, in all these cases, thiabendazole (TBZH), an anthelmintic, is used as a bidentate ligand. The copper(II) complex Tz-22 presented a distorted square pyramidal geometry in which two equatorial positions are occupied by the secondary ligand Gly-L-Val (N,O) and the other two by the thiabendazole ligand (N,N), while the axial position is occupied by an O atom from H2O.
Tz-23a was tested in four bacteria (
S. aureus,
B. subtilis,
S. typhi, and
E. coli) by the double dilution technique. The MIC value of the ligand was in the range of 320 to 512 μg/mL while for
Tz-23a, it was in the range of 128–160 μg/mL
[19].
Tz-23b showed an MIC value of 160 μg/mL in
B. subtilis,
S. aureus,
Salmonella, and
E. coli [20]. For
Tz-24, the central copper(II) ion was reported to be penta-coordinated and exhibited a distorted square pyramidal coordination geometry.
Other complexes using mixed ligands were reported, and such was the case with
Tz-25. This compound crystallized in the triclinic space group and was tested in both
Gram-positive and
Gram-negative bacteria. The best results were reported for
B. subtilis and
E. coli, with a MIC value of 0.5 μg/mL for both strains, while in
Salmonella, the MIC value was 2 μg/mL
[21]. In all three cases—
Tz-23a,
Tz-23b, and
Tz-25—the scholars attributed the result to the ability of the complex to bind to DNA in an intercalated fashion and cleave from pBR322 DNA. In the complexes
Tz-26a–
Tz-26b, the resulting coordination geometry was described as slightly distorted trigonal bipyramidal. The complexes were tested against bacteria (
B. subtilis,
S. aureus,
Salmonella and
E. coli). In addition, the MIC value was determined by the two-fold serial tube dilution method. The results showed that the antibacterial activity of
Tz-26a (16–64 µg/mL) was better than that of
Tz-26b (128–256 µg/mL) and that of the free ligand (512 µg/mL).
Thiabendazole complexes (
Tz-27a–
Tz-27d) were obtained from two chelating ligands coordinated to copper(II) centres and exhibited a trigonal bipyramidal geometry. The complexes were tested for their ability to inhibit the growth of
C. albicans at a concentration of 10 µg/mL, and the results were presented as percentage of cell growth. The free ligand presented a very poor growth inhibition (76%). However, when neutral thiabendazole was coordinated to the copper(II) ion, very potent anti-candida drugs were generated (
Tz-27a = 54%,
Tz-27b = 29%,
Tz-27c = 30%,
Tz-27d = 19%). In this case, the scholars mention that the mode of action of the complexes involves a reduction in the ergosterol content of the fungal cells
[22] (
Figure 4).
Figure 4. Copper complexes with bidentate thiazole ligands functionalized with pyridine and benzimidazole.
2.3. Copper Complexes with Tridentate Thiazole Ligands
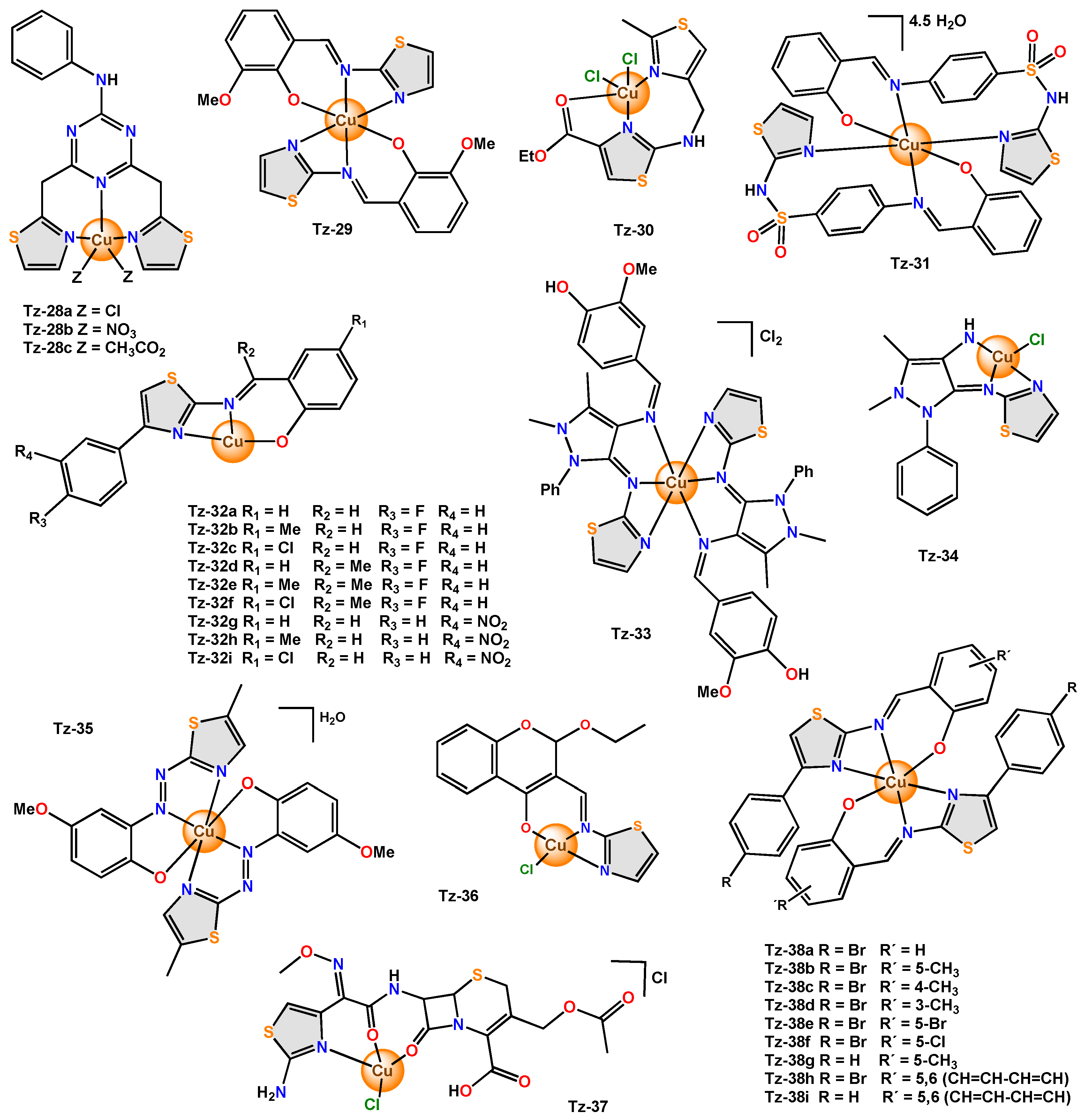
Complexes containing a tridentate thiazole ligand have also been reported, and such is the case with the Tz-28a–Tz-28c complexes, which were reported as hybrid compounds, since they contain two bioactive moieties: s-triazine and thiazole.
Tz-29 with octahedral geometry was tested for antibacterial (
M. luteus and
E. coli) and antifungal (
A. niger and
A. terreus) activity by the disk diffusion bioassay method. The results were based on the zone of inhibition for bacteria (16–11 mm) and fungi (17–15 mm), respectively, and were considerably better than those found for the free ligand for bacteria (13–12 mm) and fungi (15–14 mm). The scholars mention that metal complexes increased their lipophilic capacity as a result of chelation, which favours their uptake through the lipid membrane of microbes
[23]. A bisthiazole derivative was used in the synthesis of
Tz-30, which exhibited square pyramidal geometry. The antibacterial potential for
E. coli,
S. aureus and
P. aeruginosa, as well as the antifungal potential for
C. albicans, were determined by agar dilution tests.
Additionally,
Tz-31 was reported with a tetragonally distorted octahedral geometry and was tested against some bacteria strains (
E. coli,
P. aeruginosa,
S. aureus and
B. subtilis) by the well diffusion method. Coordination resulted in excellent inhibition in bacteria (40–45 mm), while ligand showed moderate inhibition (30–35 mm)
[24].
The antimicrobial activity of the ligand used in the synthesis of the
Tz-32a–
Tz-32f complexes (octahedral geometry) was reported using the cup plate method and by the serial dilution technique. The MIC values of the free ligand were found to be in the range of 15–20 mg/mL. In this case, the complexes were tested as insecticides and pesticides and showed a lower poisoning rate than commercial pesticides and insecticides
[25][26]. In the case of the complexes
Tz-32g–
Tz-32i reportedly with an octahedral geometry, they were tested in vitro against bacteria (
E. coli and
S. aureus) using the paper disc method at a concentration of 500 and 1000 ppm and against fungi (
A. flavus and
A. niger) using the mycelium dry weight method.
Additionally, Tz-33 was obtained using a Schiff base ligand derived from 2-aminothiazole, and it exhibited a distorted octahedral geometry. The bacteria detection effect of Tz-33 was tested against S. aureus, B. subtilis, P. aeuroginosa, and P. vulgaris by the well-diffusion method with different dilutions (20 μL, 40 μL, and 60 μL).
In the copper(II) complex Tz-34, the Schiff base acted as a tridentate ligand, and magnetic susceptibility data suggested that the geometric structure of Tz-34 was square planar. Its antimicrobial activity was examined with different species of bacteria (B. subtilis, S. aureus, E. coli and P. aeruginosa) by the well diffusion method at different concentrations (30, 60, 90 µg). As a result, it was detected that Tz-34 had greater activity (14–20 mm) than the free ligand (13 mm) at 90 µg, highlighting that for P. aeruginosa, the ligand did not present activity but that Tz-34 showed inhibition at 60 µg. (11 mm). In the case of Tz-35, an azo compound was used to functionalize the thiazole, and a tridentate chelating agent was obtained, which was coordinated through the phenolic oxygen atom, the nitrogen atom of the azo group (which is the closest phenyl ring), and from the nitrogen atom of the thiazole ring to form two chelated five-membered rings with a distorted octahedral geometry around the metal centre.
Tz-36 was reported with a distorted square planar geometry. Antimicrobial activity was determined with the disk diffusion method in bacteria (S. aureus, B. subtilis, P. aeruginosa, E. coli) and fungi (C. albicans and A. niger). A comparative study of the zones of inhibition for the free ligand and Tz-36 showed greater antibacterial activity (10–23 mm) and antifungal activity in C. albicans (16 mm) for Tz-36 with respect to the free ligand (4–18 mm and 11 mm, respectively). The scholars concluded that metal ions increased the lipophilic nature of Tz-36.
Tz-37 was obtained using cefotaxime, a third-generation cephalosporin, as a ligand. With the data acquired from the electronic spectra, the scholars found that the complex has a distorted square planar geometry. Microbiological screening was tested on S. aureus, E. coli, K. pneumoniae, S. enteritidis, P. mirabilis, and P. aeruginosa. Tz-37 exhibited superior antibacterial activity (38–54 mm) with respect to the free ligand (30–50 mm), except for P. aeruginosa.
For Tz-38a–Tz-38i, the electronic spectral data, together with the magnetic moment, suggested a distorted octahedral geometry for the complexes. The antibacterial activity of the ligand and its complexes against S. typhi was tested using the serial dilution technique.
The MIC values for the ligands were in the range of 20–26 µg/mL, while the values of the complexes were in the range of 10–15 µg/mL; that is, the complexes enhanced the antibacterial activity compared to the initial ligands
[27] (
Figure 5).
Figure 5. Copper complexes with tridentate thiazole ligands.
2.4. Copper Complexes with Tetradentate Thiazole Ligands
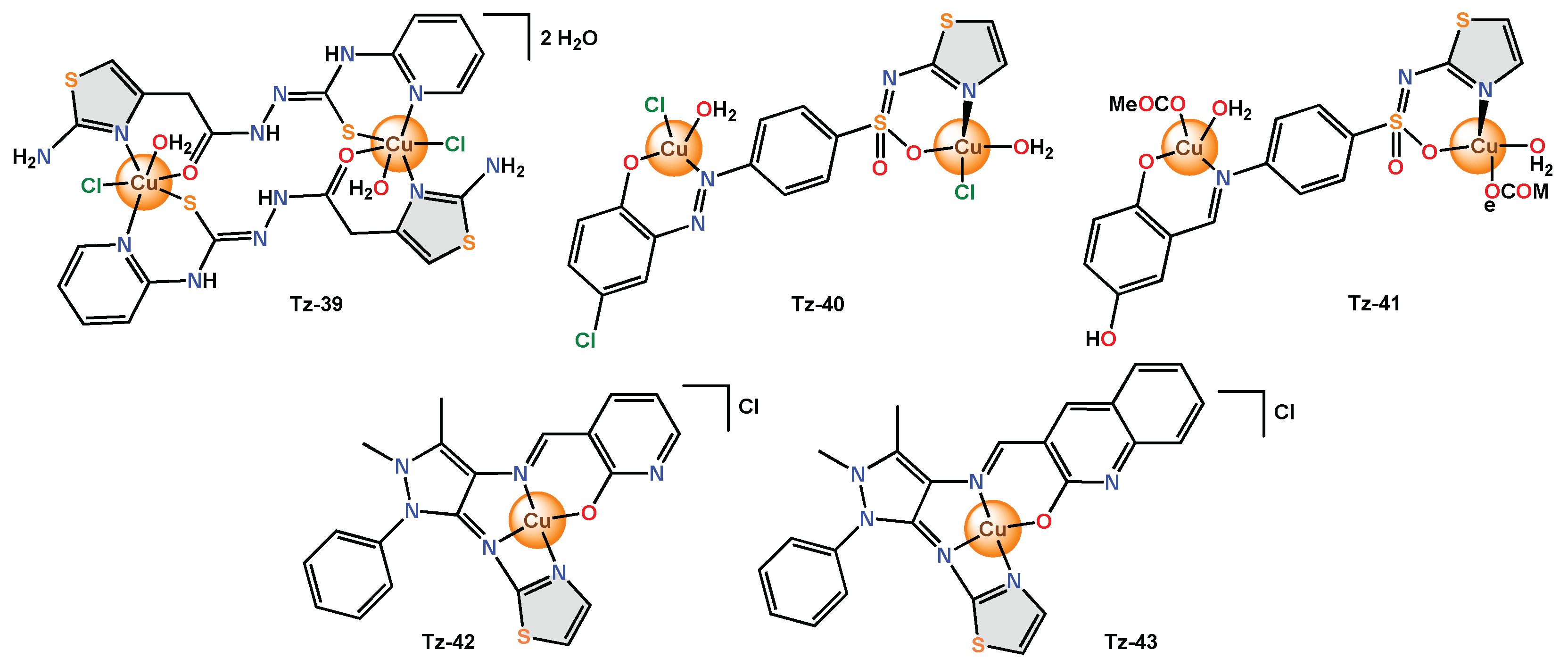
Finally, different complexes with tetradentate ligands have been reported, and such is the case with the functionalized thiazole with several donor sites that was used to obtain the Tz-39 binuclear complex with an octahedral geometry. The ligand and Tz-39 were evaluated by the disk diffusion method against bacterial strains (S. aureus and E. coli) and fungal strains (C. albicans).
The combination of sulfathiazole and an azo dye in the ligand allowed for obtaining the Tz-40 binuclear complex. The antibacterial (S. typhimurium, S. aureus) and antifungal (C. albicans, A. fumigates) activities were examined utilizing the agar diffusion technique.
On the other hand, the capacity of Schiff bases and sulfonamide derivatives was also used to obtain the binuclear copper chelate Tz-41, which exhibited an octahedral geometry. Tz-42 was reported as a mononuclear complex consisting of a Schiff base-type tetradentate ligand and a central copper atom with square planar geometry. Then, the antibacterial activity (E. coli, P. aeruginosa, B. subtilis and S. aureus) was tested by the well diffusion method at different concentrations (30, 60, 90 µg).
The metal complex
Tz-43 with a square planar geometry around the central metal ion was tested against pathogenic bacteria ((
E. coli,
P. aeruginosa,
B. subtilis and
S. aureus) and one fungal specie (
C. albicans) by the well diffusion method.
Tz-43 displayed higher antibacterial activity (13–23 mm) than the free ligand (12–14 mm) and much better fungal activity (20 and 14 mm, respectively). The in silico DNA results revealed that
Tz-43 was bound to DNA through hydrogen bonds
[28] (
Figure 6).
Figure 6. Copper complexes with tetradentate thiazole ligands.
3. Antibacterial and Antifungal Benzothiazole-Copper(II) Complexes
3.1. Copper Complexes with Monodentate Benzothiazole Ligands
The compound 2-Aminobenzothiazole and its simple metal complexes are well known for their possible biological activities, which include antimicrobial, anticancer, antifungal, anti-inflammatory, anthelmintic, antiulcer, and antitumor, among others
[29]. The 2-aminobenzothiazole derivatives contain three nucleophilic sites (N, S, NH
2); all or some of them could be potential coordination sites.
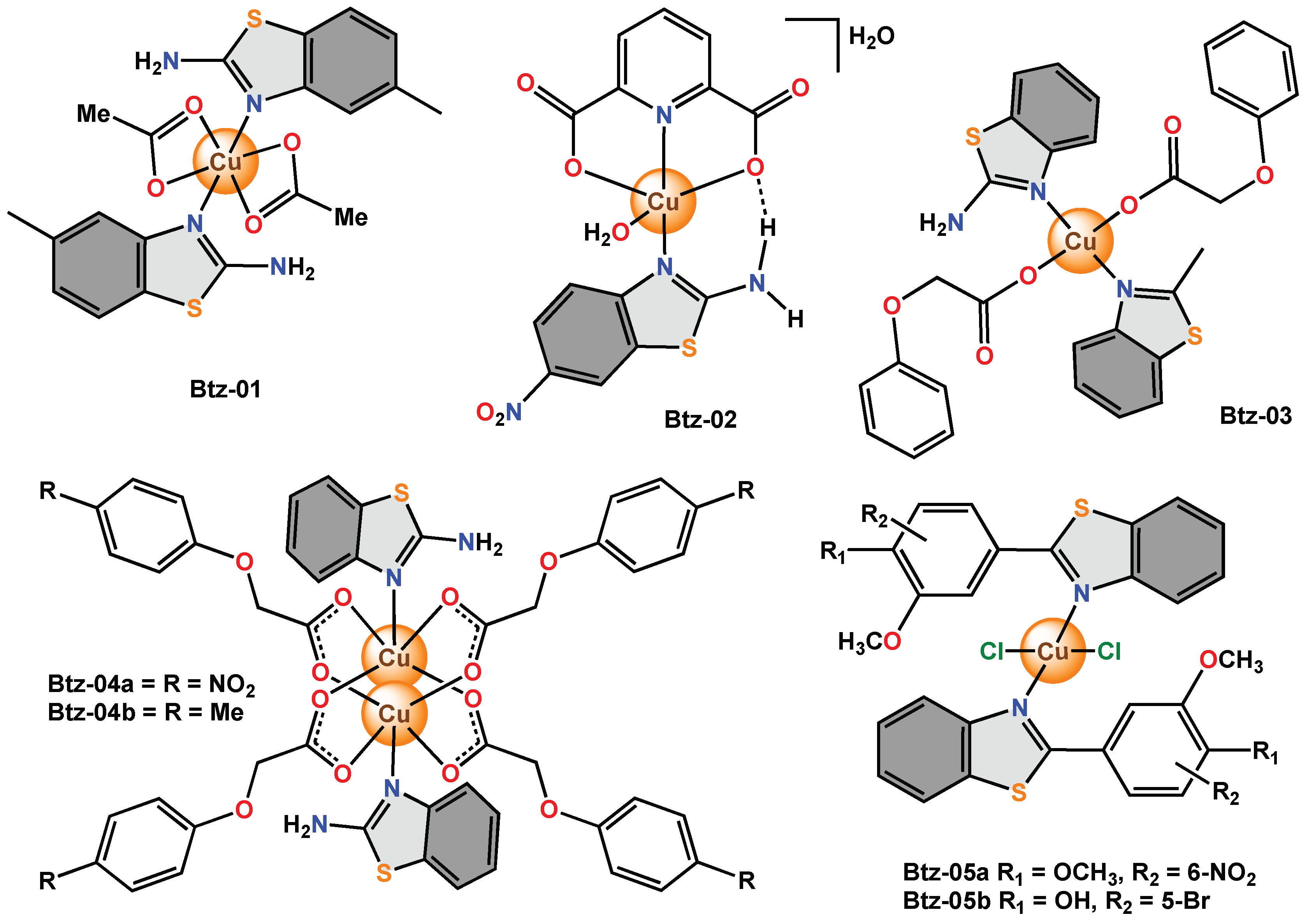
The benzothiazole used in Btz-01 contains three potential coordination sites (endocyclic nitrogen, exocyclic nitrogen, and sulphur). Between the three nucleophilic centres, the endocyclic nitrogen is the most basic and easily coordinates with the metal ion, producing a complex with an octahedral geometry. The antibacterial activity of Btz-01 was evaluated by the well assay, and the MIC value was calculated. The antifungal activity was also determined by the agar tube dilution method.
In BTz-02, the benzothiazole coordinated with the copper ion by monodentate bonding and the crystal structure showed a distorted square pyramidal structure. Intermolecular hydrogen bonds between the carboxylate group and the amino molecules play an important role in stabilizing the crystal structure.
In Btz-03, Btz-04a and Btz-04b, two benzothiazoles were coordinated with copper, forming mononuclear and binuclear complexes depending on the reaction conditions. The three complexes were tested for their antibacterial effect on E. coli and S. aureus and for their antifungal activity in R. mucilaginosa, C. albidosimilis, P. citrinum and A. niger by the double serial dilution method, and the MIC value was calculated. All three complexes showed moderate antibacterial effects between 10 and 20 µg/mL.
Considering that the change in the structure of the substituent at the C2 position of the benzothiazole commonly results in a change in its bioactivity, the derivatives
Btz-05a–
Btz-05b were synthesized. These compounds were reported with a square planar geometry and were evaluated for their antibacterial (
S. aureus,
S. pyogenus,
E. coli and
P. aeruginosa) and antifungal (
C. albicans,
A. niger,
A. clavatus) activity by the broth dilution method. The bioassays showed specific activity in the inhibition of the growth of the four bacteria studied (62.5–200 µg/mL). Meanwhile, in the fungicidal activity,
Btz-05a–
Btz-05b did not show significant activity (500–1000 µg/mL). In general,
Btz-05a–
Btz-05b exhibited better antibacterial activity (125–500 µg/mL) than their free ligands
[30] (
Figure 7).
Figure 7. Copper complexes with monodentate benzothiazole ligands.
3.2. Copper Complexes with Bidentate Benzothiazole Ligands
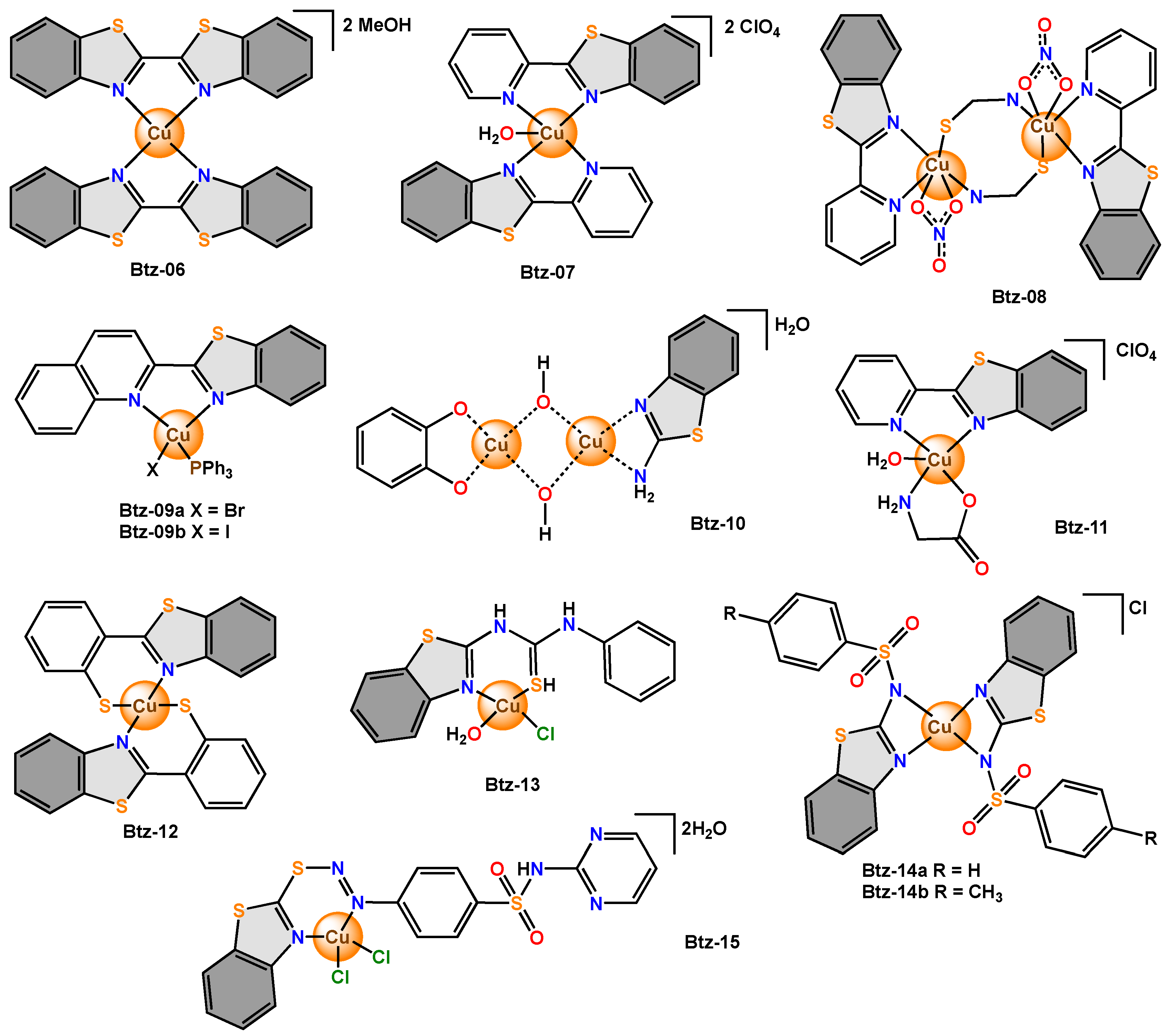
Benzothiazole and its derivatives are of great interest due to their coordination capacity, which is favoured by the delocalized π system in their structures. These compounds are capable of binding to molecules of biological interest, such as DNA, through π–π interactions [30]. In this sense, the antimicrobial activity of bidentate complexes derived from benzothiazole has been reported. In Btz-06 it was reported that the ligand acted as a bidentate agent that coordinated through the nitrogen atoms of the benzothiazole fragment, and it was also reported that the complex adopted a square planar geometry. The antimicrobial potential of Btz-06 was evaluated against bacteria (B. subtilis, S. aureus, K. oxytoca, E. coli, P. mirabilis and P. aeruginosa) and fungi (A. niger, A. flavus and R. stolonifera) by the agar diffusion method at 250 µg/mL and by the MIC value.
In 2015, two new copper(II) complexes, Btz-07 and Btz-08, were reported. The scholars found that Btz-07 was mononuclear and had a distorted square pyramidal geometry. Btz-08 was a thiocyanate bridged species in which each copper(II) ion adopted an irregular octahedral geometry.
For Btz-09a-Btz-09b, in the single crystal XRD analysis, a distorted tetrahedron was found around the central metal ion for both complexes. Then, the antibacterial activity was tested against S. aureus, B. cereus and S. typhi using the diffusion method. The inhibition zone of the ligand free is smaller (14–18 mm) than that of BTz-09a (14–24 mm) and Btz-09b (15–21 mm). These results showed that Btz-09a-Btz-09 were more bioactive than the free ligand.
BTz-10 is a copper(II) chelate and has a square planar geometry. This compound was evaluated against
S. aureus,
E. coli, and
K. pneumoniae Although Btz-10 was successfully characterized, no data was obtained indicating acceptable bioactivity against the bacteria tested. The scholars suggested that the concentration used was not sufficient to inhibit bacterial growth
[31]
Btz-11 was described as a coordinated compound with a slightly distorted square pyramid geometry. Btz-11 was tested on E. coli, Salmonella, B. subtilis and S. aureus and showed good antibacterial activities against these microorganisms (160–256 µg/mL) compared to the free ligand (1024 µg/mL).
Btz-12 with a square planar geometry was used as an antibacterial agent against
E. coli,
S. aureus,
B. subtilis and
P. aeruginosa by the cup plate method.
Btz-12 was active against
E. coli,
S. aureus, and
B. subtilis (8–10 mm), and no inhibition was detected against
P. aeruginosa. The free ligand was active in
E. coli and
B. subtilis but with increases in the inhibition zone (13 mm) with respect to
Btz-12 [32].
Btz-13 with a square planar geometry was tested in vitro against pathogenic bacteria (
E. coli and
S. aureus) and fungi (
C. albicans) by the agar streak dilution method to determine the MIC value.
In bacteria isolated under clinical conditions, Btz-14a and Btz-14b did not show antibacterial activity, while both ligands showed antimicrobial activity in E. coli and P. aeruginosa (10 mg/mL). In the bacterial strains, Btz-14a showed antimicrobial activity in S. aureus (10 mg/mL), while the free ligand (R=H) was active in P. aeruginosa, S. aureus, C. krusei and C. albicans (2.5–20 mg/mL).
In the case of
Btz-15, its potential as an antibacterial (
S. aureus,
B. cereus,
M. roseus) and antifungal (
A. flavus,
A. niger,
P. chrysogenum) agent was evaluated by the disk diffusion method.
Btz-15 showed a slight increase in the inhibition zones (7–13 mm) with respect to the free ligand (4–12 mm). The antimicrobial activity of
Btz-15 stood out in
M. roseus and
A. niger (13 and 10 mm), and it was inactive in
A flavus [33].
The results obtained with these investigations that include more sulphur atoms to the main ligand could be related to the studies that indicate that sulphur compounds regulate the potential toxic effects of metal ions such as copper
[34][35][36][37] (
Figure 8).
Figure 8. Copper complexes with bidentate benzothiazole ligands.
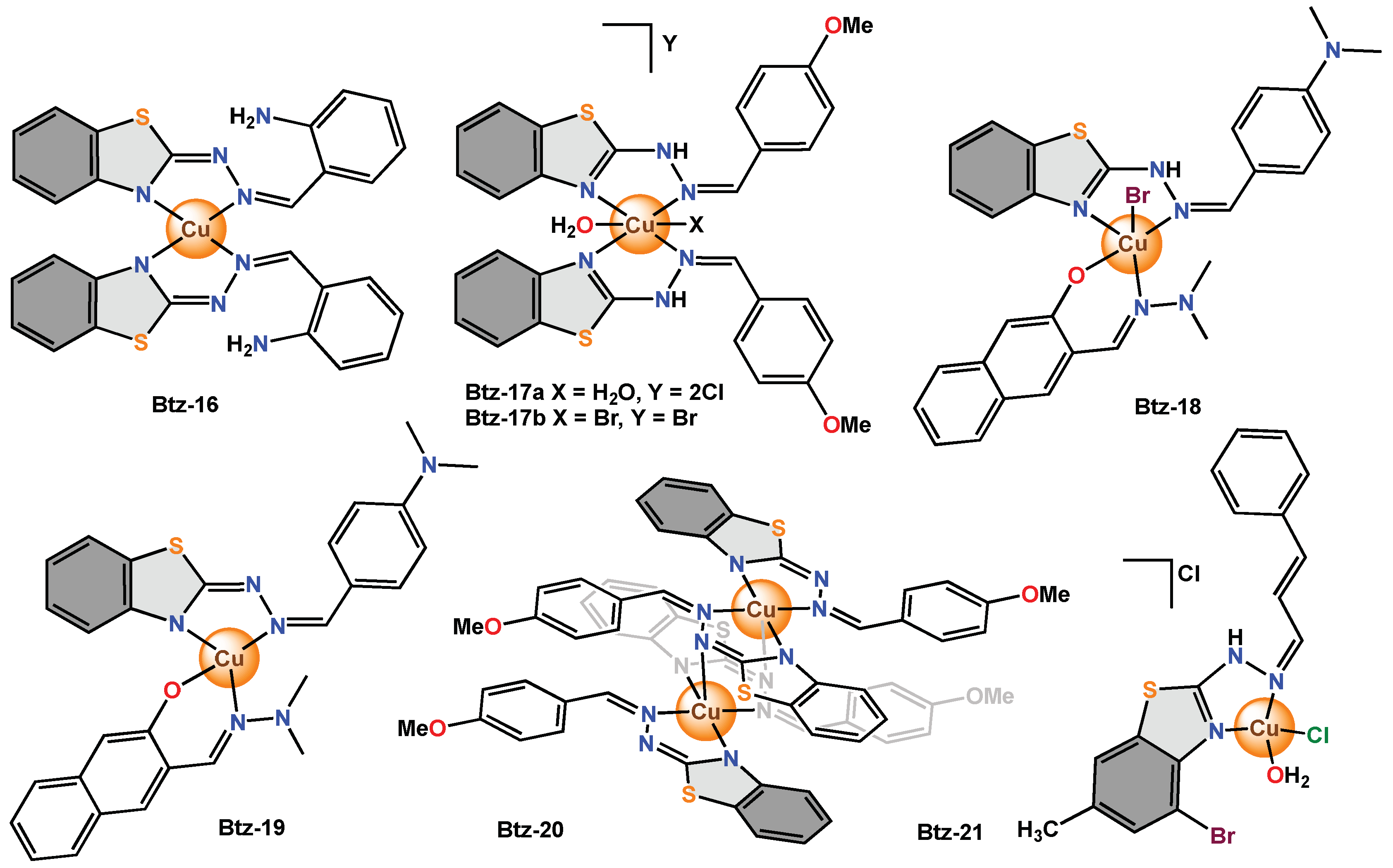
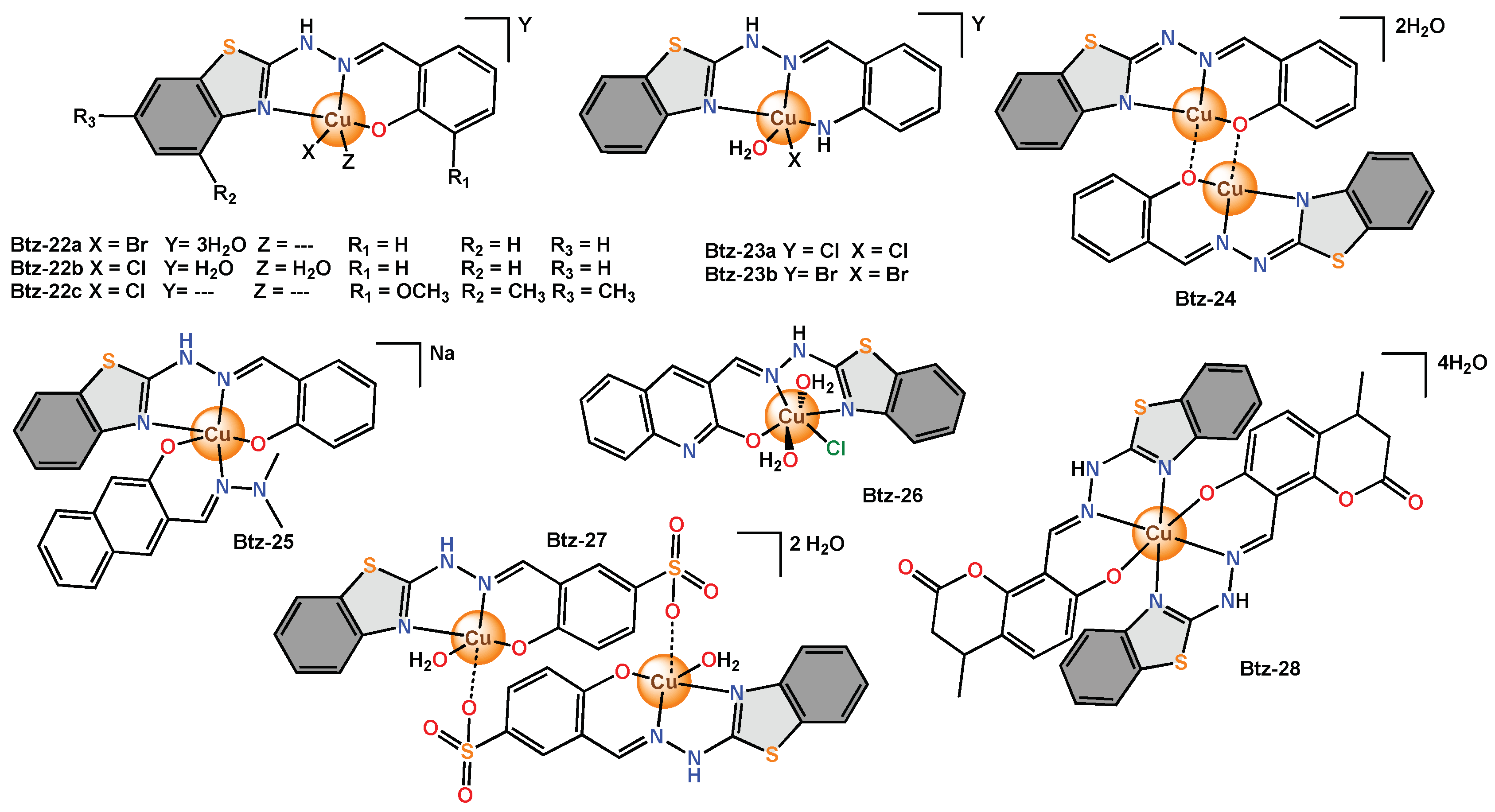
Recent research compared the use of benzothiazole derivatives containing the hydrazone moiety and different substituents on the aromatic rings. The purpose was to determine the form of coordination of these ligands and their influence on biological activity. The scholars reported different copper complexes with bidentate and tridentate ligands as in
Btz-16,
Btz-22–
Btz-24 [38],
Btz-17a,
Btz-17b,
Btz-20 [39], and even using mixed ligands as in
Btz-18,
Btz-19,
Btz-25 [40]. The complexes presented different distorted geometries; for example,
Btz-16 and
Btz-22a were pseudotetrahedral;
Btz-17a and
Btz-17b were octahedral;
Btz-18,
Btz-20,
BTz-22b,
Btz-23a,
Btz-23b and
Btz-25 were square pyramidal; and
Btz-19 and
Btz-24 were square planar. Moreover, in the IR spectrum, the absorption band of the NH group disappears, indicating the tautomeric form of the
Btz-16,
Btz-19,
Btz-20 and
Btz-24 complexes. In all cases, for antimicrobial activity, 10 μL of a 10
−4 M solution of each complex was used against bacteria (
E. coli,
P. aeruginosa,
Staphylococcus coagulase-positive,
Streptococcus β-hemolytic type A,
Streptococcus β-hemolytic type B and
S. aureus) and fungi (
C. albicans). The ligand used in the synthesis of
Btz-16,
Btz-17a,
Btz-17b and
Btz-20 with methoxy and amino groups as substituents on the phenyl ring did not show antimicrobial activity. Ligands used in the synthesis of
Btz-18,
Btz-19 and
Btz-22–
Btz-25 with dimethylamino and hydroxyl groups as substituents on the phenyl ring were weak inhibitors (0–3 mm). Nevertheless, all the complexes were biologically active. The most active bidentate ligand complexes were
Btz-17a,
Btz-18 and
Btz-19 (2–5 mm), and the most active tridentate ligand complexes were
Btz-22a,
Btz-24 and
Btz-25 (1–5 mm). The scholars mention that it is possibly due to the more rapid diffusion of these metal complexes through the cell membrane.
On the other hand,
Btz-21 was tested as an antibacterial and antifungal agent by the agar diffusion method.
Btz-21 showed good antibacterial activity against
E. coli and
B. subtilis, with inhibition zones of 2.5 and 2.0 mm, respectively. The study of antifungal activity showed inhibition zones of 1.6 and 1.4 mm against
A. niger and
A. flavus, respectively. Nonetheless, the free ligand showed antimicrobial activity only in
B. subtilis, with an inhibition halo of 1.1 mm
[41] (
Figure 9 and
Figure 10).
Figure 9. Copper complexes with bidentate benzothiazole ligands functionalized with hydrazine moieties.
Figure 10. Copper complexes with tridentate benzothiazole ligands functionalized with hydrazine moieties.
3.3. Copper Complexes with Tridentate and Tetradentate Benzothiazole Ligands
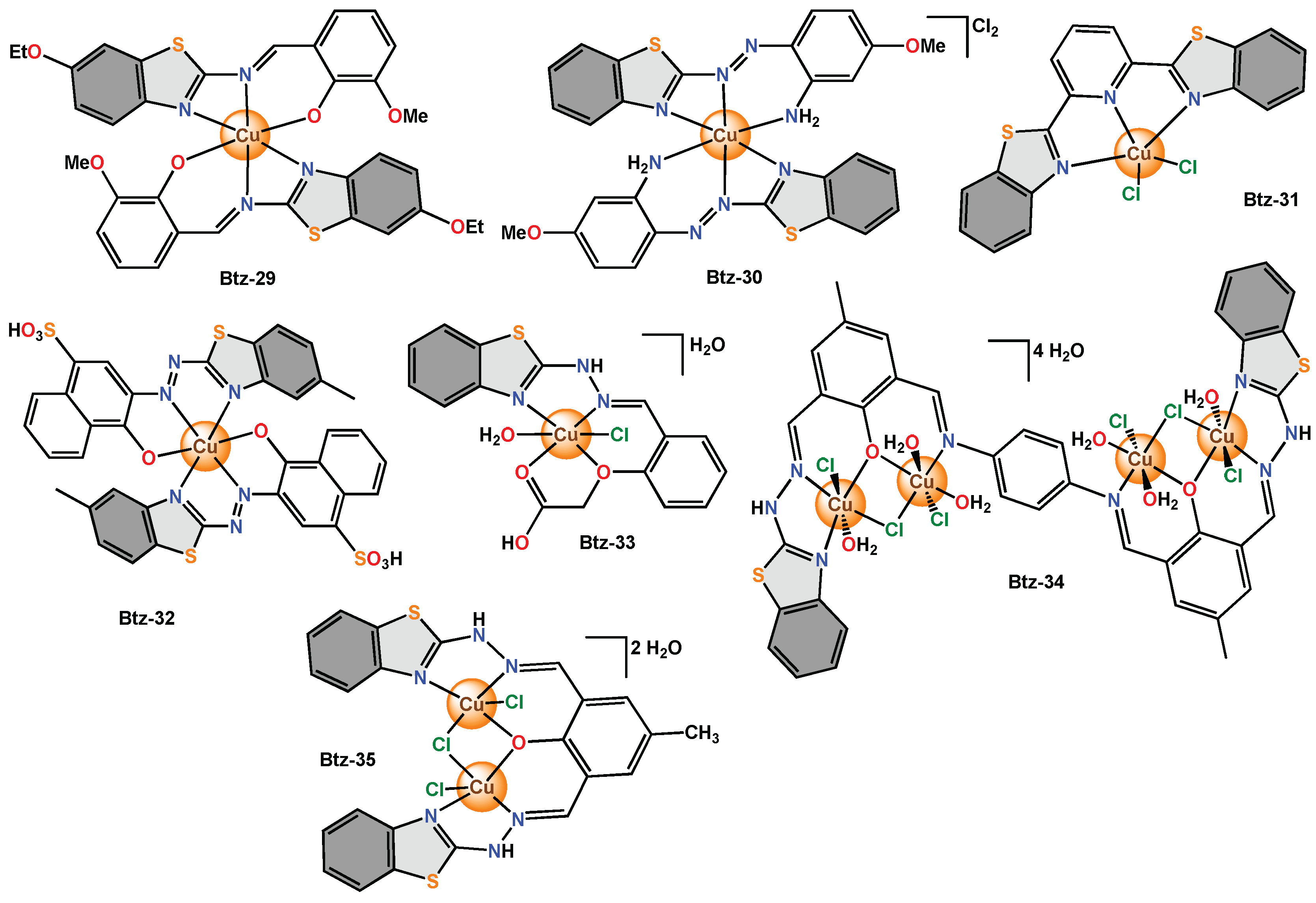
Tridentate complexes have been reported by different research groups and have been used as antimicrobial agents. For example, Btz-26 with an octahedral geometry was screened against bacteria (E. coli and P. aeruginosa) and fungi (A. niger and Cladosporium). A new Schiff base type ligand was used to obtain Btz-27, which is a dinuclear copper complex that adopts a pentacoordinate quasi-square pyramidal geometry. Btz-28 with octahedral geometry was proposed as a candidate for the evaluation of antibacterial (S. aureus and E. coli) and antifungal (C. albicans and A. fumigatus) activity by the broth microdilution method. Btz-29 with octahedral geometry was proposed as a candidate to test its antimicrobial activity against bacteria (M. luteus and E. coli) and fungi (A. niger and A. terreus) using the disk diffusion bioassay method. Btz-30 with an octahedral geometry was tested in bacteria (S. aureus, S. epidermis, P. aeruginosa, Streptococcus, E. coli, Klebsiella sp.) and fungi (C. albicans) by employing the disk diffusion method. Btz-31 is a copper(II) complex whose structural analysis revealed a distorted square pyramidal geometry. The antibacterial activity of Btz-31 against bacteria (S. epidermidis and A. baumannii) was studied. Btz-32 with an octahedral geometry was used to evaluate the antimicrobial activity against pathogenic bacteria (S. aureus, Enterococcus, S. viridans, E. coli, P. aeruginosa and K. pneumoniae) and fungi (A. niger and A. flavus).
On the other hand,
Btz-33 was described as a monomeric complex with octahedral geometry. Therefore, a bioassay of
Btz-33 and the free ligand was carried out by analysing an antibiogram to determine its antibacterial activity. Antibiograms showed that the free ligand was inactive in
E. coli, but there was an improvement in the inhibitory activity of
Btz-33 [42].
Btz-34 was reported as a phenoxyde bridged tetranuclear copper(II) complex. This compound presented an octahedral geometry and was tested in bacteria (
P. aeruginosa,
S. aureus) and fungi (
A. niger and
C. albicans) at different concentrations (5–100 μg/mL).
Btz-35 was a reported binuclear complex with a square pyramidal geometry and was tested in bacteria (
P. aeruginosa and
B. cirroflagellosus) and fungi (
A. niger and
C. albicans) by the cup plate method.
Btz-35 presented low inhibition in
P. aeruginosa (5.2%) but high antimicrobial inhibition in
B. cirroflagellosus,
A. niger and
C. albicans (61.9 to 260%)
[43] (
Figure 11).
Figure 11. Copper complexes with diverse tridentate and tetradentate benzothiazole ligands.