Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
|
Psychology, Experimental
The significant complexity of the brain can lead to the development of serious neuropsychiatric disorders, including schizophrenia. A number of mechanisms are involved in the etiopathogenesis of schizophrenia, pointing to its complexity and opening a new perspective on studying this disorder. HNE (4-hydroxynonenal) is an essential molecule that significantly impacts cell function and survival. For this reason, it is assumed that HNE might play a role in the pathophysiology of schizophrenia and other disorders.
- schizophrenia
- mitochondria
- oxidative stress
1. Introduction
Schizophrenia is a specifically human disease that affects approximately 0.5–1% of the population. Schizophrenia has been defined as a neuropsychiatric disease that causes changes in the process of thoughts, perceptions, and emotions, usually leading to mental deterioration and affective blunting [1,2].
Currently, commonly used clinical treatments can improve some symptoms but not others; for example, negative symptoms such as social withdrawal and alogia in schizophrenia are not sufficiently treated with antipsychotic medications. In addition, it is currently not possible to predict which patients will or will not respond to antipsychotic medications. Therefore, developing novel, more effective therapeutic strategies for schizophrenia is imperative [2,3,4,5].
Early detection and intervention in schizophrenia requires mechanism-based biomarkers that capture circuitry dysfunction and can allow optimised patient stratification, disease monitoring, treatment, and improved prognosis [3,4,6].
The hypothesis of mitochondrial dysfunction as a factor in the etiopathogenesis of schizophrenia is currently gaining more attention. This is the basis of so-called mitochondrial psychiatry. The basis of this hypothesis is that mitochondria are important not only as the main energy producer but also as a significant influencer of many important life processes [1,5,6,7].
Schizophrenia is an often debilitating illness that typically manifests in late adolescence and frequently has a progressive course associated with significant disability, economic burden, and early mortality. Therefore, an improved understanding of the molecular pathophysiology underlying schizophrenia is needed in order to better target future therapies.
Multiple converging lines of evidence from ex vivo, postmortem, imaging, and animal model studies indicate that proper mitochondrial function is important in the pathogenesis of schizophrenia. The mitochondrial dysfunction observed in schizophrenia is closely related to oxidative stress with the formation of free radicals (Figure 1). These changes lead to oxidative damage and the alteration of several key enzymes and antioxidant systems, as well as energy metabolism [2,6,11,38,55,56].
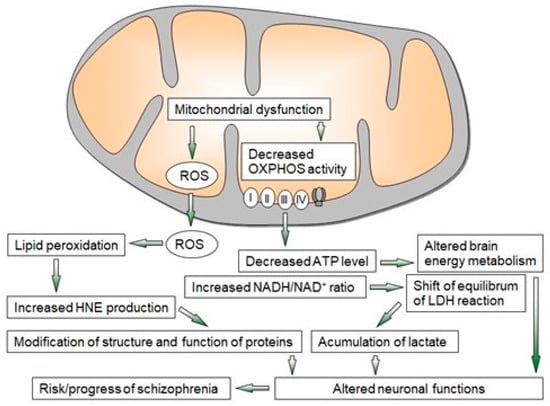
Figure 1. Overview of mechanisms of mitochondrial dysfunction leading to neuroprogressive changes in schizophrenia. LDH—lactate dehydrogenase, OXPHOS—oxidative phosphorylation, ROS—reactive oxygen species, I–IV—enzyme complexes of OXPHOS.
In vitro measurement data suggest that the mitochondrial effects of current antipsychotics are likely related to their adverse impacts and are due to drug-induced decreased ATP production and increased ROS production; however, based on the mitochondrial dysfunctions observed in neurodegenerative diseases, bipolar disorder, and schizophrenia, new psychotropic drugs are aimed at selected mitochondrial targets [5,6].
2. The Link between HNE and Schizophrenia
HNE is an essential molecule that significantly impacts cell function and survival. For this reason, it is assumed that HNE might play a role in the pathophysiology of schizophrenia and other disorders (e.g., Rett syndrome and autism spectrum disorder), although its specific influence is not yet fully understood [24,26,27,28,29]. Regarding schizophrenia, the results of a recent study showed increased levels of HNE conjugates with proteins in the brains of patients with schizophrenia compared to the control group. Interestingly, elevated levels of HNE protein conjugates have been documented in the hippocampus compared to the cortex of schizophrenia patients [24].
In one recent study [21], Western blot analysis showed HNE-modified plasma proteins with a discrete molecular mass in the range of 37–50 kDa in the plasma of schizophrenia patients, while HNE-modified plasma proteins with a discrete molecular mass of 100–200 kDa were documented in the control group.
The altered profile of plasma proteins modified by HNE may explain the statistically insignificant results obtained by fluorescence measurements [21].
Interestingly, in one study, increased levels of HNE-modified proteins with a molecular weight of around 50 kDa were observed in the plasma of patients suffering from Rett syndrome [27]. That study also documented increased levels of HNE-modified proteins with a molecular weight of 150–200 kDa. The massive modification of HNE plasma proteins in the plasma of patients with classical autistic disorder was also reported [28,29].
In another study, an increase in discrete proteins modified by HNE was observed, mainly in mitochondria isolated from rat brains, and was dependent on age [29].
It is also known that free HNE can react with proteins, thereby changing their conformation and function, as was also shown in the case of cytochrome c oxidase, the terminal complex of the mitochondrial respiratory chain. As a result of the action of HNE, various intracellular enzymes can also be inhibited (e.g., Na/K-ATPase, poly-ADP ribose polymerase, complex I of the respiratory chain, protein kinase C, NADPH oxidase, and proteasome). The inhibitory values reported in the literature indicate a wide range of enzyme sensitivity to HNE. Although HNE is primarily generated in membranes (or other hydrophobic compartments of cells), the partition coefficient of HNE allows diffusion into the cytosol or the extracellular space [23,24].
Nevertheless, it can be assumed that the HNE concentration is much higher in cell membranes than in the hydrophilic compartments of cells. HNE is considered a bifunctional aldehyde and can easily form cross-links within or between proteins. HNE is formed under physiological conditions and in pathophysiological situations as a product of ongoing oxidative stress; therefore, it is likely that many proteins are directly damaged by oxidants. The groups of HNE-peptide and HNE-protein conjugates are an integral part of the harmful effects of HNE on cellular functions [11,23,30,31].
In contrast, other pathways of HNE degradation contribute to HNE detoxification. These pathways include products of beta- and alpha-oxidation and the tricarboxylic acid cycle, such as acetyl-CoA, citrate, aconitate, malate, fumarate, succinate, carbon dioxide, and water. The most critical HNE-degrading enzymes are glutathione-S transferase, alcohol dehydrogenase, and aldehyde dehydrogenase. Among the products generated by these enzymatic substances, referred to as primary intermediates of HNE, are HNE-GSH, hydroxynonenic acid (HNA), and 1,4-dihydroxynonene (DH) [11,30,31].
The quick elimination of HNE from affected cells is a significant part of the secondary antioxidant defence mechanism of cells. Still, the processes have not yet been studied in detail with regard to schizophrenia. In addition, the increased formation of protein conjugates with HNE may also be a consequence of the activation of the inflammatory response, which is often involved in the etiopathogenesis of schizophrenia [11,30,32,33,34].
As mentioned, one of the specific targets of HNE may be cytochrome c oxidase, the terminal complex of the mitochondrial respiratory chain [32]. Modifying cytochrome c oxidase via HNE inhibits its activity, which could lead to mitochondrial dysfunction and lactate overproduction. Changes in lactate levels may be evidence of impaired energy metabolism in schizophrenia [34,35].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24097991
This entry is offline, you can click here to edit this entry!
