Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by DAVID MORALES-MORALES.
Thiazole and benzothiazole are present in different structures with interesting biological effects and are used to develop new effective antimicrobial agents. Moreover, nitrogen atoms that are present in this heterocycle allow for coordination with various metals, forming metal complexes that enhance the biological activity of organic ligands that are often used as commercial drugs.
- thiazole
- benzothiazole
- copper complexes
- heterocycles
- azoles
- antibacterial
- antifungal
1. Introduction
Heterocycles that contain nitrogen atoms in their structure play an important role in the discovery of new drugs, and such is the case with azoles, which occupy a privileged place in the chemical, biological and medical area due to their contributions to the synthesis of new compounds with therapeutic potential in humans [1]. Among the azoles, it can highlight thiazole (1,3-thiazole), which is one of the most important heterocycles in organic chemistry and in the design of molecules of biological interest [2,3][2][3]. This compound is made up of a five-membered unicycle and contains a sulphur atom (hydrogen bond acceptor) in the S1 position and a nitrogen atom (donor of an electron pair) in the N3 position [4,5][4][5] (Figure 1). The unsubstituted thiazole has a minimal formula, C3H3NS, and is a versatile building block enabling new drug development. It is found in several synthetic substances and in some natural compounds [6,7][6][7]. For example, vitamin B1 (thiamine) contains in its global structure the thiazole nucleus, which serves as an electron trap and participates in the decarboxylation of α-ketoacids and helps the normal functioning of the central nervous system (CNS) due to its role in the synthesis of acetylcholine [8]. In addition, thiazoles are part of various natural products such as alkaloids, steroids, and flavones, among others [9,10][9][10].
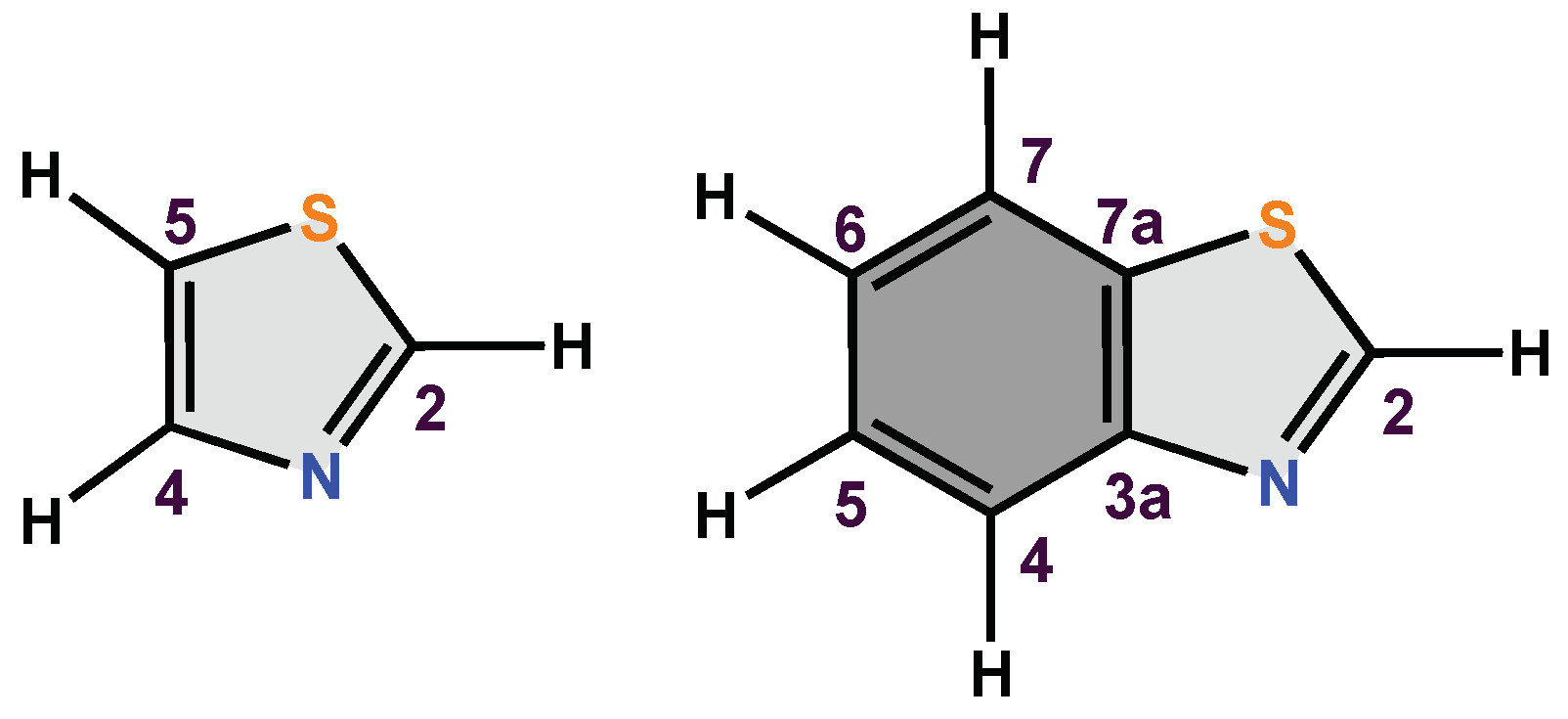
Figure 1.
General structure of thiazole and benzothiazole.
2. Antibacterial and Antifungal Thiazole-Copper(II) Complexes
2.1. Copper Complexes with Monodentate Thiazole Ligands
Sulfathiazole, a sulfa drug, is mainly used in the treatment of respiratory tract and digestive system infections, as well as skin and urinary tract infections [66][11]. This compound can act as a ligand due to the acidic behaviour of the –SO2–NH– site, forming an anionic donor supported by the presence of O, N and/or S atoms in the adjacent heterocyclic ring. This drug was used as a ligand, along with the co-ligand dien (diethylenetriamine) and the copper(II) atom in the Tz-01 complex. Another monodentate sulfathiazole-based mononuclear coordination complex (Tz-02) was obtained; this presented a slightly distorted square pyramidal environment around the copper(II) ion. In addition to sulfathiazole (STz), Tz-02 contains 1,10-phenanthroline (phen) as a secondary ligand. The antimicrobial activity of Tz-02 and its free ligand against bacteria (S. aureus, B. subtilis, P. aeruginosa and E. coli) and fungi (C. albicans and A. flavus) was determined using the microdilution technique. MIC values in S. aureus did not show antibacterial activity for free ligand and Tz-02 (>512 µg/mL). The neutral complex Tz-03 was obtained with octahedral geometry around the central metal ion. To determine their antibacterial and antifungal activity, the aminothiazole ligand and Tz-03 were tested against human pathogenic bacteria (E. coli, S. aureus) and fungi (A. niger, A. flavus) using the ditch-plate method. Tz-03 showed a larger zone of inhibition for bacteria (12 mm) and fungi (2–9 mm) than the free ligand (0–5 and 0–6 mm, respectively) [69][12]. Finally, the antibacterial activity of a new series of coordination complexes (Tz-04a–Tz-04e) with a distorted octahedral geometry was evaluated against E. coli, B. subtilis and B. cereus using the twofold serial dilution method. (Figure 2)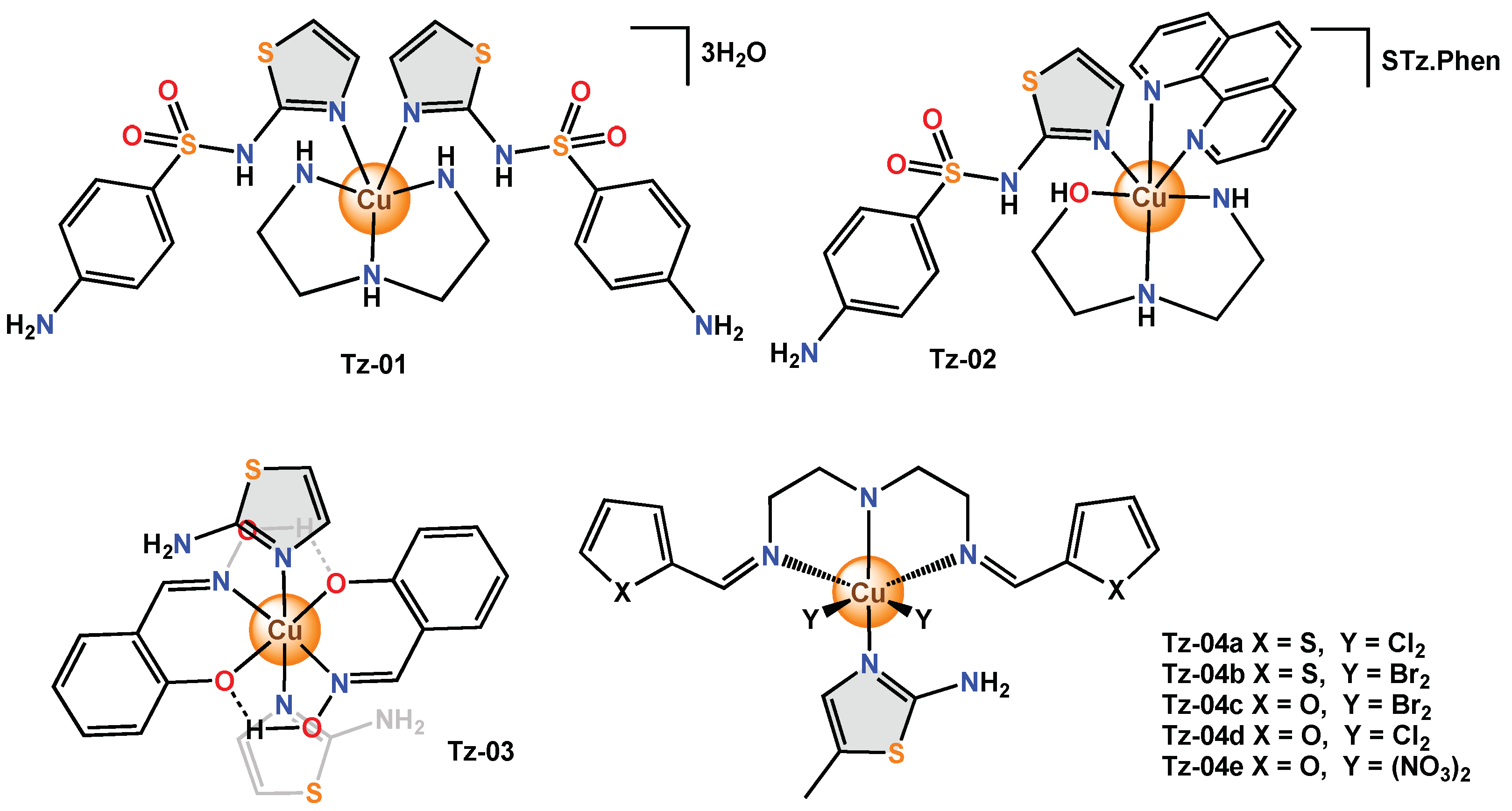
Figure 62.
Copper complexes with monodentate thiazole ligands.
Tz-05, which presented a square planar geometry around the metal centre. In this study, the antimicrobial activity was investigated using the disk diffusion method against the bacteria (2.2. Copper Complexes with Bidentate Thiazole Ligands
On the other hand, coordination complexes that use bidentate ligands that contain different thiazole derivatives in their structure have been reported; such is the case with
, which presented a square planar geometry around the metal centre. In the study, the antimicrobial activity was investigated using the disk diffusion method against the bacteria (
,
,
,
), and the fungi (
and
).
The antimicrobial activity of
was described using a new chalconoid ligand. In this compound, there are keto groups and π-olefin bonds that act as the preferred binding sites for metal ions. In
the nitrogen atom of the thiazole and the oxygen of the carbonyl group act as effective donors on the metal bond to obtain a complex with a square planar geometry.
Meloxicam, a non-steroidal anti-inflammatory drug (NSAID), has been used as an organic ligand in coordination complexes to produce better pharmacokinetic and pharmacodynamic properties and to study their chemical characteristics. In
, meloxicam acts in a monobasic bidentate manner and coordinates through the oxygen of the amide and the nitrogen of the thiazole rings, forming a complex with a slightly distorted octahedral geometry.
was tested on several species of bacteria (
,
,
,
and
) and fungi (
and
) using the disk diffusion method and calculating the value of MIC.
On the other hand,
was obtained with two bidentate ligands coordinated to the metal centre and was evaluated in
by the agar diffusion method.
did not show relevant antibacterial activity in
; however, the scholars mention that it would be important to test this hexacoordinated copper(II) complex using other microorganism species to prove its potential [13]. The heterobimetallic mixed complexes (
) presented an octahedral geometry and were evaluated in the
and
strains by the agar disk diffusion method.
and
were highly sensitive against
, while the same complexes exhibited resistance against
.
presented resistance against both bacteria.
was reported to have a distorted octahedral geometry and was tested for its antibacterial activity on
,
,
, and
and for its antifungal activity on
and
by the agar well diffusion method.
showed an antibacterial inhibition zone of 12–15 mm and an antifungal inhibition zone of 8–10 mm, respectively. In addition, the MIC values of
were determined, and ranges of 17–22 μg/mL for antibacterial activity and 13–22 μg/mL for antifungal activity were found, respectively [14]. Sulfathiazole was used again, but this time as a bidentate ligand, and the hexacoordinated copper(II) complex (
) was obtained, which presented a distorted octahedral geometry. The complex was tested in bacteria such as
,
and
by determining the MIC value.
was prepared from
-(2-thiazolyl)-1
-benzotriazole-1-carbothioamide, a polydentate ligand with several coordination sites (benzotriazole, carbothioamide, and thiazole). The thiazole donor sites (N and C-S-) of two ligand molecules were coordinated with a copper(II) ion, obtaining a structure with a square planar geometry. Preliminary biological screening was carried out at 20 mg/mL with
and
.
Two new tetracopper(II) (
–
) complexes with a square-pyramidal geometry were reported. The in vitro antibacterial activities of
were qualitatively determined by the paper disc method against
,
,
,
and
. Furthermore, the complexes were evaluated by calculating the MIC value using the microdilution broth method. Both complexes showed good antibacterial activity (10–16 mm for
and 12–20 mm for
) against the selected microorganisms. In addition,
presented MIC values in the range of 50 to 180 μM, while
was in the range of 25 to 140 μM against the selected microorganisms. The scholars deduced that the higher activity of
may be due to the better permeability of the
In Tz-14Tz-14Tz-14S. pyogenesP. aeruginosaE. coliCandidaTz-15aTz-15eP. aeruginosaK. pneumoniaeS. typhiV. choleraB. megateriumK. pneumoniaeP. aeruginosaTz-15eTz-15bP. aeruginosaK. pneumoniaeTz-15dTz-15aTz-15cTz-15aTz-15e was related to the anions present in the complexes [81].
, the organic ligand was coordinated through the nitrogen atom of the thiazole ring and the amino group linked to C2, and it acted in a bidentate manner. According to the spectroscopic results, the geometry of
was square planar. The disk diffusion method was used to measure the antimicrobial activity of
against different bacteria (
,
and
) and a fungus (
).
In the
–
complexes, the scholars did not report the structure; however, spectral studies revealed that the ligand is attached to the metal ion through the nitrogen atoms of the azomethine group and the thiazole ring. The effect of the anions on the antibacterial activity of the ligand and its metal complexes against the strains
,
,
,
and
was examined. The free ligand presented a low zone of inhibition for
and
(2 mm) and a slightly higher one for the other three bacteria (6–8 mm).
and
produced a very high inhibition halo for
(28–22 mm) and
(18–12 mm), and in other bacteria, the inhibition halo was low (8–10 mm).
showed moderate inhibition (6–18 mm) for all bacteria tested. The least effective bactericides were
(4–8 mm) and
(2–10 mm). The difference in the antibacterial activity of the
–
For Tz-162Tz-17E. coliS. aureusP. aeruginosaK. pneumoniae
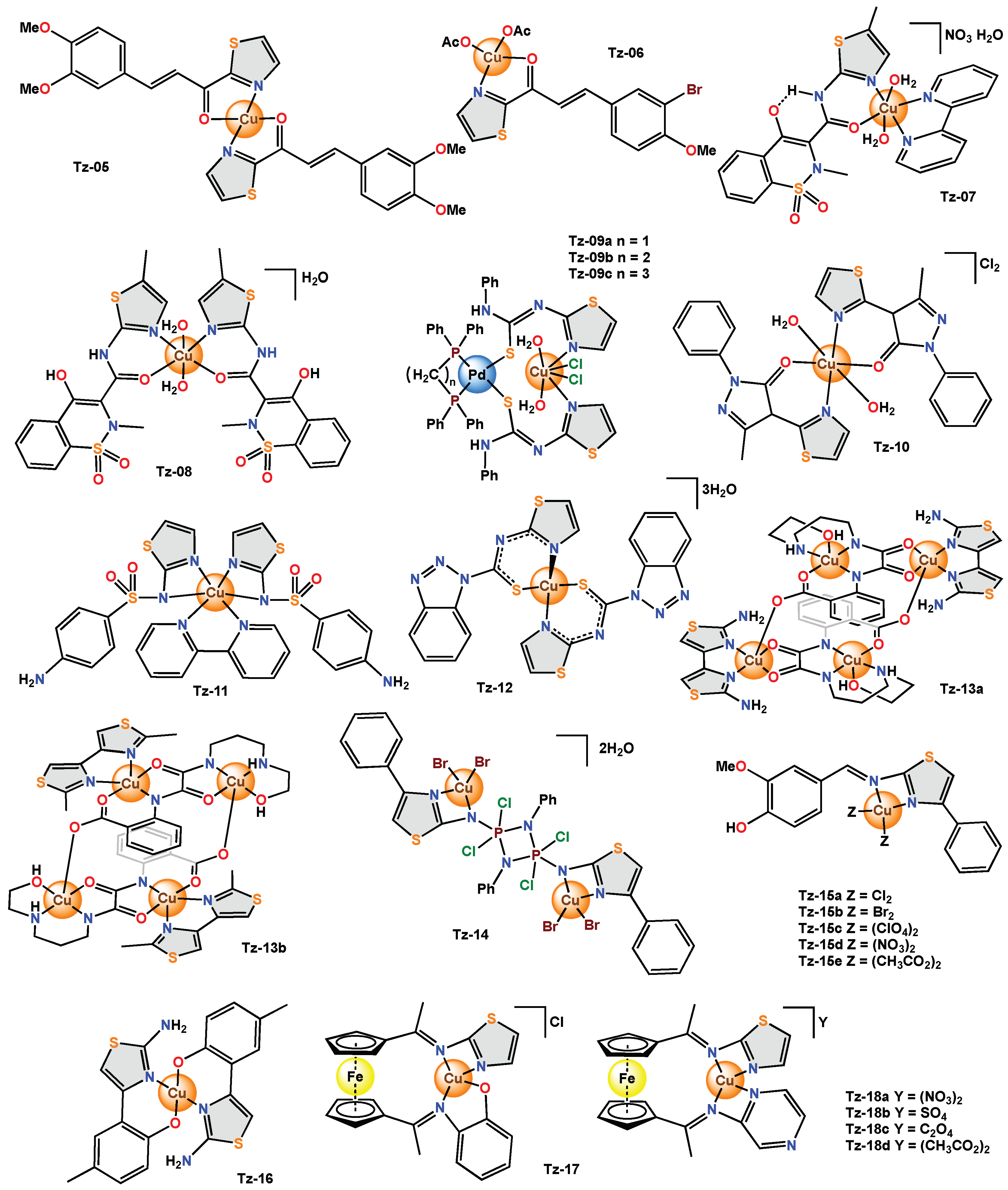
, the scholars did not report the structure; however, the data from the elemental analyses and the determined molecular weight suggest a 1:2 stoichiometry (metal-ligand) and a mononuclear nature of the complex ML
.
The mixed heterobimetallic complex
possesses a square planar structure conferred by the Schiff base ligand. Its antibacterial activity against various pathogenic bacteria (
,
,
and
) was determined using the paper disk diffusion method.
The heterobimetallic complexes (
Tz-18a–Tz-18d) with the square planar structure were proposed, and the effect of anions (sulphate, nitrate, acetate or oxalate) on antibacterial activity was studied. They were screened against various pathogenic bacteria (
E. coli,
S. aureus,
P. aeruginosa and
K. pneumoniae) using the paper disk diffusion method. The complex with the nitrate anion (
Tz-18a) presented the highest percentage of inhibition (82–100%) in
E. coli., while in
S. aureus, there was no difference in the percentage of inhibition with respect to the anions used (64–82%). On the other hand, in
P. aeruginosa, the complex with the acetate anion (
Tz-18d) presented the highest percentage of inhibition (82–100%). In
K. pneumoniae, the complexes with nitrate, acetate and oxalate anions presented a higher percentage of inhibition (64–82%). With these evaluations, it was concluded that anions play an important role in the biological behaviour of copper(II) and iron(II) complexes. Some factors, such as solubility, conductivity, dipole moment, and cell permeability mechanism, are influenced by the presence of these anions [84] (, the complexes with nitrate, acetate and oxalate anions presented a higher percentage of inhibition (64–82%). With these evaluations, it was concluded that anions play an important role in the biological behaviour of copper(II) and iron(II) complexes. Some factors, such as solubility, conductivity, dipole moment, and cell permeability mechanism, are influenced by the presence of these anions [17] (
Figure 7).3).
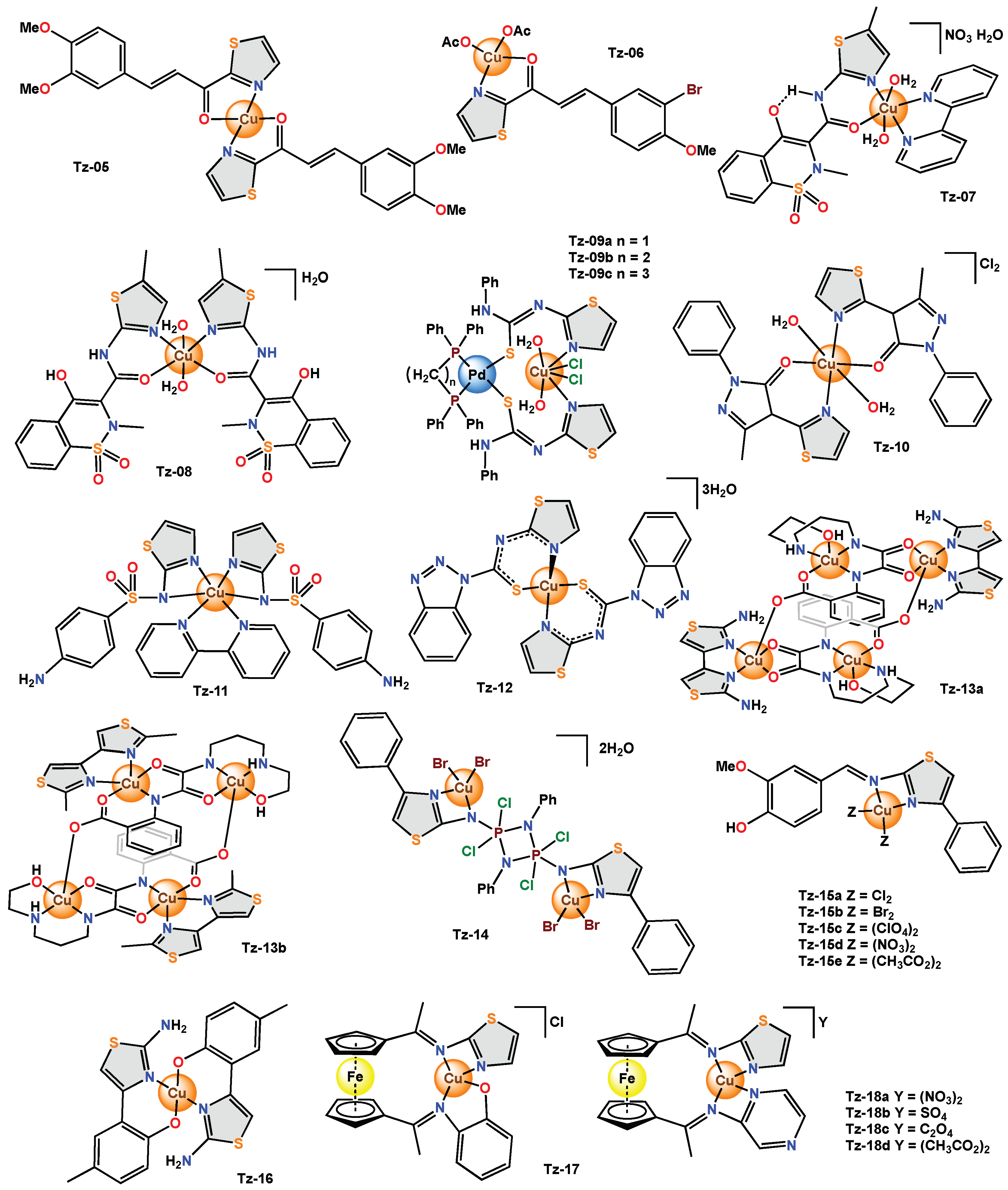
Figure 73. Copper complexes with bidentate thiazole ligands.
In the exploration of novel compounds with antimicrobial activity, complexes with bidentate ligands derived from thiazole have been reported, for example,
Tz-19a
with elongated octahedral geometry and
Tz-19b
,
Tz-20,
and
Tz-21
with distorted square-pyramidal geometry.
Tz-20
is a dinuclear complex, and
Tz-21
is a polymeric complex. Both complexes did not affect the bacterial growth of
P. aeruginosa
and
S. aureus
. However, moderate antifungal activity was observed, especially in the case of
Tz-19b
and
Tz-21,
with MIC values of 31.25 µg/mL for both compounds against
C. albicans
and no significant activity against
C. parapsilosis
. Activity in
Furthermore, in all these cases, thiabendazole (TBZH), an anthelmintic, is used as a bidentate ligand. The copper(II) complex
Tz-22
presented a distorted square pyramidal geometry in which two equatorial positions are occupied by the secondary ligand Gly-L-Val (N,O) and the other two by the thiabendazole ligand (N,N), while the axial position is occupied by an O atom from H
2
O.
Tz-23a
was tested in four bacteria (
S. aureus
,
B. subtilis
,
S. typhi
, and
E. coli
) by the double dilution technique. The MIC value of the ligand was in the range of 320 to 512 μg/mL while for
Tz-23b
showed an MIC value of 160 μg/mL in
B. subtilis
,
S. aureus
,
Salmonella
, and
Tz-24,
the central copper(II) ion was reported to be penta-coordinated and exhibited a distorted square pyramidal coordination geometry.
Other complexes using mixed ligands were reported, and such was the case with
Tz-25
. This compound crystallized in the triclinic space group and was tested in both
Gram
-positive and
Gram
-negative bacteria. The best results were reported for
B. subtilis
and
E. coli
, with a MIC value of 0.5 μg/mL for both strains, while in
Tz-23a
,
Tz-23b
, and
Tz-25
—the scholars attributed the result to the ability of the complex to bind to DNA in an intercalated fashion and cleave from pBR322 DNA. In the complexes
Tz-26a
–
Tz-26b
, the resulting coordination geometry was described as slightly distorted trigonal bipyramidal. The complexes were tested against bacteria (
B. subtilis
,
S. aureus
,
Salmonella
and
E. coli
). In addition, the MIC value was determined by the two-fold serial tube dilution method. The results showed that the antibacterial activity of
Tz-26a
(16–64 µg/mL) was better than that of
Tz-26b
(128–256 µg/mL) and that of the free ligand (512 µg/mL).
Thiabendazole complexes (
Tz-27a
–
Tz-27d
) were obtained from two chelating ligands coordinated to copper(II) centres and exhibited a trigonal bipyramidal geometry. The complexes were tested for their ability to inhibit the growth of
C. albicans
at a concentration of 10 µg/mL, and the results were presented as percentage of cell growth. The free ligand presented a very poor growth inhibition (76%). However, when neutral thiabendazole was coordinated to the copper(II) ion, very potent anti-candida drugs were generated (
Tz-27a
= 54%,
Tz-27b
= 29%,
Tz-27c
= 30%,
Tz-27d = 19%). In this case, the scholars mention that the mode of action of the complexes involves a reduction in the ergosterol content of the fungal cells [92] (
= 19%). In this case, the scholars mention that the mode of action of the complexes involves a reduction in the ergosterol content of the fungal cells [22] (
Figure 8).
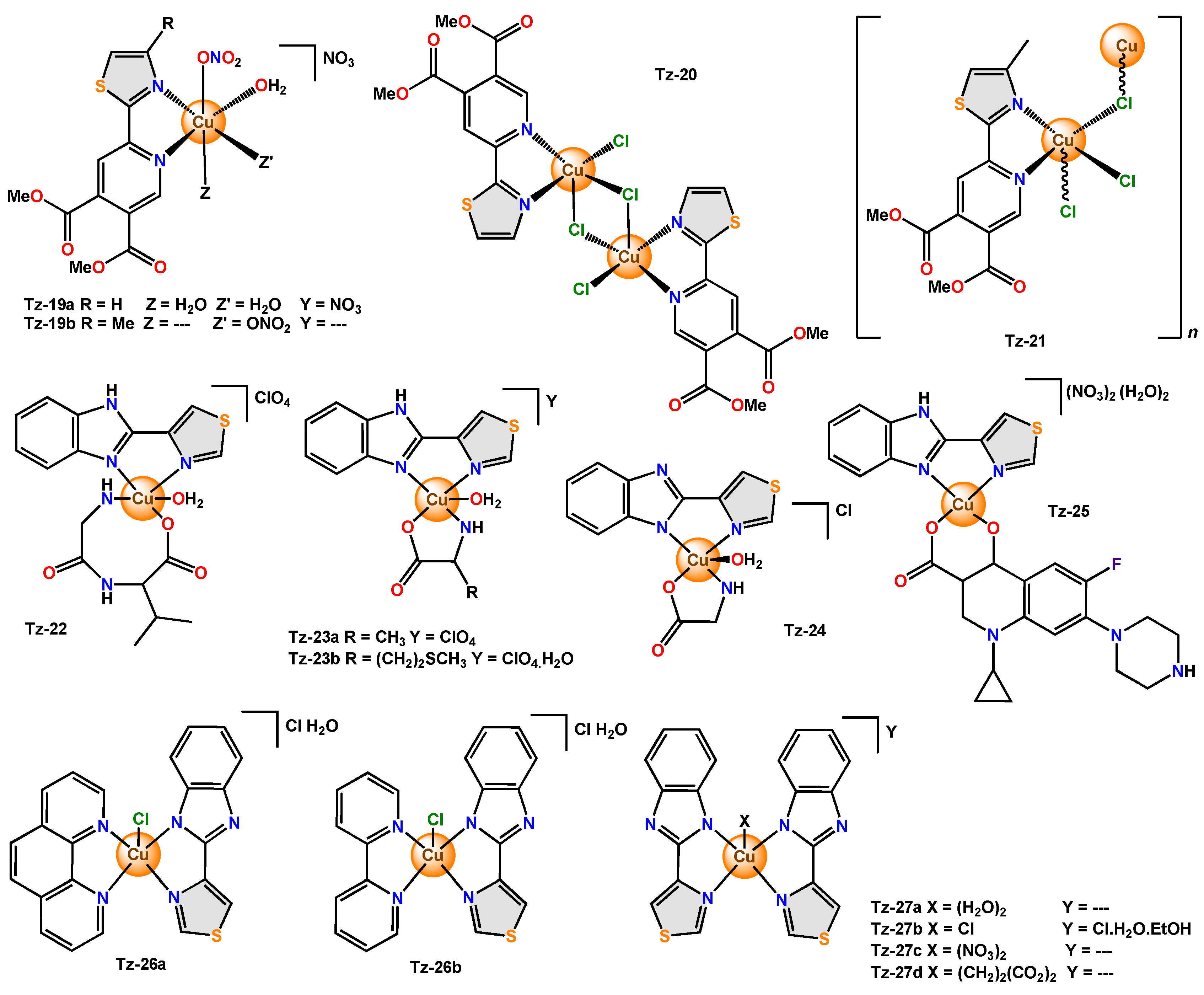
4).

Figure 84. Copper complexes with bidentate thiazole ligands functionalized with pyridine and benzimidazole.
2.3. Copper Complexes with Tridentate Thiazole Ligands
Complexes containing a tridentate thiazole ligand have also been reported, and such is the case with the
Tz-28a
–
Tz-28c
complexes, which were reported as hybrid compounds, since they contain two bioactive moieties: s-triazine and thiazole.
Tz-29
with octahedral geometry was tested for antibacterial (
M. luteus
and
E. coli
) and antifungal (
A. niger
and
A. terreus) activity by the disk diffusion bioassay method. The results were based on the zone of inhibition for bacteria (16–11 mm) and fungi (17–15 mm), respectively, and were considerably better than those found for the free ligand for bacteria (13–12 mm) and fungi (15–14 mm). The scholars mention that metal complexes increased their lipophilic capacity as a result of chelation, which favours their uptake through the lipid membrane of microbes [94]. A bisthiazole derivative was used in the synthesis of
) activity by the disk diffusion bioassay method. The results were based on the zone of inhibition for bacteria (16–11 mm) and fungi (17–15 mm), respectively, and were considerably better than those found for the free ligand for bacteria (13–12 mm) and fungi (15–14 mm). The scholars mention that metal complexes increased their lipophilic capacity as a result of chelation, which favours their uptake through the lipid membrane of microbes [23]. A bisthiazole derivative was used in the synthesis of
Tz-30
, which exhibited square pyramidal geometry. The antibacterial potential for
E. coli
,
S. aureus
and
P. aeruginosa
, as well as the antifungal potential for
C. albicans
, were determined by agar dilution tests.
Additionally,
Tz-31
was reported with a tetragonally distorted octahedral geometry and was tested against some bacteria strains (
E. coli
,
P. aeruginosa
,
S. aureus
and
B. subtilis) by the well diffusion method. Coordination resulted in excellent inhibition in bacteria (40–45 mm), while ligand showed moderate inhibition (30–35 mm) [96].
) by the well diffusion method. Coordination resulted in excellent inhibition in bacteria (40–45 mm), while ligand showed moderate inhibition (30–35 mm) [24].
The antimicrobial activity of the ligand used in the synthesis of the
Tz-32a
–
Tz-32f complexes (octahedral geometry) was reported using the cup plate method and by the serial dilution technique. The MIC values of the free ligand were found to be in the range of 15–20 mg/mL. In this case, the complexes were tested as insecticides and pesticides and showed a lower poisoning rate than commercial pesticides and insecticides [97,98]. In the case of the complexes
complexes (octahedral geometry) was reported using the cup plate method and by the serial dilution technique. The MIC values of the free ligand were found to be in the range of 15–20 mg/mL. In this case, the complexes were tested as insecticides and pesticides and showed a lower poisoning rate than commercial pesticides and insecticides [25][26]. In the case of the complexes
Tz-32g
–
Tz-32i
reportedly with an octahedral geometry, they were tested in vitro against bacteria (
E. coli
and
S. aureus
) using the paper disc method at a concentration of 500 and 1000 ppm and against fungi (
A. flavus
and
A. niger
) using the mycelium dry weight method.
Additionally,
Tz-33
was obtained using a Schiff base ligand derived from 2-aminothiazole, and it exhibited a distorted octahedral geometry. The bacteria detection effect of
Tz-33
was tested against
S. aureus
,
B. subtilis
,
P. aeuroginosa
, and
P. vulgaris
by the well-diffusion method with different dilutions (20 μL, 40 μL, and 60 μL).
In the copper(II) complex
Tz-34
, the Schiff base acted as a tridentate ligand, and magnetic susceptibility data suggested that the geometric structure of
Tz-34
was square planar. Its antimicrobial activity was examined with different species of bacteria (
B. subtilis
,
S. aureus
,
E. coli
and
P. aeruginosa
) by the well diffusion method at different concentrations (30, 60, 90 µg). As a result, it was detected that
Tz-34
had greater activity (14–20 mm) than the free ligand (13 mm) at 90 µg, highlighting that for
P. aeruginosa
, the ligand did not present activity but that
Tz-34
showed inhibition at 60 µg. (11 mm). In the case of
Tz-35
, an azo compound was used to functionalize the thiazole, and a tridentate chelating agent was obtained, which was coordinated through the phenolic oxygen atom, the nitrogen atom of the azo group (which is the closest phenyl ring), and from the nitrogen atom of the thiazole ring to form two chelated five-membered rings with a distorted octahedral geometry around the metal centre.
Tz-36
was reported with a distorted square planar geometry. Antimicrobial activity was determined with the disk diffusion method in bacteria (
S. aureus
