Colorectal cancer diagnosed in individuals under 50 years old is called early-onset colorectal cancer (EOCRC), and its incidence has been rising worldwide. Simultaneously occurring with increasing obesity, this worrisome trend is partly explained by the strong influence of dietary elements, particularly fatty, meaty, and sugary food. An animal-based diet, the so-called Western diet, causes a shift in dominant microbiota and their metabolic activity, which may disrupt the homeostasis of hydrogen sulfide concentration. Bacterial sulfur metabolism is recognized as a critical mechanism of EOCRC pathogenesis.
- colorectal neoplasm
- exposome
- Western diet
1. Sulfur Metabolism of Gut Microbiota and Its Association with CRC Development
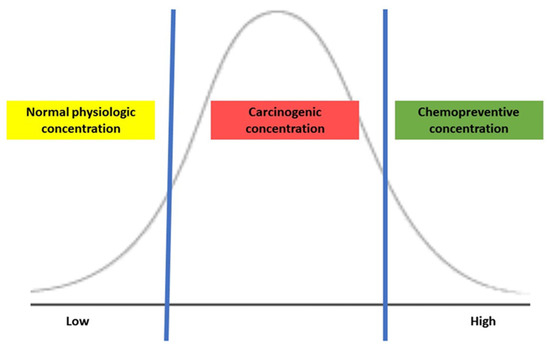
2. Status of Evaluating the Sulfur Microbial Diet and Its Association with CRC
| Authors | Year | Study Type | Cohort | Comparatives | Findings |
|---|---|---|---|---|---|
| Magee, E.A. et al. [35] | 2000 | Clinical trial | 5 healthy men | The intervention of change in dietary components: vegetarian diet vs. high meat diet
|
|
| Sikavi, D.R. et al. [18] | 2021 | Prospective observational | 51,529 men enrolled in the Health Professionals Follow-up Study |
Cancer tissues obtained from CRC patients
|
|
| Nguyen, L.H. et al. [2] | 2020 | Prospective observational | 51,529 men enrolled in the Health Professionals Follow-up Study |
CRC patients vs. Healthy individuals
|
|
| Wang, Y. et al. [17] | 2021 | Prospective observational |
|
CRC patients vs. Healthy individuals
|
|
| Nguyen, L.H. et al. [19] | 2021 | Prospective observational |
|
Individuals with polyps vs without polyps |
|
This entry is adapted from the peer-reviewed paper 10.3390/nu15081966
References
- Lin, H.; Yu, Y.; Zhu, L.; Lai, N.; Zhang, L.; Guo, Y.; Lin, X.; Yang, D.; Ren, N.; Zhu, Z.; et al. Implications of hydrogen sulfide in colorectal cancer: Mechanistic insights and diagnostic and therapeutic strategies. Redox. Biol. 2023, 59, 102601.
- Zhang, W.; An, Y.; Qin, X.; Wu, X.; Wang, X.; Hou, H.; Song, X.; Liu, T.; Wang, B.; Huang, X.; et al. Gut Microbiota-Derived Metabolites in Colorectal Cancer: The Bad and the Challenges. Front. Oncol. 2021, 11, 739648.
- Wallace, J.L.; Motta, J.P.; Buret, A.G. Hydrogen sulfide: An agent of stability at the microbiome-mucosa interface. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G143–G149.
- Song, M.; Chan, A.T.; Sun, J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology 2020, 158, 322–340.
- Kushkevych, I.; Dordević, D.; Vítězová, M. Possible synergy effect of hydrogen sulfide and acetate produced by sulfate-reducing bacteria on inflammatory bowel disease development. J. Adv. Res. 2021, 27, 71–78.
- Mathai, J.C.; Missner, A.; Kügler, P.; Saparov, S.M.; Zeidel, M.L.; Lee, J.K.; Pohl, P. No facilitator required for membrane transport of hydrogen sulfide. Proc. Natl. Acad. Sci. USA 2009, 106, 16633–16638.
- Olson, K.R.; Straub, K.D. The Role of Hydrogen Sulfide in Evolution and the Evolution of Hydrogen Sulfide in Metabolism and Signaling. Physiology 2016, 31, 60–72.
- Khattak, S.; Rauf, M.A.; Khan, N.H.; Zhang, Q.Q.; Chen, H.J.; Muhammad, P.; Ansari, M.A.; Alomary, M.N.; Jahangir, M.; Zhang, C.Y.; et al. Hydrogen Sulfide Biology and Its Role in Cancer. Molecules 2022, 27, 3389.
- Zhang, D.; Du, J.; Tang, C.; Huang, Y.; Jin, H. H(2)S-Induced Sulfhydration: Biological Function and Detection Methodology. Front. Pharmacol. 2017, 8, 608.
- Zhao, K.; Ju, Y.; Li, S.; Altaany, Z.; Wang, R.; Yang, G. S-sulfhydration of MEK1 leads to PARP-1 activation and DNA damage repair. EMBO Rep. 2014, 15, 792–800.
- Degirmenci, U.; Wang, M.; Hu, J. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells 2020, 9, 198.
- Szabo, C.; Coletta, C.; Chao, C.; Módis, K.; Szczesny, B.; Papapetropoulos, A.; Hellmich, M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 12474–12479.
- Untereiner, A.A.; Oláh, G.; Módis, K.; Hellmich, M.R.; Szabo, C. H(2)S-induced S-sulfhydration of lactate dehydrogenase a (LDHA) stimulates cellular bioenergetics in HCT116 colon cancer cells. Biochem. Pharmacol. 2017, 136, 86–98.
- Cai, W.J.; Wang, M.J.; Ju, L.H.; Wang, C.; Zhu, Y.C. Hydrogen sulfide induces human colon cancer cell proliferation: Role of Akt, ERK and p21. Cell Biol. Int. 2010, 34, 565–572.
- Rose, P.; Moore, P.K.; Ming, S.H.; Nam, O.C.; Armstrong, J.S.; Whiteman, M. Hydrogen sulfide protects colon cancer cells from chemopreventative agent beta-phenylethyl isothiocyanate induced apoptosis. World J. Gastroenterol. 2005, 11, 3990–3997.
- Magee, E.A.; Richardson, C.J.; Hughes, R.; Cummings, J.H. Contribution of dietary protein to sulfide production in the large intestine: An in vitro and a controlled feeding study in humans. Am. J. Clin. Nutr. 2000, 72, 1488–1494.
- Nguyen, L.H.; Ma, W.; Wang, D.D.; Cao, Y.; Mallick, H.; Gerbaba, T.K.; Lloyd-Price, J.; Abu-Ali, G.; Hall, A.B.; Sikavi, D.; et al. Association Between Sulfur-Metabolizing Bacterial Communities in Stool and Risk of Distal Colorectal Cancer in Men. Gastroenterology 2020, 158, 1313–1325.
- Yamagishi, K.; Onuma, K.; Chiba, Y.; Yagi, S.; Aoki, S.; Sato, T.; Sugawara, Y.; Hosoya, N.; Saeki, Y.; Takahashi, M.; et al. Generation of gaseous sulfur-containing compounds in tumour tissue and suppression of gas diffusion as an antitumour treatment. Gut 2012, 61, 554–561.
- Phillips, C.M.; Zatarain, J.R.; Nicholls, M.E.; Porter, C.; Widen, S.G.; Thanki, K.; Johnson, P.; Jawad, M.U.; Moyer, M.P.; Randall, J.W.; et al. Upregulation of Cystathionine-β-Synthase in Colonic Epithelia Reprograms Metabolism and Promotes Carcinogenesis. Cancer Res. 2017, 77, 5741–5754.
- Ascenção, K.; Szabo, C. Emerging roles of cystathionine β-synthase in various forms of cancer. Redox. Biol. 2022, 53, 102331.
- Guo, S.; Li, J.; Huang, Z.; Yue, T.; Zhu, J.; Wang, X.; Liu, Y.; Wang, P.; Chen, S. The CBS-H(2)S axis promotes liver metastasis of colon cancer by upregulating VEGF through AP-1 activation. Br. J. Cancer. 2022, 126, 1055–1066.
- Ramasamy, S.; Singh, S.; Taniere, P.; Langman, M.J.; Eggo, M.C. Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G288–G296.
- Piran, M.; Sepahi, N.; Moattari, A.; Rahimi, A.; Ghanbariasad, A. Systems Biomedicine of Primary and Metastatic Colorectal Cancer Reveals Potential Therapeutic Targets. Front. Oncol. 2021, 11, 597536.
- Attene-Ramos, M.S.; Wagner, E.D.; Gaskins, H.R.; Plewa, M.J. Hydrogen sulfide induces direct radical-associated DNA damage. Mol. Cancer Res. 2007, 5, 455–459.
- Figliuolo, V.R.; Coutinho-Silva, R.; Coutinho, C. Contribution of sulfate-reducing bacteria to homeostasis disruption during intestinal inflammation. Life Sci. 2018, 215, 145–151.
- Wolf, P.G.; Cowley, E.S.; Breister, A.; Matatov, S.; Lucio, L.; Polak, P.; Ridlon, J.M.; Gaskins, H.R.; Anantharaman, K. Diversity and distribution of sulfur metabolic genes in the human gut microbiome and their association with colorectal cancer. Microbiome 2022, 10, 64.
- Buret, A.G.; Allain, T.; Motta, J.P.; Wallace, J.L. Effects of Hydrogen Sulfide on the Microbiome: From Toxicity to Therapy. Antioxid. Redox Signal. 2022, 36, 211–219.
- Rong, F.; Wang, T.; Zhou, Q.; Peng, H.; Yang, J.; Fan, Q.; Li, P. Intelligent polymeric hydrogen sulfide delivery systems for therapeutic applications. Bioact. Mater. 2023, 19, 198–216.
- Chattopadhyay, M.; Kodela, R.; Nath, N.; Dastagirzada, Y.M.; Velázquez-Martínez, C.A.; Boring, D.; Kashfi, K. Hydrogen sulfide-releasing NSAIDs inhibit the growth of human cancer cells: A general property and evidence of a tissue type-independent effect. Biochem. Pharmacol. 2012, 83, 715–722.
- Wang, Y.; Ni, X.; Chadha, R.; McCartney, C.; Lam, Y.; Brummett, B.; Ramush, G.; Xian, M. Methods for Suppressing Hydrogen Sulfide in Biological Systems. Antioxid. Redox Signal. 2022, 36, 294–308.
- Truong, D.H.; Mihajlovic, A.; Gunness, P.; Hindmarsh, W.; O’Brien, P.J. Prevention of hydrogen sulfide (H2S)-induced mouse lethality and cytotoxicity by hydroxocobalamin (vitamin B(12a)). Toxicology 2007, 242, 16–22.
- Wang, Y.; Nguyen, L.H.; Mehta, R.S.; Song, M.; Huttenhower, C.; Chan, A.T. Association Between the Sulfur Microbial Diet and Risk of Colorectal Cancer. JAMA Netw. Open 2021, 4, e2134308.
- Sikavi, D.R.; Nguyen, L.H.; Haruki, K.; Ugai, T.; Ma, W.; Wang, D.D.; Thompson, K.N.; Yan, Y.; Branck, T.; Wilkinson, J.E.; et al. The Sulfur Microbial Diet and Risk of Colorectal Cancer by Molecular Subtypes and Intratumoral Microbial Species in Adult Men. Clin. Transl. Gastroenterol. 2021, 12, e00338.
- Nguyen, L.H.; Cao, Y.; Hur, J.; Mehta, R.S.; Sikavi, D.R.; Wang, Y.; Ma, W.; Wu, K.; Song, M.; Giovannucci, E.L.; et al. The Sulfur Microbial Diet Is Associated with Increased Risk of Early-Onset Colorectal Cancer Precursors. Gastroenterology 2021, 161, 1423–1432.e4.
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123.
