Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Montserrat Fernandez-Guarino | -- | 3129 | 2023-05-06 03:54:54 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fernandez-Guarino, M.; Bacci, S.; Pérez González, L.A.; Bermejo-Martínez, M.; Cecilia-Matilla, A.; Hernández-Bule, M.L. Physical Therapies in Wound Healing and Assisted Scarring. Encyclopedia. Available online: https://encyclopedia.pub/entry/43912 (accessed on 07 February 2026).
Fernandez-Guarino M, Bacci S, Pérez González LA, Bermejo-Martínez M, Cecilia-Matilla A, Hernández-Bule ML. Physical Therapies in Wound Healing and Assisted Scarring. Encyclopedia. Available at: https://encyclopedia.pub/entry/43912. Accessed February 07, 2026.
Fernandez-Guarino, Montserrat, Stefano Bacci, Luis Alfonso Pérez González, Mariano Bermejo-Martínez, Almudena Cecilia-Matilla, Maria Luisa Hernández-Bule. "Physical Therapies in Wound Healing and Assisted Scarring" Encyclopedia, https://encyclopedia.pub/entry/43912 (accessed February 07, 2026).
Fernandez-Guarino, M., Bacci, S., Pérez González, L.A., Bermejo-Martínez, M., Cecilia-Matilla, A., & Hernández-Bule, M.L. (2023, May 06). Physical Therapies in Wound Healing and Assisted Scarring. In Encyclopedia. https://encyclopedia.pub/entry/43912
Fernandez-Guarino, Montserrat, et al. "Physical Therapies in Wound Healing and Assisted Scarring." Encyclopedia. Web. 06 May, 2023.
Copy Citation
Wound healing (WH) is a complex multistep process in which a failure could lead to a chronic wound (CW). CW is a major health problem and includes leg venous ulcers, diabetic foot ulcers, and pressure ulcers. CW is difficult to treat and affects vulnerable and pluripathological patients. On the other hand, excessive scarring leads to keloids and hypertrophic scars causing disfiguration and sometimes itchiness and pain. Treatment of WH includes the cleaning and careful handling of injured tissue, early treatment and prevention of infection, and promotion of healing. Treatment of underlying conditions and the use of special dressings promote healing.
chronic wound
electromagnetic fields
hypertrophic scar
keloid
laser
physical therapies
photobiomodulation
photodynamic therapy
radiofrequency
ultrasound therapy
wound healing
1. Introduction
Wound healing (WH) is a main health problem in current society. Firstly, acute wounds could lead to scars and disfiguring lesions, and secondly, chronic wounds (CW) cause morbidity and high economic cost. AWs occur, in general, after surgery, trauma, or burns, whereas in CWs occur, in general, with an underlying systemic condition, such as diabetes, elderly, vascular alterations, or malnutrition.
Guidelines for care in wounds are useful, clear, and concise [1]. They represent the principal approach in clinical practice. The main CWs presented in clinical practice include leg ulcers (LU), diabetic foot ulcer (DFU), and pressure ulcer (PU). The main treatment of CWs include adequate dressing, debridement, and pressure control. Nevertheless, undoubtedly, there is an uncovered gap in this pathology, as scarring is sometimes unavoidable, and CWs could persist for months.
Lots of research works are nowadays focused on solving the problem of WH, most of them searching for very advanced therapies, such as cellular transplantation therapy [2][3], vascular enhancers [4], regenerative materials [5], or nanoparticles in hydrogels [6]. Despite the highly anticipated novel therapies in development, right now, they are very far from being used in real practice.
Physical therapy (PT) is present in daily clinical consultations and has demonstrated a certain utility in WH [7].
2. General Approach to Wounds
2.1. Epidemiology
The data in the literature referring to failure of WH show the seriousness of the problem.
WH failure, dermal fibrosis and scarring affect all ethnicities, while keloids or hypertrophic scars are more prevalent in American, African, and Asian populations, which can reach up to 16% of the population [8]. Factors associated with excessive scarring include genetic predisposition, hypertension, endocrine dysfunction, autoimmune diseases, and endocrine alterations [9]. The genetic factors described have been found to be associated with polymorphism alterations in genes such as TGF-beta, which evolved in fibrosis formation, opening, and interested therapeutic target [10]. The subsequent endothelial malfunction in hypertension has recently been associated with the risk of scarring and other diseases which have fibrosis and remodeling in their pathogenic [11].
On the other hand, the type of injury has also been associated with the risk of scarring and other factors often seen in clinical practice [12]. Two types of scars are described: keloids and hypertrophic scars (HS). HS are limited to the wound with an increase in cicatricial tissue, whereas keloids are invasive, going through the limit of the wound [12].
Conversely, the failure of healing a wound also produces a high impact on the patients. A chronic wound (CW) is a wound that fails to repair and restore the skin in three months [13]. It is estimated that 1–2% of the population suffer from CWs [14], for example, in the United States more than 6.5 million patients are affected [15].
2.2. Process and Stages of Wound Healing
WH is a complex process evolving multiple biological pathways and mechanisms. Classically, it is divided into different phases, including hemostasis/inflammatory stage, proliferation, and remodeling (Figure 1) [2][16].
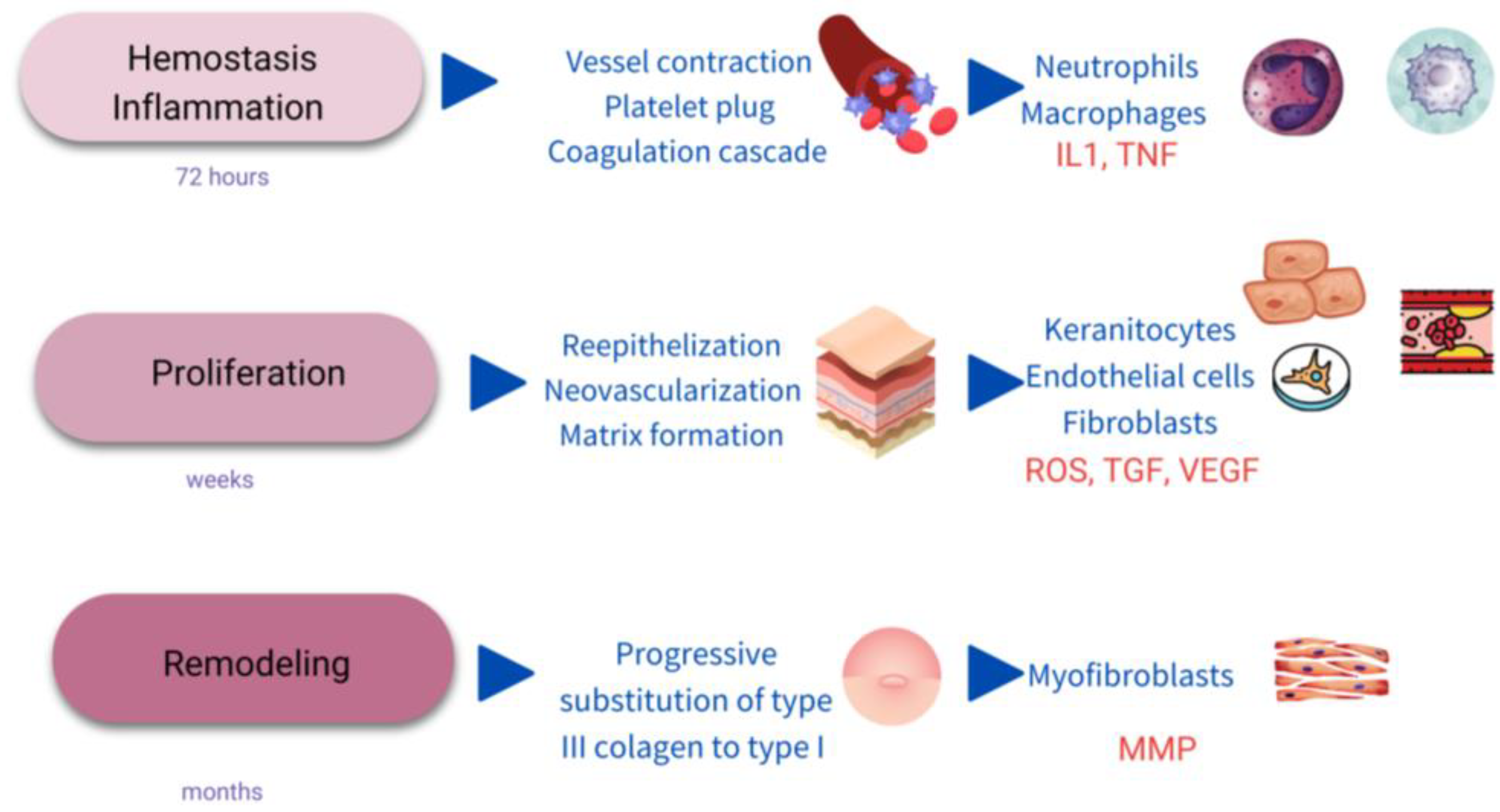
Figure 1. Scheme of the stages of wound healing. IL1: interkeukin1; TNF: Tumor necrosis factor; ROS: single oxygen radicals; TGF: transforming growth factor; VEGF: vascular endothelial growth factor; MMP: metalloproteinases.
2.3. Chronic Wounds
A chronic wound (CW) is described as a wound that fails to repair itself or remains unhealed after 12 weeks [1].
Most of the CW are classified as diabetic ulcers (DU), pressure ulcers (PU), or venous leg ulcers (VU), in relation to their clinical findings and cause.
2.4. General Management of Chronic Wounds
WH and scarring is a complex process with multiple influencing and interacting factors. Additionally, some of those factors are not under the control of the dermatologist, such as age, vascular abnormalities, comorbidities, malnutrition, or smoking [1]. The management is challenging, and multiple approaches and visits are needed with the implication of different health care workers [1] and arisen important indirect costs.
All CW should be treated according to the TIME principles: tissue debridement, infection control, moisture balance and edges of the wound [13]. Debridement is the first step in the treatment of a CW, it must be carried out weekly and it increases the speed of healing by over 72% [17].
Biofilm is presented in the extracellular matrix and is considered the cause of 80% of the infections in CW [18]. Biofilm is invisible to the naked eye, and different techniques to assess its presence are being developed, apart from a cutaneous biopsy. Nevertheless, the biofilm must be removed because it maintains the CW in the inflammatory stage [19]. The risk of infection is usually controlled by topical antibiotics, silver dressing, or with other topical components.
The wound should not be exposed to air, and if the skin appears dry, moisturizer should be added to the dressing. On the other hand, if excessive drainage is present, it needs to be clean and dried. The wound edge, in case of overgrowth, must be excised for epithelization [20].
2.5. General Prevention of Scarring
Excess WH or scarring is caused by an overproduction of extracellular matrix generated by myofibroblasts, which in this type of lesion are not replaced by fibroblasts during the proliferative phase. In this fibrosis, matrix proteins such as alpha-smooth muscle actin (alpha-SMA) are overexpressed, and the expression of MMP decreases, which induces an accumulation of collagen [12].
Keloid and HS are clinical expressions, and both can be considered successive stages of the same proliferative disorder. The initial common process is a purulent inflammatory skin lesion, the hyperfunction of the fibroblasts and excessive extracellular matrix deposition. HS consists of mainly type III collagen, whereas keloids contain type I and III [21].
3. The Role of Physical Therapies in Hard-To-Heal Chronic Wounds
Principles and Basis
Once it is known what fails in WH, the possibility of understanding the role of physical therapies arises more clearly. Figure 2 shows a scheme of possible targets for increasing WH, and Figure 3 shows how to promote regeneration rather than scarring with physical therapies (PT). It is of notice that with their theoretical mechanisms, we can impact in all the phases completing and fostering traditional treatments with innocuity.
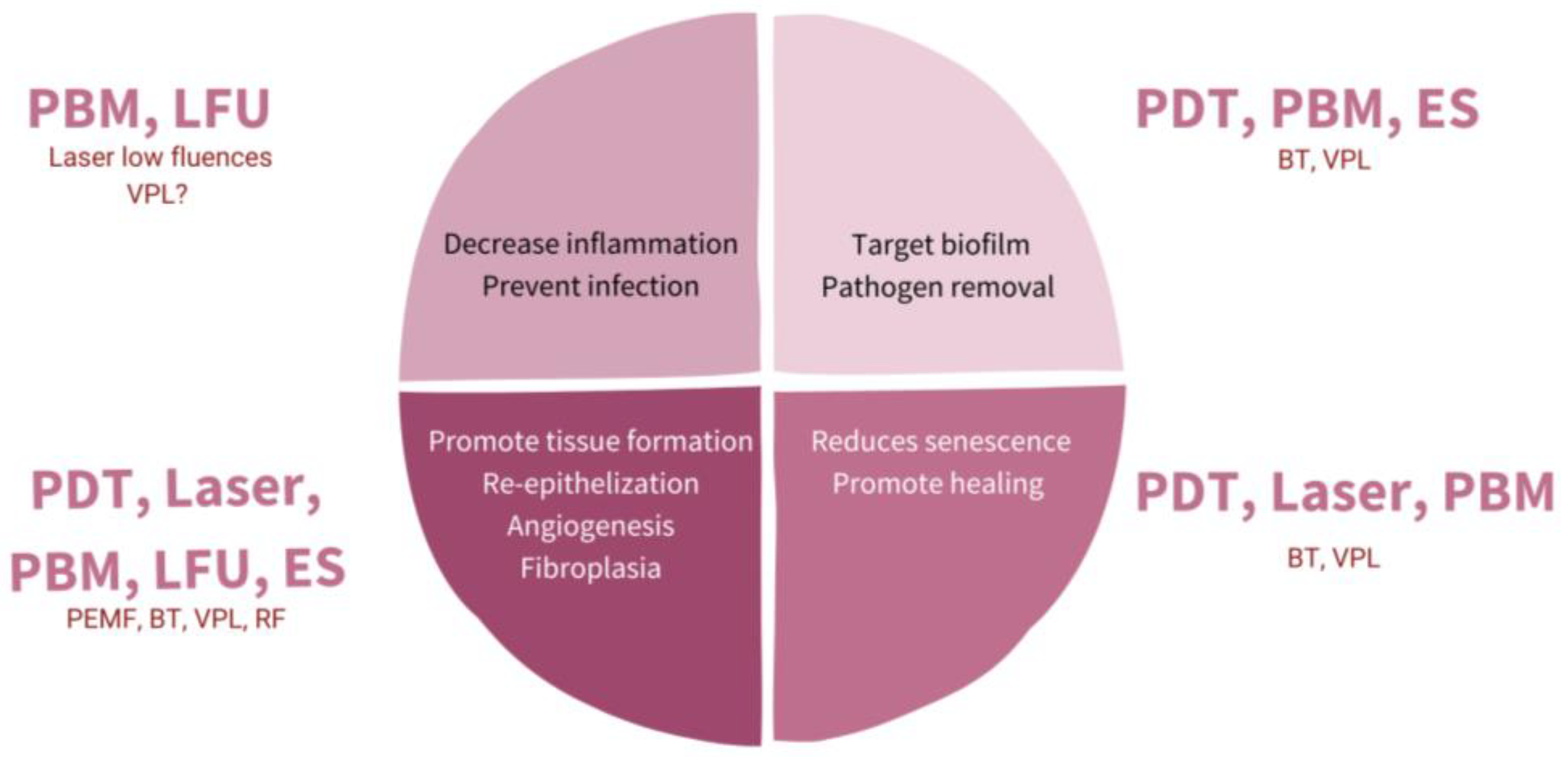
Figure 2. Diagram representing different targets with physical therapies (PT) for promoting wound healing (WH). PBM: photobiomodulation; LFU: low frequent ultrasound; PDT: photodynamic therapy; ES: electrostimulation; VPL: visible pulsed light; PEMF: pulsed electromagnetic fields; BT: biophotonic therapy; RF: radiofrequency.
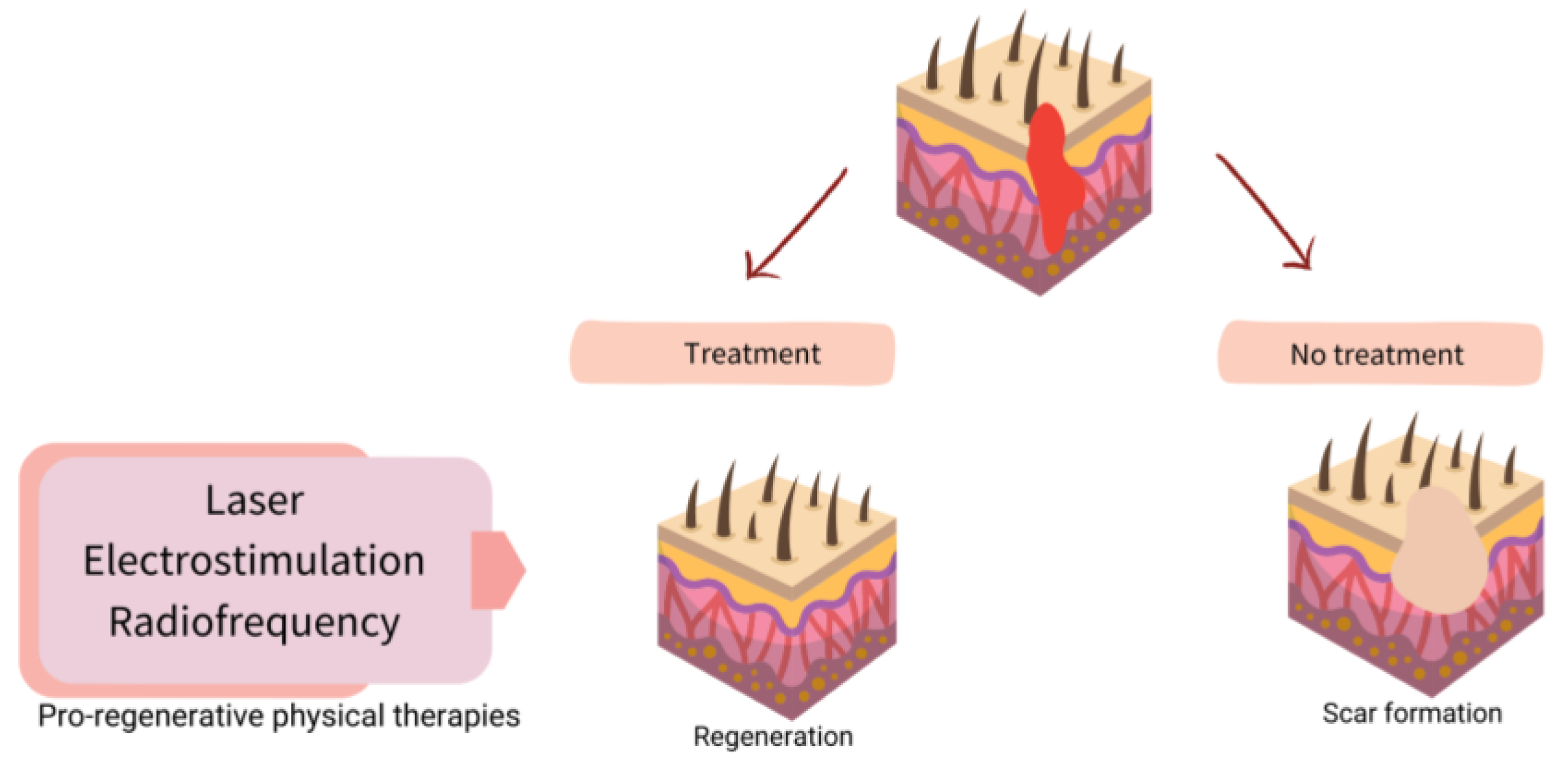
Figure 3. Scheme of strategies for physical therapies (PT) in assisted well-scaring.
The guidelines for the management of CW are extensive, but the pillars are promoting patient adherence to treatment, debridement control of the possible infection, covering with an appropriate dressing and effective compression if necessary [1].
Two options appear when PT are introduced in the treatment, either CW or scarring. One is proactive management, starting the treatment in the initial phases of the wound, as a prevention or adjuvant therapy. The other one is using those therapies when a CW, HS or keloid has appeared. Both situations not only depend on the physician but also the patient consultation.
4. Physical Therapies in Assisted Healing and Scar Prevention
4.1. Laser
The main target of laser therapy is the treatment and prevention of scarring, and there are few studies published in its assistance in WH. Among the different issues presented in an HS or keloids, different lasers could be used to target each objective [22]. Laser treatment is flexible and allows for their combination in a single treatment session. The most widely applied are fractional lasers in combination with vascular lasers and lasers targeting melanin [23]. Basically, there are two different types of fractional laser: ablative (wavelengths of 2790–10,600 nm) and non-ablative (1320–1927 nm). Both ablative and non-ablative lasers have become the gold standard for the treatment of scarring, although ablative lasers are probably the most used [24].
Erbio and CO2 lasers are ablative lasers that target water, producing a selective burn in the skin. In a fractional mode, they work in separated columns, allowing for a better regeneration throughout the non-damage columns of skin. Both induce selective thermal necrosis in the skin, increasing in the first weeks of the inflammatory stage of the scar, but after three months, collagen remodeling in a thin bundle due to collagen III appears [25]. The clinical results show a decreasing dermis thickness and increasing skin flexibility [23]. On the other hand, vascular lasers target small vessels and are used for decreasing erythema in HS and keloids, causing excessive neovascularization. Pulsed dye laser (PDL) is probably the most used. PDL has been demonstrated to decrease connective tissue growth factor expression in keloids, despite targeting vessels [26] and inhibiting fibroblast proliferation in vitro [27]. After the vessel coagulation and subsequent hypoxia, PDL leads to an increase in collagen type III [28].
Apart from the treatment of scars, some studies of lasers in assisted WH and preventing scarring from the first day of surgery have been published showing different results. Curiously, the immediate application of lasers differs from other physical therapies, which need some healing days before they start to be applied. In a split-face study, no differences were found in the area treated with CO2 laser immediately after surgery, but in other similar studies the scars treated exhibit better healing and cosmetic outcome [29][30][31]. PDL and non-ablative fractional laser have also been shown to improve scaring when used early; however, the differences with the untreated area were not statistically significant [32]. Different types of lasers could be applied in the same session, PDL plus ablative fractional CO2 laser have been suggested to be the best combination [33].
An early start of the treatment is recommended in the literature reviewed when lasers are used to assist scarring. The optimal interval between sessions has been found to be 5 to 6 weeks during a period of months [34]. All the lasers applied in early treatment times have also been used under lower parameters [28]. Further clinical trials with long-term follow-up are needed to support the evidence of laser treatment in HS, keloids and WH alone or in combination with other options for treatment [35]; however, lasers are recommended for expert panels as a first-line therapy in scarring [36].
4.2. Photobiomodulation (Low-Level Light Therapy-LLLT)
LLLT has been intensely studied in WH, the near-infrared light (NIR) between 800 and 900 nm and red light (600–700 nm) being the most used. The use of light in a non-thermal effect is supported by the photon’s absorption of the cells’ receptors. The main three chromophores in the skin are melanin in the epidermis, hemoglobin in the dermis and water in all the skin [37] and longer wavelengths achieve deeper penetration (Figure 4).
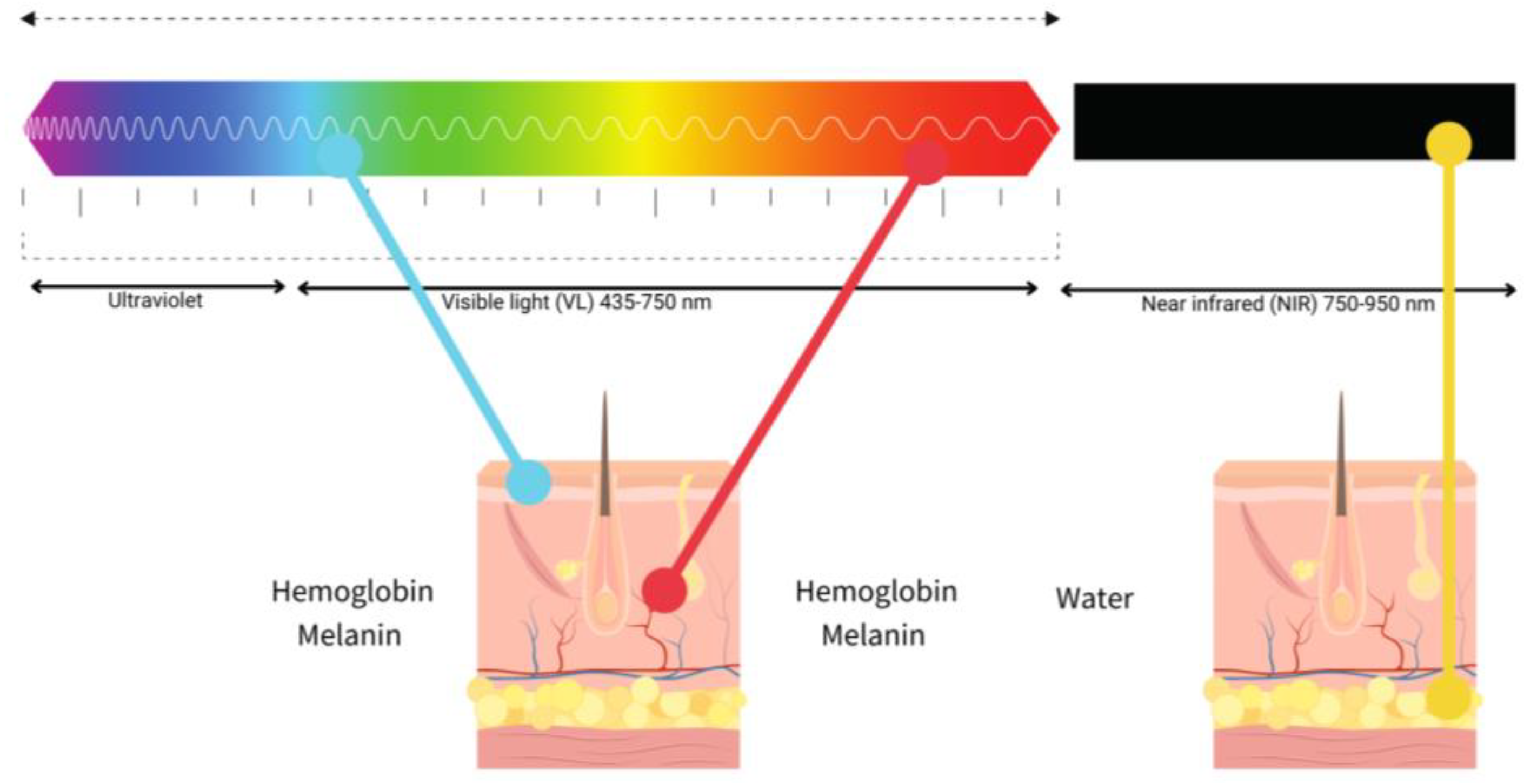
Figure 4. Diagram of the relationship between visible light (blue, red) and near-infrared (NIR), penetration and chromophores.
Hormesis responses occur in WH in response to low doses of light (LLLT) or photobiomodulation (PBM). Hormesis or biomodulation are terms used to describe a natural biological process in which low doses of an input, for example, light or energy, induce activation, but high doses produce an inhibition [38]. PBM induces the production of nitric oxide (NO), a vasodilator, and anti-inflammatory agent [39]. LLLT can trigger natural mechanisms involved in WH, including TGF-beta families of molecules, transforming growth platelet factor, interleukins (IL6, 13, 15), and matrix metalloproteinases (MMPs) associated with alterations in WH. TGF-beta is crucial in fibroblast proliferation [7][38][39]. Thus, PBM has been demonstrated to be useful in all the steps of WH.
In animal models, LLLT increases collagen and reduces oxidative and nitroxidative stress in diabetic wounded mouse skin [39]. In vitro studies have also found an increased expression in keratinocytes after LLLT of cyclin D1 and cytokeratin, suggesting an increase in proliferation and maturation [40][41].
LLLT is not as widely used as laser despite being safer, without adverse reactions such as swelling, crusting or purpura. With respect to laser, LLLT is easy to apply, allows the treatment of bigger areas, a wearable device is available, self-treating is an opportunity and it is not as expensive. The main disadvantage of LLLT is the necessity of near-daily repeated sessions [42].
There are few studies of LLLT in WH with different results. In VU, red light did not demonstrate any additional benefit to conventional treatment [43]. Whereas in PU and DU, red light increases healing with better outcomes when compared with NIR [44]. A prophylactic treatment in the prevention of keloid in three patients was shown effective with NIR (LED 805 nm). In this small study, patients self-treated at home daily for one month [45].
LLLT improves inflammation, releases pain, and fosters healing in clinical practice. Even though it has been deeply investigated, further studies in the daily clinical application are necessary as no standard protocol has been developed [42].
4.3. Photodynamic Therapy
Photodynamic therapy (PDT) is a safe and easy procedure to enhance WH, nevertheless, further studies are necessary to determine an exact protocol. Anyhow, PDT is versatile, with the limitation of pain during the treatment and repeat sessions.
PDT is indicated in dermatology for the treatment of actinic keratosis, basal cell carcinoma and Bowen disease [46]. PDT has been explored in WH and prevents scarring, whereas no results have been found in the treatment of keloids and HS. The main difference between PDT and other PT in WH is the ability to scope infections without resistance to antibiotics.
4.4. Electrical Stimulation
Endogenous bioelectric fields (EBF) take place during WH, produced by the cells generated by the Na+/K+ ATPase of the epidermis. EBFs influence cell migration, proliferation, and function, but also gene and protein expression [47]. The underlying mechanisms presented in a CW could be targeted with electrical stimulation (ES) mimicking the natural process (Figure 2).
There are different forms of ES, including direct current, alternating current and pulsed current on mono or bipolar devices. That huge variability limits knowing the real exact beneficial protocol. Moreover, no comparative study between those modalities has been conducted, whereas it is supposed that the pulsed current is the most similar to the physiological(25) [47][48][49]. Theoretically, not all forms of ES are beneficial in all phases of WH, the alternative current only being useful in the first days [48]. There is also a lack of literature about standard protocols [50].
According to the mechanism of action of ES, it would be more effective in the proliferative and remodeling phase of WH, that implies, from days to months after the injury, either in acute wounds (AW) to prevent scarring or in CW to enhance healing [50].
If ES is applied, it should be added to the conventional treatment of the wound as a complementary treatment. The ES devices are usually applied by setting electrodes around the wound. Repeated-weekly sessions are necessarily, lasting from 45 min to hours [48]. The therapy could last months, which is a great limitation due to time consumption and displacement. Therefore, ES might be used in selected patients with a risk of failure in WH. Novel devices are emerging, offering different possibilities such as home devices, electric dressings, or electric fields, providing a practical future [47].
4.5. Others
4.5.1. Ultrasound Therapy
Ultrasound therapy (UT) consists of sound waves that cause thermal and non-thermal effects in tissues. When UT is strongly applied to the skin, the temperature will rise to 40 Celsius degrees and produces an increase in vessel flow, cell proliferation, collagen synthesis, and tissue regeneration. Moreover, UT has anti-inflammatory properties. The non-thermal effects comply with acoustic streaming with a displacement of the particles and cavitation with the generation of microenvironmental gases [51]. Cavitation cleans necrotic tissue preserving the healthy one [7].
UT accelerates the decrease in the wound area with respect to controls in LU, and it is approved as an adjuvant therapy in WH by the FDA [52][53].
Two types of therapeutic US exits are low frequency ultrasound (LFU) from 30 to 40 kHz and high-frequency ultrasound (HFU), ranging from 1 to 3 MHz. HFU has been used for decades for the treatment of muscular diseases in sports medicine. A variant of HFU, micro focused ultrasound in high intense mode (MFU, HIFU), is being widely studied because of its benefits in aesthetic medicine reducing wrinkles and laxity of the skin [52]. In contrast, LFU has demonstrated efficacy in WH and has been applied with good results in LU.
LFU is used directly on the skin, around the wound for 5 to 10 min. A topical gel is usually needed between the skin surface and the applicator [51]. UT is contraindicated in a patient carrying a metal prosthesis in the leg, neuropathy, infection, or thrombophlebitis [52].
UT has a possible application in WH; nevertheless, there are no clinical studies of the effects of UT in WH or scar prevention, most of the evidence is limited to LU and further randomized clinical essays and protocols are necessary [52][53].
4.5.2. Electromagnetic Fields
Low frequency pulsed electromagnetic fields (PEMF) can accelerate WH, generating connective tissue, enhancing the VEGF pathway and the production of collagen type I. There are some published studies with good results in PU, VLU and DU. PEMF are possible to apply at home on portable devices as multiple sessions are necessary [54][55].
4.5.3. Biophotonic Therapy
Biophotonic therapy (BT) consists of the application of the PBM applying a special gel over the CW containing the chromophores. Afterwards, an LED lamp with a hyper pulsed beam and low energy is used to activate the photoconverter gel. One concrete device known as “Lumihel®” was evaluated showing improvement in the healing of the CW, increasing the life quality of the patient without adverse events. The main limitations of the study were the simultaneous inclusion of VU, LU and PU and the weekly treatment sessions lasting 8 weeks [56].
4.5.4. Visible Polarized Light
Visible polarized light (VPL) has been used as a complementary therapy in WH. The device used emitted light like the sun but without ultraviolet radiation. Thus, the light used was safe, low energy light, polychromatic, incoherent, and polarized. The polarization allows it to work on flat surfaces and enhances light penetration. The molecular mechanism of action of VL is not well documented; however, some studies showing improvement in treated CW have been published [57][58]. PVL seems a promising possible treatment for WH but needs to be more deeply studied [59].
4.5.5. Radiofrequency
Radiofrequency therapies consist of the application of a high-frequency electromagnetic field (3 kHz and 300 GHz) that induces oscillation and friction in the molecules of the target tissue, which causes tissue hyperthermia. This electrically induced hyperthermia can degrade collagen, which stimulates neocollagenogenesis and tissue remodeling [60]. The main indicators of RF are skin tightening, the reduction of wrinkles and the treatment of scars. There are some studies assessing the efficacy of RF in WH with good results in releasing pain. Nevertheless, multiple sessions are necessary for 2–4 weeks. RF technology is rapidly developing, with new micro-needling devices and fractionated delivery, which shows good results in acne scars, HS, and keloids [61][62].
References
- Gupta, S.; Andersen, C.; Black, J.; Fife, C.; Lantis, J.I.; Niezgoda, J.; Snyder, R.; Sumpio, B.; Tettelbach, W.; Treadwell, T.; et al. Management of Chronic Wounds: Diagnosis, Preparation, Treatment, and Follow-Up. Wounds Compend. Clin. Res. Pract. 2017, 29, S19–S36.
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101.
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706.
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125.
- Monavarian, M.; Kader, S.; Moeinzadeh, S.; Jabbari, E. Regenerative Scar-Free Skin Wound Healing. Tissue Eng. Part B Rev. 2019, 25, 294–311.
- Bai, Q.; Han, K.; Dong, K.; Zheng, C.; Zhang, Y.; Long, Q.; Lu, T. Potential Applications of Nanomaterials and Technology for Diabetic Wound Healing. Int. J. Nanomed. 2020, 15, 9717–9743.
- Palmieri, B.; Vadalà, M.; Laurino, C. Electromedical devices in wound healing management: A narrative review. J. Wound Care 2020, 29, 408–418.
- Lu, W.-S.; Zheng, X.-D.; Yao, X.-H.; Zhang, L.-F. Clinical and epidemiological analysis of keloids in Chinese patients. Arch. Dermatol. Res. 2015, 307, 109–114.
- Berman, B.; Maderal, A.; Raphael, B. Keloids and Hypertrophic Scars: Pathophysiology, Classification, and Treatment. Dermatol. Surg. 2017, 43, S3–S18.
- He, Y.; Deng, Z.; Alghamdi, M.; Lu, L.; Fear, M.W.; He, L. From genetics to epigenetics: New insights into keloid scarring. Cell Prolif. 2017, 50, e12326.
- Huang, C.; Ogawa, R. Systemic factors that shape cutaneous pathological scarring. FASEB J. 2020, 34, 13171–13184.
- Huang, C.; Ogawa, R. Keloidal pathophysiology: Current notions. Scars Burn. Health 2021, 7.
- Bowers, S.; Franco, E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician 2020, 101, 159–166.
- Gottrup, F. A specialized wound-healing center concept: Importance of a multidisciplinary department structure and surgical treatment facilities in the treatment of chronic wounds. Am. J. Surg. 2004, 187, S38–S43.
- Fife, C.E.; Carter, M.J. Wound Care Outcomes and Associated Cost among Patients Treated in US Outpatient Wound Centers: Data from the US Wound Registry. Wounds A Compend. Clin. Res. Pract. 2012, 24, 10–17.
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321.
- Wilcox, J.R.; Carter, M.J.; Covington, S. Frequency of debridements and time to heal: A retrospective cohort study of 312 744 wounds. JAMA Dermatol. 2013, 149, 1050–1058.
- Azevedo, M.-M.; Lisboa, C.; Cobrado, L.; Pina-Vaz, C.; Rodrigues, A.G. Hard-to-heal wounds, biofilm and wound healing: An intricate interrelationship. Br. J. Nurs. 2020, 29, S6–S13.
- Sun, F.; Qu, F.; Ling, Y.; Mao, P.; Xia, P.; Chen, H.; Zhou, D. Biofilm-associated infections: Antibiotic resistance and novel therapeutic strategies. Futur. Microbiol. 2013, 8, 877–886.
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464.
- Huang, C.; Akaishi, S.; Hyakusoku, H.; Ogawa, R. Are keloid and hypertrophic scar different forms of the same disorder? A fibroproliferative skin disorder hypothesis based on keloid findings. Int. Wound J. 2014, 11, 517–522.
- Kauvar, A.N.B.; Kubicki, S.L.; Suggs, A.K.; Friedman, P.M. Laser Therapy of Traumatic and Surgical Scars and an Algorithm for Their Treatment. Lasers Surg. Med. 2020, 52, 125–136.
- Altemir, A.; Boixeda, P. Laser Treatment of Burn Scars. Actas Dermosifiliogr. 2022, 113, T938–T944.
- Clementoni, M.T.; Pedrelli, V.; Zaccaria, G.; Pontini, P.; Motta, L.R.; Azzopardi, E.A. New Developments for Fractional CO2 Resurfacing for Skin Rejuvenation and Scar Reduction. Facial Plast. Surg. Clin. N. Am. 2020, 28, 17–28.
- Azzam, O.A.; Bassiouny, D.A.; El-Hawary, M.S.; El Maadawi, Z.M.; Sobhi, R.M.; El-Mesidy, M.S. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: A clinical, histological, and immunohistochemical study. Lasers Med. Sci. 2016, 31, 9–18.
- Yang, Q.; Ma, Y.; Zhu, R.; Huang, G.; Guan, M.; Avram, M.M.; Lu, Z. The effect of flashlamp pulsed dye laser on the expression of connective tissue growth factor in keloids. Lasers Surg. Med. 2012, 44, 377–383.
- Zhibo, X.; Miaobo, Z. Molecular mechanism of pulsed-dye laser in treatment of keloids: An in vitro study. Adv. Skin Wound Care 2010, 23, 29–33.
- Lv, K.; Xia, Z.; Chinese Consensus Panel on the Prevention and Treatment of Scars. Chinese expert consensus on clinical prevention and treatment of scar+. Burn. Trauma 2018, 6, 27.
- Sobanko, J.F.; Vachiramon, V.; Rattanaumpawan, P.; Miller, C.J. Early postoperative single treatment ablative fractional lasing of Mohs micrographic surgery facial scars: A split-scar, evaluator-blinded study. Lasers Surg. Med. 2015, 47, 1–5.
- Shin, H.W.; Suk, S.; Chae, S.W.; Yoon, K.C.; Kim, J. Early postoperative treatment of mastectomy scars using a fractional carbon dioxide laser: A randomized, controlled, split-scar, blinded study. Arch. Plast. Surg. 2021, 48, 347–352.
- Lee, S.H.; Zheng, Z.; Roh, M.R. Early Postoperative Treatment of Surgical Scars Using a Fractional Carbon Dioxide Laser: A Split-Scar, Evaluator-Blinded Study. Dermatol. Surg. 2013, 39, 1190–1196.
- Kim, D.H.; Ryu, H.J.; Choi, J.E.; Ahn, H.H.; Kye, Y.C.; Seo, S.H. A Comparison of the Scar Prevention Effect between Carbon Dioxide Fractional Laser and Pulsed Dye Laser in Surgical Scars. Dermatol. Surg. 2014, 40, 973–978.
- Liu, X.-J.; Liu, W.-H.; Fang, S.-W.; Zhou, X.-L.; Xu, J.-X.; Li, G.-S. Lasers and Intense Pulsed Light for the Treatment of Pathological Scars: A Network Meta-Analysis. Aesthetic Surg. J. 2022, 42, NP675–NP687.
- Brewin, M.P.; Lister, T.S. Prevention or treatment of hypertrophic burn scarring: A review of when and how to treat with the Pulsed Dye Laser. Burns 2014, 40, 797–804.
- Leszczynski, R.; da Silva, C.A.; Pinto, A.; Kuczynski, U.; da Silva, E.M. Laser therapy for treating hypertrophic and keloid scars. Cochrane Database Syst. Rev. 2022, 9, CD011642.
- Seago, M.; Shumaker, P.R.; Spring, L.K.; Alam, M.; Al-Niaimi, F.; Anderson, R.R.; Artzi, O.; Bayat, A.; Cassuto, D.; Chan, H.H.; et al. Laser Treatment of Traumatic Scars and Contractures: 2020 International Consensus Recommendations. Lasers Surg. Med. 2020, 52, 96–116.
- Mosca, R.C.; Ong, A.A.; Albasha, O.; Bass, K.; Arany, P. Photobiomodulation Therapy for Wound Care: A Potent, Noninvasive, Photoceutical Approach. Adv. Ski. Wound Care 2019, 32, 157–167.
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Agathokleous, E.; Calabrese, V. Hormesis: Wound healing and fibroblasts. Pharmacol. Res. 2022, 184, 106449.
- Tatmatsu-Rocha, J.C.; Ferraresi, C.; Hamblin, M.R.; Maia, F.D.; do Nascimento, N.R.; Driusso, P.; Parizotto, N. Low-level laser therapy (904 nm) can increase collagen and reduce oxidative and nitrosative stress in diabetic wounded mouse skin. J. Photochem. Photobiol. B Biol. 2016, 164, 96–102.
- Sperandio, F.F.; Simões, A.; Corrêa, L.; Aranha, A.C.C.; Giudice, F.S.; Hamblin, M.R.; Sousa, S.C. Low-level laser irradiation promotes the proliferation and maturation of keratinocytes during epithelial wound repair. J. Biophotonics 2014, 8, 795–803.
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Agathokleous, E.; Calabrese, V. Hormesis: Wound healing and keratinocytes. Pharmacol. Res. 2022, 183, 106393.
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017.
- Vitse, J.; Bekara, F.; Byun, S.; Herlin, C.; Teot, L. A Double-Blind, Placebo-Controlled Randomized Evaluation of the Effect of Low-Level Laser Therapy on Venous Leg Ulcers. Int. J. Low. Extremity Wounds 2017, 16, 29–35.
- Taradaj, J.; Halski, T.; Kucharzewski, M.; Urbanek, T.; Halska, U.; Kucio, C. Effect of Laser Irradiation at Different Wavelengths (940, 808, and 658 nm) on Pressure Ulcer Healing: Results from a Clinical Study. Evid. Based Complement. Altern. Med. 2013, 2013, 960240.
- Barolet, D.; Boucher, A. Prophylactic low-level light therapy for the treatment of hypertrophic scars and keloids: A case series. Lasers Surg. Med. 2010, 42, 597–601.
- Morton, C.A.; Szeimies, R.M.; Basset-Seguin, N.; Calzavara-Pinton, P.; Gilaberte, Y.; Haedersdal, M.; Hofbauer, G.F.L.; Hunger, R.E.; Karrer, S.; Piaserico, S.; et al. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 1: Treatment delivery and established indications—Actinic keratoses, Bowen’s disease and basal cell carcinomas. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2225–2238.
- Rajendran, S.B.; Challen, K.; Wright, K.L.; Hardy, J.G. Electrical Stimulation to Enhance Wound Healing. J. Funct. Biomater. 2021, 12, 40.
- Aleksandrowicz, H.; Owczarczyk-Saczonek, A.; Placek, W. Venous Leg Ulcers: Advanced Therapies and New Technologies. Biomedicines 2021, 9, 1569.
- Ud-Din, S.; Bayat, A. Electrical Stimulation and Cutaneous Wound Healing: A Review of Clinical Evidence. Healthcare 2014, 2, 445–467.
- Koel, G.; Houghton, P.E. Electrostimulation: Current Status, Strength of Evidence Guidelines, and Meta-Analysis. Adv. Wound Care 2014, 3, 118–126.
- Cullum, N.; Nelson, E.A.; Flemming, K.; Sheldon, T. Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy. Health Technol. Assess. 2001, 5, 9.
- Beheshti, A.; Shafigh, Y.; Parsa, H.; Zangivand, A.A. Comparison of High-Frequency and MIST Ultrasound Therapy for the Healing of Venous Leg Ulcers. Adv. Clin. Exp. Med. 2014, 23, 969–975.
- Cullum, N.; Liu, Z. Therapeutic ultrasound for venous leg ulcers. Cochrane Database Syst. Rev. 2017, 5, CD001180.
- Guerriero, F.; Botarelli, E.; Mele, G.; Polo, L.; Zoncu, D.; Renati, P.; Sgarlata, C.; Rollone, M.; Ricevuti, G.; Maurizi, N.; et al. Effectiveness of an Innovative Pulsed Electromagnetic Fields Stimulation in Healing of Untreatable Skin Ulcers in the Frail Elderly: Two Case Reports. Case Rep. Dermatol. Med. 2015, 2015, 576580.
- Kwan, R.L.; Wong, W.C.; Yip, S.L.; Chan, K.L.; Zheng, Y.P.; Cheing, G.L. Pulsed electromagnetic field therapy promotes healing and microcirculation of chronic diabetic foot ulcers: A pilot study. Adv. Skin Wound Care 2015, 28, 212–219.
- Romanelli, M.; Piaggesi, A.; Scapagnini, G.; Dini, V.; Janowska, A.; Iacopi, E.; Scarpa, C.; Fauverghe, S.; Bassetto, F.; EUREKA Study Group. Evaluation of fluorescence biomodulation in the real-life management of chronic wounds: The EUREKA trial. J. Wound Care 2018, 27, 744–753.
- Taha, M.M.; El-Nagar, M.M.; Elrefaey, B.H.; Elkholy, R.M.; Ali, O.I.; Alkhamees, N.; Felaya, E.-S.E.E.-S. Effect of Polarized Light Therapy (Bioptron) on Wound Healing and Microbiota in Diabetic Foot Ulcer: A Randomized Controlled Trial. Photobiomodulation Photomed. Laser Surg. 2022, 40, 792–799.
- M. Allam, N.; Eladl, H.M.; Eid, M.M. Polarized Light Therapy in the Treatment of Wounds: A Review. Int. J. Low Extrem. Wounds 2022, 15347346221113991.
- Feehan, J.; Burrows, S.P.; Cornelius, L.; Cook, A.M.; Mikkelsen, K.; Apostolopoulos, V.; Husaric, M.; Kiatos, D. Therapeutic applications of polarized light: Tissue healing and immunomodulatory effects. Maturitas 2018, 116, 11–17.
- Ekelem, C.; Thomas, L.; Van Hal, M.; Valdebran, M.; Lotfizadeh, A.; Mlynek, K.; Mesinkovska, N.A. Radiofrequency Therapy and Noncosmetic Cutaneous Conditions. Dermatol. Surg. 2019, 45, 908–930.
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7.
- Cucu, C.; Butacu, A.; Niculae, B.D.; Tiplica, G.S. Benefits of fractional radiofrequency treatment in patients with atrophic acne scars—Literature review. J. Cosmet. Dermatol. 2021, 20, 381–385.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revision:
1 time
(View History)
Update Date:
06 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




