Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jozef Dragašek | -- | 1095 | 2023-05-05 18:19:12 | | | |
| 2 | Conner Chen | Meta information modification | 1095 | 2023-05-08 03:40:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fizíková, I.; Dragašek, J.; Račay, P. The Link between 4-Hydroxynonenal and Schizophrenia. Encyclopedia. Available online: https://encyclopedia.pub/entry/43890 (accessed on 07 February 2026).
Fizíková I, Dragašek J, Račay P. The Link between 4-Hydroxynonenal and Schizophrenia. Encyclopedia. Available at: https://encyclopedia.pub/entry/43890. Accessed February 07, 2026.
Fizíková, Iveta, Jozef Dragašek, Peter Račay. "The Link between 4-Hydroxynonenal and Schizophrenia" Encyclopedia, https://encyclopedia.pub/entry/43890 (accessed February 07, 2026).
Fizíková, I., Dragašek, J., & Račay, P. (2023, May 05). The Link between 4-Hydroxynonenal and Schizophrenia. In Encyclopedia. https://encyclopedia.pub/entry/43890
Fizíková, Iveta, et al. "The Link between 4-Hydroxynonenal and Schizophrenia." Encyclopedia. Web. 05 May, 2023.
Copy Citation
The significant complexity of the brain can lead to the development of serious neuropsychiatric disorders, including schizophrenia. A number of mechanisms are involved in the etiopathogenesis of schizophrenia, pointing to its complexity and opening a new perspective on studying this disorder. HNE (4-hydroxynonenal) is an essential molecule that significantly impacts cell function and survival. For this reason, it is assumed that HNE might play a role in the pathophysiology of schizophrenia and other disorders.
schizophrenia
mitochondria
oxidative stress
1. Introduction
Schizophrenia is a specifically human disease that affects approximately 0.5–1% of the population. Schizophrenia has been defined as a neuropsychiatric disease that causes changes in the process of thoughts, perceptions, and emotions, usually leading to mental deterioration and affective blunting [1][2].
Currently, commonly used clinical treatments can improve some symptoms but not others; for example, negative symptoms such as social withdrawal and alogia in schizophrenia are not sufficiently treated with antipsychotic medications. In addition, it is currently not possible to predict which patients will or will not respond to antipsychotic medications. Therefore, developing novel, more effective therapeutic strategies for schizophrenia is imperative [2][3][4][5].
Early detection and intervention in schizophrenia requires mechanism-based biomarkers that capture circuitry dysfunction and can allow optimised patient stratification, disease monitoring, treatment, and improved prognosis [3][4][6].
The hypothesis of mitochondrial dysfunction as a factor in the etiopathogenesis of schizophrenia is currently gaining more attention. This is the basis of so-called mitochondrial psychiatry. The basis of this hypothesis is that mitochondria are important not only as the main energy producer but also as a significant influencer of many important life processes [1][5][6][7].
Schizophrenia is an often debilitating illness that typically manifests in late adolescence and frequently has a progressive course associated with significant disability, economic burden, and early mortality. Therefore, an improved understanding of the molecular pathophysiology underlying schizophrenia is needed in order to better target future therapies.
Multiple converging lines of evidence from ex vivo, postmortem, imaging, and animal model studies indicate that proper mitochondrial function is important in the pathogenesis of schizophrenia. The mitochondrial dysfunction observed in schizophrenia is closely related to oxidative stress with the formation of free radicals (Figure 1). These changes lead to oxidative damage and the alteration of several key enzymes and antioxidant systems, as well as energy metabolism [2][6][8][9][10][11].
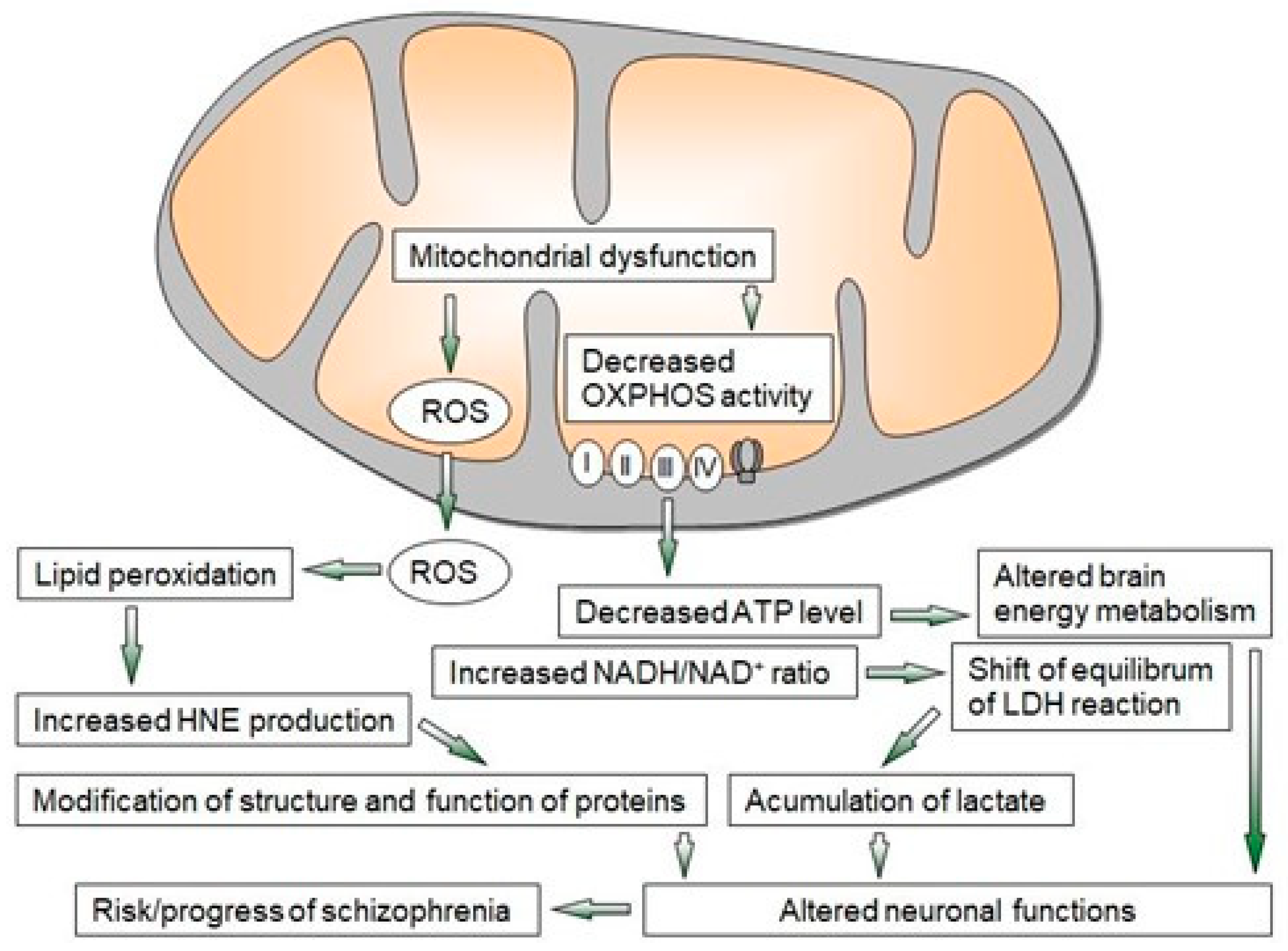
Figure 1. Overview of mechanisms of mitochondrial dysfunction leading to neuroprogressive changes in schizophrenia. LDH—lactate dehydrogenase, OXPHOS—oxidative phosphorylation, ROS—reactive oxygen species, I–IV—enzyme complexes of OXPHOS.
In vitro measurement data suggest that the mitochondrial effects of current antipsychotics are likely related to their adverse impacts and are due to drug-induced decreased ATP production and increased ROS production; however, based on the mitochondrial dysfunctions observed in neurodegenerative diseases, bipolar disorder, and schizophrenia, new psychotropic drugs are aimed at selected mitochondrial targets [5][6].
2. The Link between HNE and Schizophrenia
HNE is an essential molecule that significantly impacts cell function and survival. For this reason, it is assumed that HNE might play a role in the pathophysiology of schizophrenia and other disorders (e.g., Rett syndrome and autism spectrum disorder), although its specific influence is not yet fully understood [12][13][14][15][16]. Regarding schizophrenia, the results of a recent study showed increased levels of HNE conjugates with proteins in the brains of patients with schizophrenia compared to the control group. Interestingly, elevated levels of HNE protein conjugates have been documented in the hippocampus compared to the cortex of schizophrenia patients [12].
In one recent study [17], Western blot analysis showed HNE-modified plasma proteins with a discrete molecular mass in the range of 37–50 kDa in the plasma of schizophrenia patients, while HNE-modified plasma proteins with a discrete molecular mass of 100–200 kDa were documented in the control group.
The altered profile of plasma proteins modified by HNE may explain the statistically insignificant results obtained by fluorescence measurements [17].
Interestingly, in one study, increased levels of HNE-modified proteins with a molecular weight of around 50 kDa were observed in the plasma of patients suffering from Rett syndrome [14]. That study also documented increased levels of HNE-modified proteins with a molecular weight of 150–200 kDa. The massive modification of HNE plasma proteins in the plasma of patients with classical autistic disorder was also reported [15][16].
In another study, an increase in discrete proteins modified by HNE was observed, mainly in mitochondria isolated from rat brains, and was dependent on age [16].
It is also known that free HNE can react with proteins, thereby changing their conformation and function, as was also shown in the case of cytochrome c oxidase, the terminal complex of the mitochondrial respiratory chain. As a result of the action of HNE, various intracellular enzymes can also be inhibited (e.g., Na/K-ATPase, poly-ADP ribose polymerase, complex I of the respiratory chain, protein kinase C, NADPH oxidase, and proteasome). The inhibitory values reported in the literature indicate a wide range of enzyme sensitivity to HNE. Although HNE is primarily generated in membranes (or other hydrophobic compartments of cells), the partition coefficient of HNE allows diffusion into the cytosol or the extracellular space [12][18].
Nevertheless, it can be assumed that the HNE concentration is much higher in cell membranes than in the hydrophilic compartments of cells. HNE is considered a bifunctional aldehyde and can easily form cross-links within or between proteins. HNE is formed under physiological conditions and in pathophysiological situations as a product of ongoing oxidative stress; therefore, it is likely that many proteins are directly damaged by oxidants. The groups of HNE-peptide and HNE-protein conjugates are an integral part of the harmful effects of HNE on cellular functions [8][18][19][20].
In contrast, other pathways of HNE degradation contribute to HNE detoxification. These pathways include products of beta- and alpha-oxidation and the tricarboxylic acid cycle, such as acetyl-CoA, citrate, aconitate, malate, fumarate, succinate, carbon dioxide, and water. The most critical HNE-degrading enzymes are glutathione-S transferase, alcohol dehydrogenase, and aldehyde dehydrogenase. Among the products generated by these enzymatic substances, referred to as primary intermediates of HNE, are HNE-GSH, hydroxynonenic acid (HNA), and 1,4-dihydroxynonene (DH) [8][19][20].
The quick elimination of HNE from affected cells is a significant part of the secondary antioxidant defence mechanism of cells. Still, the processes have not yet been studied in detail with regard to schizophrenia. In addition, the increased formation of protein conjugates with HNE may also be a consequence of the activation of the inflammatory response, which is often involved in the etiopathogenesis of schizophrenia [8][19][21][22][23].
As mentioned, one of the specific targets of HNE may be cytochrome c oxidase, the terminal complex of the mitochondrial respiratory chain [21]. Modifying cytochrome c oxidase via HNE inhibits its activity, which could lead to mitochondrial dysfunction and lactate overproduction. Changes in lactate levels may be evidence of impaired energy metabolism in schizophrenia [23][24].
References
- Nestler, E.; Hyman, E.S.; Malenka, R.C.; Piško. Molekulárna Neuropsychofarmakológia, Základy Klinických Neurovied; 1. Vyd.; Vydavateľstvo F: Trenčín, Slovakia, 2012; ISBN 978-80-88952-70-1.
- Vita, A.; Minelli, A.; Barlati, S.; Deste, G.; Giacopuzzi, E.; Valsecchi, P.; Turrina, C.; Gennarelli, M. Treatment-Resistant Schizophrenia: Genetic and Neuroimaging Correlates. Front. Pharmacol. 2019, 10, 402.
- Fusar-Poli, P.; Papanastasiou, E.; Stahl, D.; Rocchetti, M.; Carpenter, W.; Shergill, S.; McGuire, P. Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophr. Bull. 2015, 41, 892–899.
- Do, K.Q. Bridging the gaps towards precision psychiatry: Mechanistic biomarkers for early detection and intervention. Psychiatry Res. 2023, 321, 115064.
- Peiyan, N. Mitochondrial dysfunction in psychiatric disorders. Schizophr. Res. 2022, in press.
- Fišar, Z. Biological hypotheses, risk factors, and biomarkers of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 120, 110626.
- Zilocchi, M.; Broderick, K.; Phanse, S.; Aly, K.A.; Babu, M. Mitochondria under the spotlight: On the implications of mitochondrial dysfunction and its connectivity to neuropsychiatric disorders. Comput. Struct. Biotechnol. J. 2020, 14, 2535–2546.
- Ciobica, A.; Padurariu, M.; Dobrin, M.; Stefanescu, C.; Dobrin, R. Oxidative stress in schizophrenia—Focusing on the main markers. Psychiatr. Danub. 2011, 23, 237–245.
- Pruett, B.S.; Meador-Woodruff, J.H. Evidence for altered energy metabolism, increased lactate, and decreased pH in schizophrenia brain: A focused review and meta-analysis of human postmortem and magnetic resonance spectroscopy studies. Schizophr. Res. 2020, 223, 29–42.
- Ľuptak, M.; Fišar, Z.; Hroudova, J. Effect of novel antipsychotics on energy metabolism—In vitro study in pig brain mitochondria. Mol. Neurobiol. 2021, 58, 5548–5563.
- Bar-Yosef, T.; Hussein, W.; Yitzhaki, O.; Damri, O.; Givon, L.; Marom, C.; Gurman, V.; Levine, J.; Bersudsky, Y.; Agam, G.; et al. Mitochondrial function parameters as a tool for tailored drug treatment of an individual with psychosis: A proof of concept study. Sci. Rep. 2020, 10, 12258.
- Manzoor, S.; Khan, A.; Hasan, B.; Mushtag, S.; Ahmed, N. Expression Analysis of 4-hydroxynonenal Modified Proteins in Schizophrenia Brain; Relevance to Involvement in Redox Dysregulation. Curr. Proteom. 2022, 19, 102–113.
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630.
- Pecorelli, A.; Ciccoli, L.; Signorini, C.; Leoncini, S.; Giardini, A.; D’Esposito, M.; Filosa, S.; Hayek, J.; De Felice, C.; Valacchi, G. Increased levels of 4HNE-protein plasma adducts in Rett syndrome. Clin. Biochem. 2011, 44, 368–371.
- Pecorelli, A.; Leoncini, S.; De Felice, C.; Signorini, C.; Cerrone, C.; Valacchi, G.; Ciccoli, L.; Hayek, J. Non-protein-bound iron and 4-hydroxynonenal protein adducts in classic autism. Brain Dev. 2013, 35, 146–154.
- Pecorelli, A.; Leoncini, C.; Ciccoli, S.; Valacchi, G.; Signorini, C.; De Felice, C.; Hayek, C. 4HNE Protein Adducts in Autistic Spectrum Disorders: Rett Syndrome and Autism. In Comprehensive Guide to Autism; Springer: New York, NY, USA, 2014; pp. 2667–2688.
- Fizikova, I.; Racay, P. Oxidative modifications of plasma proteins and decreased leukocyte mitochondrial DNA of schizophrenia patients. Act. Nerv. Super. Rediviva 2022, 64, 25–32.
- Moller, I.M.; Rogowska-wrzesinska, R.S.; Rao, R.S. Protein carbonylation and metal-catalysed Protein oxidation in a cellular perspective. J. Proteom. 2011, 74, 2228–2242.
- Tatarkova, Z.; Kovalska, M.; Timkova, V.; Racay, P.; Lehotsky, J.; Kaplan, P. The Effect of Aging on Mitochondrial Complex I and the Extent of Oxidative Stress in the Rat Brain Cortex. Neurochem. Res. 2016, 41, 2160–2172.
- Siems, W.; Grune, T. Intracellular metabolism of 4-hydroxynonenal. Mol. Asp. Med. 2003, 24, 167–175.
- Castro, J.P.; Jung, T.; Grune, T.; Siems, W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free. Radic. Biol. Med. 2017, 111, 309–315.
- Feigenson, K.A.; Kusnecov, A.W.; Silverstein, S.M. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci. Biobehav. Rev. 2014, 38, 72–93.
- Fragaus, D.; Diaz-Caneja, C.M.; Ayora, M.; Hernández-Álvarez, F.; Rodríguez-Quiroga, A.; Recio, S.; Leza, J.C.; Arango, C. Oxidative Stress and Inflammation in First-Episode Psychosis: A Systematic Review and Meta-analysis. Schizophr. Bull. 2019, 18, 742–751.
- Kaplán, P.; Tatarková, Z.; Račay, P.; Doborota, D.; Lehotsky, J.; Pavlikova, M. Oxidative modifications of cardiac mitochondria and inhibition of cytochrome c oxidase activity by 4-hydroxynonenal. Redox Rep. 2007, 12, 211–218.
More
Information
Subjects:
Others; Psychology, Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
923
Revisions:
2 times
(View History)
Update Date:
08 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




