Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Moon, J.; Kye, B.; Ko, S.; Yoo, R.N. Sulfur Metabolism of the Gut Microbiome/Colorectal Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/43918 (accessed on 08 February 2026).
Moon J, Kye B, Ko S, Yoo RN. Sulfur Metabolism of the Gut Microbiome/Colorectal Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/43918. Accessed February 08, 2026.
Moon, Ji-Yeon, Bong-Hyeon Kye, Seung-Hyun Ko, Ri Na Yoo. "Sulfur Metabolism of the Gut Microbiome/Colorectal Cancer" Encyclopedia, https://encyclopedia.pub/entry/43918 (accessed February 08, 2026).
Moon, J., Kye, B., Ko, S., & Yoo, R.N. (2023, May 06). Sulfur Metabolism of the Gut Microbiome/Colorectal Cancer. In Encyclopedia. https://encyclopedia.pub/entry/43918
Moon, Ji-Yeon, et al. "Sulfur Metabolism of the Gut Microbiome/Colorectal Cancer." Encyclopedia. Web. 06 May, 2023.
Copy Citation
Colorectal cancer diagnosed in individuals under 50 years old is called early-onset colorectal cancer (EOCRC), and its incidence has been rising worldwide. Simultaneously occurring with increasing obesity, this worrisome trend is partly explained by the strong influence of dietary elements, particularly fatty, meaty, and sugary food. An animal-based diet, the so-called Western diet, causes a shift in dominant microbiota and their metabolic activity, which may disrupt the homeostasis of hydrogen sulfide concentration. Bacterial sulfur metabolism is recognized as a critical mechanism of EOCRC pathogenesis.
colorectal neoplasm
exposome
Western diet
1. Sulfur Metabolism of Gut Microbiota and Its Association with CRC Development
Hydrogen sulfide (H2S) is widely accepted as a critical signaling molecule in humans, identified as a gasotransmitter with various chemical properties, reaction mechanisms, and the ability to alter proteins and participate in many metal redox processes [1]. Endogenous H2S is predominantly produced by gut microbiota from metabolizing inorganic sulfur (sulfate and sulfite) from preservatives in processed food and organic sulfur compounds, mostly cysteine or taurine from red meat [2][3]. Sulfate-reducing bacteria, such as Bilophila, Desulfovibrio, Desulfomicrobium, and Fusobacterium, can colonize the gut in the human intestinal tract and generate endogenous H2S from metabolizing inorganic or organic sulfur compounds [4][5]. Several microbial enzymes, including cystathionine β-synthase (CBS), cystathionine γ-layse (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST), are responsible for the production of endogenous H2S from catabolizing cysteine and homocysteine [5].
As H2S is produced from microbial metabolic reactions, luminal H2S permeates easily through the biofilms that cover the colonocyte and epithelial cell membrane due to its high permeability [6]. Entering the colonocytes, H2S is catabolized through intracellular oxidative metabolism in the mitochondria and cytoplasm [7]. Composed of several mitochondrial enzymes in the colonocyte, including sulfide quinone oxidoreductase (SQR), ethylmalonic encephalopathy protein 1 (ETHE1), and thiosulfate thiotransferase (TST), the sulfide oxidation unit oxidizes H2S and produces persulfides, a highly reactive molecule binds to proteins [8]. This physiological post-translational modification of proteins (S-sulfuration) is known to regulate and affect the processes of cell survival and death, cell differentiation, cell proliferation and hypertrophy, cellular metabolism, mitochondrial bioenergetics and biogenesis, vasorelaxation, inflammation, oxidative stress [9]. It has been shown that the S-sulfuration regulates the DNA damage repair system by activating the RAS–RAF–MEK–ERK cascade by sulfhydrated MEK1, influencing tumor growth [10][11]. Additionally, the persulfidation to the NF-κB induces metastasis-promoting gene expression and activates NF-κB/IL-1β signaling, which may result in cancer progression and metastasis via VEGF activation [12].
The biological effects of H2S depend on its concentration in the colonic lumen, and the luminal concentration is mainly determined by endogenous production by bacterial metabolism, which influences H2S-mediated tumorigenesis. Several in vitro studies of treating CRC cell lines with exogenous H2S reveal bell-shaped concentration responses in cancer, representing the dual effects of H2S [1]. When CRC cells were exposed to a slow-release H2S donor at a low concentration (0.2–0.3 micromole), mitochondrial function and glycolysis for energy production enhanced cancer cell proliferation by activating H2S-generating enzymes within cancer cells but were typically not present in colonic epithelial cells [13][14]. Additionally, the expression of H2S-producing enzymes was higher in CRC tissue than in normal surrounding tissues, possibly maintaining its optimal concentration for tumor growth and proliferation [12]. Conversely, treating CRC cells with a high concentration (1 millimole) of an H2S donor in the form of isothiocyanate, a molecular derivative from a cruciferous plant, induced the apoptosis of CRC cells [15]. As shown in Figure 1, the exogenous H2S demonstrates a concentration-dependent effect: maintenance of normal physiology at low, carcinogenic once reaching an upper threshold, then possibly chemopreventive at a high. Thus, maintaining an appropriate concentration of H2S may be critical to balance the cell cycle and regulating apoptosis and tumorigenesis.
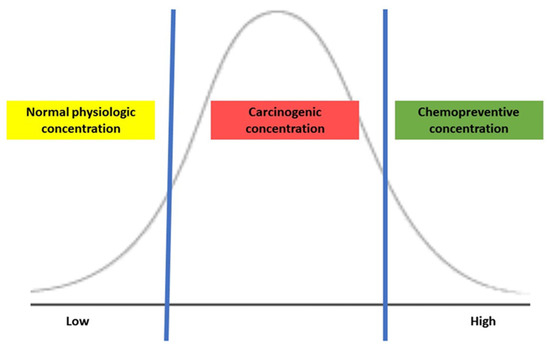
Figure 1. The action of H2S is based on its concentration.
The upregulation of H2S and sulfidogenic bacteria positively correlates with a diet high in fat and protein [4][16]. A high concentration of sulfidogenic bacteria in stool is associated with the risk of distal CRC [17]. Moreover, comparing the flatus samples from patients with CRC to those from healthy individuals, the concentration of the sulfur compounds was significantly higher in the patients with CRC [18]. In vitro study using colon cancer-derived epithelial cell lines demonstrated selective upregulation in the ability of H2S-producing enzymes, which increased H2S concentration compared to the nonmalignant colonic mucosa cells [12]. In mice with loss of the H2S-producing enzyme function, the blood flow to the tumor was decreased, inhibiting tumor growth and angiogenesis [12]. The level of CBS in human samples is low in the healthy colonic mucosa but gradually increases as the epithelial cells are transformed into polyps, hyperplastic polyps, tubular adenoma, and adenocarcinoma [19]. The CBS protein levels in human colon cancer specimens closely correlated to the disease severity and tumor stage, and more advanced tumors expressed higher levels of CBS with higher expression of vascular endothelial growth factor (VEGF) [20][21]. Furthermore, it has been shown that expression of H2S-detoxifying enzymes, e.g., TST, located in colonocytes in the lumen is markedly reduced in advanced colon cancer [22]. A meta-analysis flowchart by identifying differentially expressed genes among normal colonic mucosa, primary tumor sites, and metastatic samples in the liver and lung demonstrated that the expression of mitochondrial oxidation enzymes, including SQR, ETHE1, and TST, decreased during the evolving process from the normal epithelium to the primary tumor and metastatic lesions [23]. These findings suggest that dysregulated expression and activity of sulfide-detoxifying or -producing enzymes may contribute to disruption in the homeostasis of the sulfur-containing compound. Consequently, increased endogenous H2S concentration may play a role as a tumor growth factor, inducing tumor growth and proliferation and promoting angiogenesis and vasorelaxation.
Interestingly, H2S can have dual effects, harmful or beneficial, depending on its source and concentration. In a previous in vitro study evaluating the underlying mechanism of H2S action causing carcinogenesis, sulfide at concentrations similar to those in the human colon (e.g., millimole) induced direct genomic DNA damage in mammalian cells [24]. Furthermore, H2S can cause mucosal damage by breaking disulfide bonds in the mucus layer. Consequently, luminal bacteria and their metabolites can penetrate the epithelial lining, induce apoptosis of epithelial cells, and activate the inflammatory cascade [2][25]. This evidence is consistent with the finding that a Westernized diet increases CRC, particularly in the distal location where sulfur-metabolizing bacteria are found at a higher concentration than in the proximal colon [17]. Intriguingly, some studies have demonstrated that H2S has a protective and reparative effect on the colonic epithelium. Endogenous H2S at a low concentration (e.g., micromole) can act as a vasorelaxant, reduce endoplasmic reticulum stress, and prevent apoptosis [26]. Additionally, exogenous H2S exists in garlic, onions, and cruciferous vegetables, such as cabbage, cauliflower, kale, and broccoli, which are known to be beneficial to colonocytes and enterocytes, acting as an energy source for microbial metabolism. Inorganic plant-derived H2S helps colonocyte respiration and stimulates mitochondria to detoxify and recover from epithelial injury [1]. Thus, oral consumption of exogenous H2S stabilizes gut microbiota biofilm integrity and prevents the formation of the pathogenic shift in colonies, eventually inhibiting inflammation and tumorigenesis [27]. However, the specific mechanism of H2S action connected to the interaction between dietary sources and gut microbiota needs further investigation.
This unique biological property of H2S provides new approaches to CRC treatment, targeting H2S modulation by delivering exogenous H2S in high doses or inhibiting endogenous H2S expression [1]. Researchers have developed exogenous H2S compounds that can release in a site-specific and time-dependent manner. Various biocompatible polymers of H2S have been developed as donors, demonstrating the ability to specifically target the lesions, respond to the pathological microenvironment, and monitor changes in the microenvironment after the delivery [28]. H2S-releasing non-steroidal anti-inflammatory drugs (H2S-NSAIDS) have been proposed as anticancer drugs [29]. After covalently attaching H2S to NSAIDS, the researchers tested the growth properties of different human cell lines from six different tissues. They found that H2S-NSAIDS inhibited the growth of all cancer cell lines studied, with potencies of 28- to >3000-fold greater than traditional NSAIDS [29]. HS-NSAIDs inhibited cell proliferation, induced apoptosis, and caused G(0)/G(1) cell cycle block [29]. Additionally, inhibition of endogenous H2S production mainly focuses on targeting enzymes related to endogenous H2S synthesis [30]. Several small molecule inhibitor models have been designed and synthesized to inhibit CBS, CSE, and 3-MST, mainly inducing anti-proliferative activity [1]. Aminooxyacetic acid (AOAA) is a well-known CBS inhibitor that reacts with vitamin B6, transforming vitamin B6 into a biologically inactive form [30]. Because CBS requires a biologically active cofactor derived from vitamin B6, pyridoxal-5′-phosphate (PLP), CBS is inhibited in the presence of AOAA [30]. Another attractive approach to reducing endogenous H2S concentration is the development of endogenous H2S scavengers [30]. For example, hydroxocobalamin has been investigated as a potential scavenger for H2S overdose [31]. At all concentrations, hydroxocobalamin prevented mice treated with sodium hydrosulfide from death [31]. Although inhibitors or scavengers may effectively reduce H2S concentration levels, they may have undesirable consequences during practical use due to the ubiquity of enzymes and systemic impact, inevitably causing damage to the body. A comprehensive assessment is mandatory to develop a therapeutic agent to eliminate potential side effects. Further translational studies searching for viable therapeutics are necessary.
2. Status of Evaluating the Sulfur Microbial Diet and Its Association with CRC
Only a limited number of clinical studies have evaluated a dietary pattern associated with microbial sulfur metabolism for the development of CRC, as shown in Table 1. Nguyen, L.H. et al. developed a sulfur microbial dietary scoring system based on dietary elements associated with bacterial species involved in sulfur metabolism. Analyzing serial stool metagenomes and metatranscriptomes from CRC patients in association with sulfur microbial dietary scores, the authors identified that high sulfur microbial dietary scores were associated with increased consumption of high intakes of low-calorie beverages, french fries, red meats, and processed meats and low intakes of fruits, yellow vegetables, whole grains, legumes, leafy vegetables, and cruciferous vegetables [17][32]. Namely, the sulfur microbial diet on long-term adherence was associated with a high concentration of sulfur-metabolizing bacteria in the feces of CRC patients compared to healthy individuals [17]. Furthermore, tight adherence to the microbial sulfur diet was associated with an increased risk of CRC, especially in the distal location [32][33]. Similarly, a large prospective cohort study of women with detailed information on adult and adolescent diets revealed that long-term adherence to a sulfur microbial diet might be associated with an Increased risk of developing adenoma with malignant potentials before age 50 [34]. The authors suggested that the risk might begin as early as adolescence [34].
Table 1. Clinical studies are evaluating the association between the sulfur microbial diet and CRC.
| Authors | Year | Study Type | Cohort | Comparatives | Findings |
|---|---|---|---|---|---|
| Magee, E.A. et al. [35] | 2000 | Clinical trial | 5 healthy men | The intervention of change in dietary components: vegetarian diet vs. high meat diet
|
|
| Sikavi, D.R. et al. [18] | 2021 | Prospective observational | 51,529 men enrolled in the Health Professionals Follow-up Study |
Cancer tissues obtained from CRC patients
|
|
| Nguyen, L.H. et al. [2] | 2020 | Prospective observational | 51,529 men enrolled in the Health Professionals Follow-up Study |
CRC patients vs. Healthy individuals
|
|
| Wang, Y. et al. [17] | 2021 | Prospective observational |
|
CRC patients vs. Healthy individuals
|
|
| Nguyen, L.H. et al. [19] | 2021 | Prospective observational |
|
Individuals with polyps vs without polyps |
|
Yet, the previous studies are based on the hypothesis speculating that a high concentration of the sulfur-metabolizing bacteria may be related to the development of CRC and CRC precursors. Because microbial metabolism in the gut is complex and intertwined with numerous exposomal factors, further clinical studies should be reproduced in different regions and cultures of food habits. Furthermore, one accurate way to determine whether endogenous H2S concentration produced by gut microbes causes the carcinogenesis of CRC may be a directly measuring H2S concentration in the gut. However, a direct measurement of H2S concentration is not available and technically challenging [1]. Thus, it would be essential to develop a diagnostic method to measure H2S concentration to assess the relationship between dietary habits and bacterial metabolism.
References
- Lin, H.; Yu, Y.; Zhu, L.; Lai, N.; Zhang, L.; Guo, Y.; Lin, X.; Yang, D.; Ren, N.; Zhu, Z.; et al. Implications of hydrogen sulfide in colorectal cancer: Mechanistic insights and diagnostic and therapeutic strategies. Redox. Biol. 2023, 59, 102601.
- Zhang, W.; An, Y.; Qin, X.; Wu, X.; Wang, X.; Hou, H.; Song, X.; Liu, T.; Wang, B.; Huang, X.; et al. Gut Microbiota-Derived Metabolites in Colorectal Cancer: The Bad and the Challenges. Front. Oncol. 2021, 11, 739648.
- Wallace, J.L.; Motta, J.P.; Buret, A.G. Hydrogen sulfide: An agent of stability at the microbiome-mucosa interface. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G143–G149.
- Song, M.; Chan, A.T.; Sun, J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology 2020, 158, 322–340.
- Kushkevych, I.; Dordević, D.; Vítězová, M. Possible synergy effect of hydrogen sulfide and acetate produced by sulfate-reducing bacteria on inflammatory bowel disease development. J. Adv. Res. 2021, 27, 71–78.
- Mathai, J.C.; Missner, A.; Kügler, P.; Saparov, S.M.; Zeidel, M.L.; Lee, J.K.; Pohl, P. No facilitator required for membrane transport of hydrogen sulfide. Proc. Natl. Acad. Sci. USA 2009, 106, 16633–16638.
- Olson, K.R.; Straub, K.D. The Role of Hydrogen Sulfide in Evolution and the Evolution of Hydrogen Sulfide in Metabolism and Signaling. Physiology 2016, 31, 60–72.
- Khattak, S.; Rauf, M.A.; Khan, N.H.; Zhang, Q.Q.; Chen, H.J.; Muhammad, P.; Ansari, M.A.; Alomary, M.N.; Jahangir, M.; Zhang, C.Y.; et al. Hydrogen Sulfide Biology and Its Role in Cancer. Molecules 2022, 27, 3389.
- Zhang, D.; Du, J.; Tang, C.; Huang, Y.; Jin, H. H(2)S-Induced Sulfhydration: Biological Function and Detection Methodology. Front. Pharmacol. 2017, 8, 608.
- Zhao, K.; Ju, Y.; Li, S.; Altaany, Z.; Wang, R.; Yang, G. S-sulfhydration of MEK1 leads to PARP-1 activation and DNA damage repair. EMBO Rep. 2014, 15, 792–800.
- Degirmenci, U.; Wang, M.; Hu, J. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells 2020, 9, 198.
- Szabo, C.; Coletta, C.; Chao, C.; Módis, K.; Szczesny, B.; Papapetropoulos, A.; Hellmich, M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 12474–12479.
- Untereiner, A.A.; Oláh, G.; Módis, K.; Hellmich, M.R.; Szabo, C. H(2)S-induced S-sulfhydration of lactate dehydrogenase a (LDHA) stimulates cellular bioenergetics in HCT116 colon cancer cells. Biochem. Pharmacol. 2017, 136, 86–98.
- Cai, W.J.; Wang, M.J.; Ju, L.H.; Wang, C.; Zhu, Y.C. Hydrogen sulfide induces human colon cancer cell proliferation: Role of Akt, ERK and p21. Cell Biol. Int. 2010, 34, 565–572.
- Rose, P.; Moore, P.K.; Ming, S.H.; Nam, O.C.; Armstrong, J.S.; Whiteman, M. Hydrogen sulfide protects colon cancer cells from chemopreventative agent beta-phenylethyl isothiocyanate induced apoptosis. World J. Gastroenterol. 2005, 11, 3990–3997.
- Magee, E.A.; Richardson, C.J.; Hughes, R.; Cummings, J.H. Contribution of dietary protein to sulfide production in the large intestine: An in vitro and a controlled feeding study in humans. Am. J. Clin. Nutr. 2000, 72, 1488–1494.
- Nguyen, L.H.; Ma, W.; Wang, D.D.; Cao, Y.; Mallick, H.; Gerbaba, T.K.; Lloyd-Price, J.; Abu-Ali, G.; Hall, A.B.; Sikavi, D.; et al. Association Between Sulfur-Metabolizing Bacterial Communities in Stool and Risk of Distal Colorectal Cancer in Men. Gastroenterology 2020, 158, 1313–1325.
- Yamagishi, K.; Onuma, K.; Chiba, Y.; Yagi, S.; Aoki, S.; Sato, T.; Sugawara, Y.; Hosoya, N.; Saeki, Y.; Takahashi, M.; et al. Generation of gaseous sulfur-containing compounds in tumour tissue and suppression of gas diffusion as an antitumour treatment. Gut 2012, 61, 554–561.
- Phillips, C.M.; Zatarain, J.R.; Nicholls, M.E.; Porter, C.; Widen, S.G.; Thanki, K.; Johnson, P.; Jawad, M.U.; Moyer, M.P.; Randall, J.W.; et al. Upregulation of Cystathionine-β-Synthase in Colonic Epithelia Reprograms Metabolism and Promotes Carcinogenesis. Cancer Res. 2017, 77, 5741–5754.
- Ascenção, K.; Szabo, C. Emerging roles of cystathionine β-synthase in various forms of cancer. Redox. Biol. 2022, 53, 102331.
- Guo, S.; Li, J.; Huang, Z.; Yue, T.; Zhu, J.; Wang, X.; Liu, Y.; Wang, P.; Chen, S. The CBS-H(2)S axis promotes liver metastasis of colon cancer by upregulating VEGF through AP-1 activation. Br. J. Cancer. 2022, 126, 1055–1066.
- Ramasamy, S.; Singh, S.; Taniere, P.; Langman, M.J.; Eggo, M.C. Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G288–G296.
- Piran, M.; Sepahi, N.; Moattari, A.; Rahimi, A.; Ghanbariasad, A. Systems Biomedicine of Primary and Metastatic Colorectal Cancer Reveals Potential Therapeutic Targets. Front. Oncol. 2021, 11, 597536.
- Attene-Ramos, M.S.; Wagner, E.D.; Gaskins, H.R.; Plewa, M.J. Hydrogen sulfide induces direct radical-associated DNA damage. Mol. Cancer Res. 2007, 5, 455–459.
- Figliuolo, V.R.; Coutinho-Silva, R.; Coutinho, C. Contribution of sulfate-reducing bacteria to homeostasis disruption during intestinal inflammation. Life Sci. 2018, 215, 145–151.
- Wolf, P.G.; Cowley, E.S.; Breister, A.; Matatov, S.; Lucio, L.; Polak, P.; Ridlon, J.M.; Gaskins, H.R.; Anantharaman, K. Diversity and distribution of sulfur metabolic genes in the human gut microbiome and their association with colorectal cancer. Microbiome 2022, 10, 64.
- Buret, A.G.; Allain, T.; Motta, J.P.; Wallace, J.L. Effects of Hydrogen Sulfide on the Microbiome: From Toxicity to Therapy. Antioxid. Redox Signal. 2022, 36, 211–219.
- Rong, F.; Wang, T.; Zhou, Q.; Peng, H.; Yang, J.; Fan, Q.; Li, P. Intelligent polymeric hydrogen sulfide delivery systems for therapeutic applications. Bioact. Mater. 2023, 19, 198–216.
- Chattopadhyay, M.; Kodela, R.; Nath, N.; Dastagirzada, Y.M.; Velázquez-Martínez, C.A.; Boring, D.; Kashfi, K. Hydrogen sulfide-releasing NSAIDs inhibit the growth of human cancer cells: A general property and evidence of a tissue type-independent effect. Biochem. Pharmacol. 2012, 83, 715–722.
- Wang, Y.; Ni, X.; Chadha, R.; McCartney, C.; Lam, Y.; Brummett, B.; Ramush, G.; Xian, M. Methods for Suppressing Hydrogen Sulfide in Biological Systems. Antioxid. Redox Signal. 2022, 36, 294–308.
- Truong, D.H.; Mihajlovic, A.; Gunness, P.; Hindmarsh, W.; O’Brien, P.J. Prevention of hydrogen sulfide (H2S)-induced mouse lethality and cytotoxicity by hydroxocobalamin (vitamin B(12a)). Toxicology 2007, 242, 16–22.
- Wang, Y.; Nguyen, L.H.; Mehta, R.S.; Song, M.; Huttenhower, C.; Chan, A.T. Association Between the Sulfur Microbial Diet and Risk of Colorectal Cancer. JAMA Netw. Open 2021, 4, e2134308.
- Sikavi, D.R.; Nguyen, L.H.; Haruki, K.; Ugai, T.; Ma, W.; Wang, D.D.; Thompson, K.N.; Yan, Y.; Branck, T.; Wilkinson, J.E.; et al. The Sulfur Microbial Diet and Risk of Colorectal Cancer by Molecular Subtypes and Intratumoral Microbial Species in Adult Men. Clin. Transl. Gastroenterol. 2021, 12, e00338.
- Nguyen, L.H.; Cao, Y.; Hur, J.; Mehta, R.S.; Sikavi, D.R.; Wang, Y.; Ma, W.; Wu, K.; Song, M.; Giovannucci, E.L.; et al. The Sulfur Microbial Diet Is Associated with Increased Risk of Early-Onset Colorectal Cancer Precursors. Gastroenterology 2021, 161, 1423–1432.e4.
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
834
Revisions:
2 times
(View History)
Update Date:
06 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




