
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marta Slavkova | -- | 1731 | 2023-05-05 10:24:57 | | | |
| 2 | Catherine Yang | -5 word(s) | 1726 | 2023-05-05 10:59:57 | | |
Video Upload Options
Chemotherapy of skin cancer can be either oral, parenteral, or topical. In the first case, only a limited amount of the drug reaches the target site, while the rest reaches other organs, tissues, and cells, and can cause harmful side effects. The case is quite similar in parenteral application together with its invasiveness. Therefore, the local application on different skin cancer forms can gain in therapeutic efficacy and safety. The various types of gel formulations applied topically in the treatment of skin cancer was discussed. The most common gelling agents, their concentration and mechanism of action is also provided. The possibility to combine nanotechnology for improvement of drug loading and delivery by incorporation of nanoparticles into hydrogels is also evaluated. The main methods for gel characterization in the light of topical delivery of chemotherapeutics were summarized.
1. Introduction
2. Skin Structure
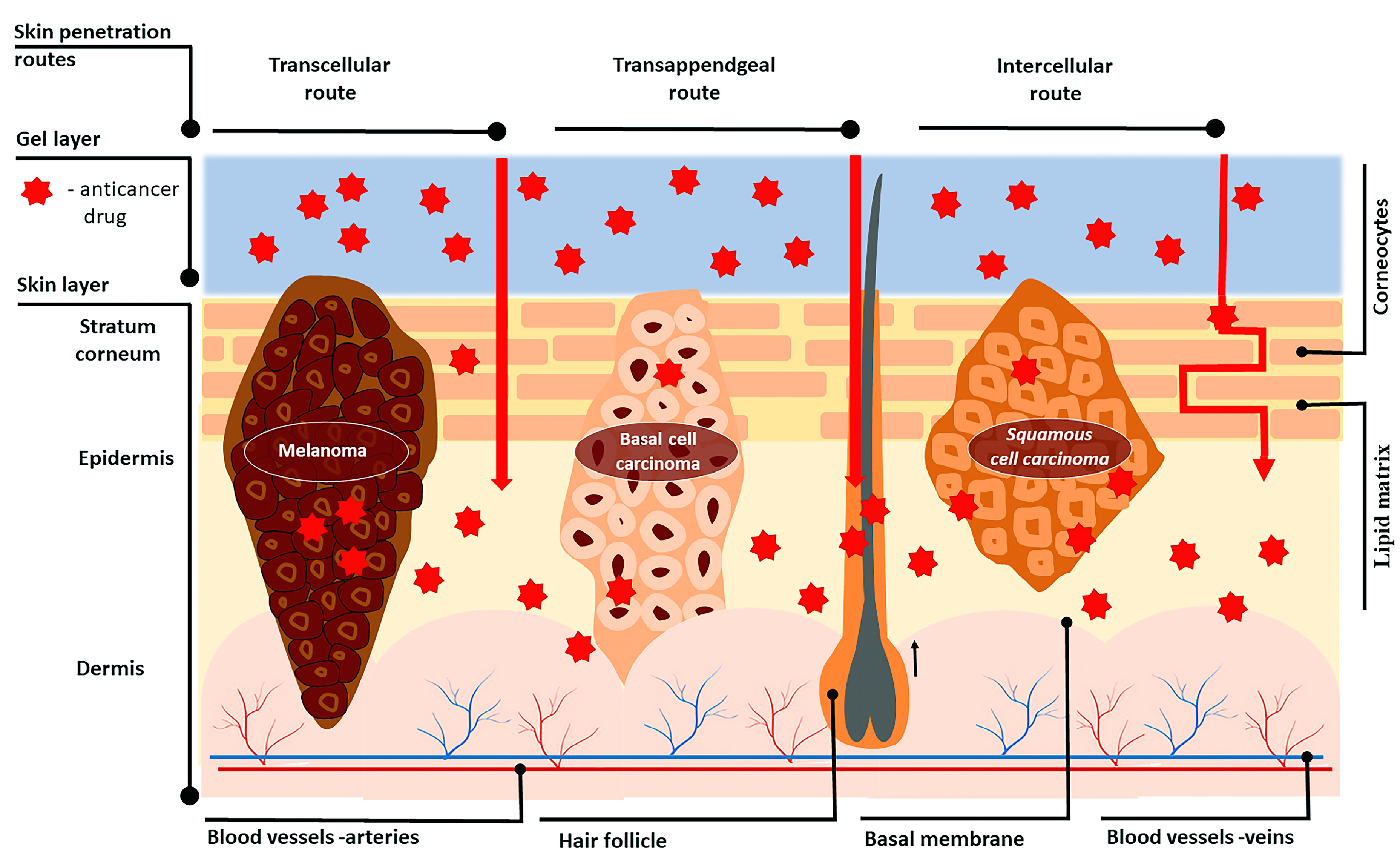
3. Skin Penetration Routes and Factors Influencing Skin Penetration
4. Opportunities for Increasing Skin Penetration
References
- Linares, M.A.; Zakaria, A.; Nizran, P. Skin Cancer. Prim. Care Clin. Off. Pract. 2015, 42, 645–659.
- Craythorne, E.; Al-Niami, F. Skin Cancer. Medicine 2017, 45, 431–434.
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; del Marmol, V.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and Treatment of Basal Cell Carcinoma: European Consensus–Based Interdisciplinary Guidelines. Eur. J. Cancer 2019, 118, 10–34.
- Nawaz, A.; Ullah, S.; Alnuwaiser, M.A.; Rehman, F.U.; Selim, S.; Al Jaouni, S.K.; Farid, A. Formulation and Evaluation of Chitosan-Gelatin Thermosensitive Hydrogels Containing 5FU-Alginate Nanoparticles for Skin Delivery. Gels 2022, 8, 537.
- Barrera, M.V.; Herrera, E. Topical Chemotherapy for Actinic Keratosis and Nonmelanoma Skin Cancer: Current Options and Future Perspectives. Actas Dermo-Sifiliográficas Engl. Ed. 2007, 98, 556–562.
- Safwat, M.A.; Soliman, G.M.; Sayed, D.; Attia, M.A. Fluorouracil-Loaded Gold Nanoparticles for the Treatment of Skin Cancer: Development, in Vitro Characterization, and in Vivo Evaluation in a Mouse Skin Cancer Xenograft Model. Mol. Pharm. 2018, 15, 2194–2205.
- Ghezzi, M.; Pescina, S.; Delledonne, A.; Ferraboschi, I.; Sissa, C.; Terenziani, F.; De Freitas Rosa Remiro, P.; Santi, P.; Nicoli, S. Improvement of Imiquimod Solubilization and Skin Retention via Tpgs Micelles: Exploiting the Co-Solubilizing Effect of Oleic Acid. Pharmaceutics 2021, 13, 1476.
- Lapteva, M.; Mignot, M.; Mondon, K.; Möller, M.; Gurny, R.; Kalia, Y.N. Self-Assembled MPEG-HexPLA Polymeric Nanocarriers for the Targeted Cutaneous Delivery of Imiquimod. Eur. J. Pharm. Biopharm. 2019, 142, 553–562.
- Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, S.M.; Carvalho, I.C.; Chagas, P.; de Oliveira, L.C.A.; Mansur, H.S. Bioengineered Carboxymethyl Cellulose-Doxorubicin Prodrug Hydrogels for Topical Chemotherapy of Melanoma Skin Cancer. Carbohydr. Polym. 2018, 195, 401–412.
- Gamal, F.A.; Sayed, O.M.; El-Ela, F.I.A.; Kharshoum, R.M.; Salem, H.F. Treatment of Basal Cell Carcinoma Via Binary Ethosomes of Vismodegib: In Vitro and In Vivo Studies. AAPS PharmSciTech 2020, 21, 51.
- Dummer, R.; Ascierto, P.A.; Basset-Seguin, N.; Dréno, B.; Garbe, C.; Gutzmer, R.; Hauschild, A.; Krattinger, R.; Lear, J.T.; Malvehy, J.; et al. Sonidegib and Vismodegib in the Treatment of Patients with Locally Advanced Basal Cell Carcinoma: A Joint Expert Opinion. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1944–1956.
- Mousa, I.A.; Hammady, T.M.; Gad, S.; Zaitone, S.A.; El-Sherbiny, M.; Sayed, O.M. Formulation and Characterization of Metformin-Loaded Ethosomes for Topical Application to Experimentally Induced Skin Cancer in Mice. Pharmaceuticals 2022, 15, 657.
- Kollipara, R.K.; Tallapaneni, V.; Sanapalli, B.K.R.; Kumar, G.V.; Karri, V.V.S.R. Curcumin Loaded Ethosomal Vesicular Drug Delivery System for the Treatment of Melanoma Skin Cancer. Res. J. Pharm. Technol. 2019, 12, 1783–1792.
- Priya, P.; Raj, R.M.; Vasanthakumar, V.; Raj, V. Curcumin-Loaded Layer-by-Layer Folic Acid and Casein Coated Carboxymethyl Cellulose/Casein Nanogels for Treatment of Skin Cancer. Arab. J. Chem. 2020, 13, 694–708.
- Alhakamy, N.A.; Aldawsari, H.M.; Ali, J.; Gupta, D.K.; Warsi, M.H.; Bilgrami, A.L.; Asfour, H.Z.; Noor, A.O.; Md, S. Brucine-Loaded Transliposomes Nanogel for Topical Delivery in Skin Cancer: Statistical Optimization, in Vitro and Dermatokinetic Evaluation. 3 Biotech 2021, 11, 288.
- Iqbal, B.; Ali, J.; Ganguli, M.; Mishra, S.; Baboota, S. Silymarin-Loaded Nanostructured Lipid Carrier Gel for the Treatment of Skin Cancer. Nanomedicine 2019, 14, 1077–1093.
- Nagaraja, S.; Basavarajappa, G.M.; Attimarad, M.; Pund, S. Topical Nanoemulgel for the Treatment of Skin Cancer: Proof-of-Technology. Pharmaceutics 2021, 13, 902.
- Kaplan, A.; Cetin, M.; Orgul, D.; Taghizadehghalehjoughi, A.; Hacimuftuoglu, A.; Hekimoglu, S. Formulation and in Vitro Evaluation of Topical Nanoemulsion and Nanoemulsion-Based Gels Containing Daidzein. J. Drug Deliv. Sci. Technol. 2019, 52, 189–203.
- Taylor, K.M.G. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2013.
- Shende, P.; Vaidya, J.; Gaud, R.S. Pharmacotherapeutic Approaches for Transportation of Anticancer Agents via Skin. Artif. Cells Nanomed. Biotechnol. 2018, 46, S423–S433.
- Depieri, L.V.; Praça, F.S.G.; Campos, P.M.; Bentley, M.V.L.B. Advances in the Bioanalytical Study of Drug Delivery across the Skin. Ther. Deliv. 2015, 6, 571–594.
- Khan, N.H.; Mir, M.; Qian, L.; Baloch, M.; Khan, M.F.A.; Rehman, A.; Ngowi, E.E.; Wu, D.-D.; Ji, X.-Y. Skin Cancer Biology and Barriers to Treatment: Recent Applications of Polymeric Micro/Nanostructures. J. Adv. Res. 2022, 36, 223–247.
- Bolzinger, M.-A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of Drugs through Skin, a Complex Rate-Controlling Membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165.
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current Status and Future Potential of Transdermal Drug Delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124.
- Matsui, T.; Amagai, M. Dissecting the Formation, Structure and Barrier Function of the Stratum Corneum. Int. Immunol. 2015, 27, 269–280.
- Sahu, P.; Kashaw, S.K.; Jain, S.; Sau, S.; Iyer, A.K. Assessment of Penetration Potential of PH Responsive Double Walled Biodegradable Nanogels Coated with Eucalyptus Oil for the Controlled Delivery of 5-Fluorouracil: In Vitro and Ex Vivo Studies. J. Control. Release 2017, 253, 122–136.
- Georgescu, S.R.; Tampa, M.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Caruntu, A.; Lupu, M.; Matei, C.; Constantin, C.; Neagu, M. Tumour Microenvironment in Skin Carcinogenesis. Adv. Exp. Med. Biol. 2020, 1226, 123–142.
- Barua, S.; Mitragotri, S. Challenges Associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243.
- McGrath, J.A.; Eady, R.A.J.; Pope, F.M. Anatomy and Organization of Human Skin. In Rook’s Textbook of Dermatology; Wiley: Hoboken, NJ, USA, 2004; Volume 1, Chapter 3; pp. 1–84.
- Taveira, S.F.; Lopez, R.F.V.; Taveira, S.F.; Lopez, R.F.V. Topical Administration of Anticancer Drugs for Skin Cancer Treatment; IntechOpen: London, UK, 2011; ISBN 978-953-307-722-2.
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Iyer, A.K. Discovering PH Triggered Charge Rebound Surface Modulated Topical Nanotherapy against Aggressive Skin Papilloma. Mater. Sci. Eng. C 2020, 107, 110263.
- Amasya, G.; Aksu, B.; Badilli, U.; Onay-Besikci, A.; Tarimci, N. QbD Guided Early Pharmaceutical Development Study: Production of Lipid Nanoparticles by High Pressure Homogenization for Skin Cancer Treatment. Int. J. Pharm. 2019, 563, 110–121.
- Williams, A. Transdermal and Topical Drug Delivery from Theory to Clinical Practice; Pharmaceutical Press: London, UK, 2003; ISBN 978-0-85369-489-2.
- National Research Council (US) Commission on Engineering and Technical Systems; Wartell, M.A.; Kleinman, M.T.; Huey, B.M. Strategies to Protect the Health of Deployed U.S. Forces: Force Protection and Decontamination. Washington (DC): National Academies Press (US); 1999. Appendix E, Percutaneous Absorption. Available online: https://www.ncbi.nlm.nih.gov/books/NBK225150/ (accessed on 1 March 2023).
- Touitou, E.; Barry, B.W. (Eds.) Enhancement in Drug Delivery; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-0-429-12231-6.
- Taylor, K.M.G.; Wenande, E.; Olesen, U.H.; Nielsen, M.M.B.; Janfelt, C.; Hansen, S.H.; Anderson, R.R.; Haedersdal, M. Fractional Laser-Assisted Topical Delivery Leads to Enhanced, Accelerated and Deeper Cutaneous 5-Fluorouracil Uptake: Expert Opinion on Drug Delivery: Volume 14, No 3. Available online: https://www.tandfonline.com/doi/abs/10.1080/17425247.2017.1260119 (accessed on 3 March 2023).
- De Oliveira, B.E.; Amorim, O.H.J.; Lima, L.L.; Rezende, R.A.; Mestnik, N.C.; Bagatin, E.; Leonardi, G.R. 5-Fluorouracil, Innovative Drug Delivery Systems to Enhance Bioavailability for Topical Use. J. Drug Deliv. Sci. Technol. 2021, 61, 102155.
- Diasio, R.B.; Harris, B.E. Clinical Pharmacology of 5-Fluorouracil. Clin. Pharmacokinet. 1989, 16, 215–237.
- Love, W.E.; Bernhard, J.D.; Bordeaux, J.S. Topical Imiquimod or Fluorouracil Therapy for Basal and Squamous Cell Carcinoma: A Systematic Review. Arch. Dermatol. 2009, 145, 1431–1438.
- Telò, I.; Pescina, S.; Padula, C.; Santi, P.; Nicoli, S. Mechanisms of Imiquimod Skin Penetration. Int. J. Pharm. 2016, 511, 516–523.
- Al-Mayahy, M.H.; Sabri, A.H.; Rutland, C.S.; Holmes, A.; McKenna, J.; Marlow, M.; Scurr, D.J. Insight into Imiquimod Skin Permeation and Increased Delivery Using Microneedle Pre-Treatment. Eur. J. Pharm. Biopharm. 2019, 139, 33–43.
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The Challenge of Drug Resistance in Cancer Treatment: A Current Overview. Clin. Exp. Metastasis 2018, 35, 309–318.
- Gottesman, M.M. Mechanisms of Cancer Drug Resistance. Annu. Rev. Med. 2002, 53, 615–627.
- Kalal, B.S.; Upadhya, D.; Pai, V.R. Chemotherapy Resistance Mechanisms in Advanced Skin Cancer. Oncol. Rev. 2017, 11, 326.
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting Multidrug Resistance in Cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234.
- Ichihashi, N.; Kitajima, Y. Chemotherapy Induces or Increases Expression of Multidrug Resistance-Associated Protein in Malignant Melanoma Cells. Br. J. Dermatol. 2001, 144, 745–750.
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339.
- Grottke, C.; Mantwill, K.; Dietel, M.; Schadendorf, D.; Lage, H. Identification of Differentially Expressed Genes in Human Melanoma Cells with Acquired Resistance to Various Antineoplastic Drugs. Int. J. Cancer 2000, 88, 535–546.
- Marangolo, M.; Bengala, C.; Conte, P.F.; Danova, M.; Pronzato, P.; Rosti, G.; Sagrada, P. Dose and Outcome: The Hurdle of Neutropenia (Review). Oncol. Rep. 2006, 16, 233–248.
- Williams, A.C.; Barry, B.W. Penetration Enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618.
- Vishnubhakthula, S.; Elupula, R.; Durán-Lara, E.F. Recent Advances in Hydrogel-Based Drug Delivery for Melanoma Cancer Therapy: A Mini Review. J. Drug Deliv. 2017, 2017, 7275985.
- Tsou, Y.-H.; Khoneisser, J.; Huang, P.-C.; Xu, X. Hydrogel as a Bioactive Material to Regulate Stem Cell Fate. Bioact. Mater. 2016, 1, 39–55.
- Ma, J.; Wang, Y.; Lu, R. Mechanism and Application of Chitosan and Its Derivatives in Promoting Permeation in Transdermal Drug Delivery Systems: A Review. Pharmaceuticals 2022, 15, 459.
- Collaud, S.; Peng, Q.; Gurny, R.; Lange, N. Thermosetting Gel for the Delivery of 5-Aminolevulinic Acid Esters to the Cervix. J. Pharm. Sci. 2008, 97, 2680–2690.
- Sepantafar, M.; Maheronnaghsh, R.; Mohammadi, H.; Radmanesh, F.; Hasani-Sadrabadi, M.M.; Ebrahimi, M.; Baharvand, H. Engineered Hydrogels in Cancer Therapy and Diagnosis. Trends Biotechnol. 2017, 35, 1074–1087.
- Gerecke, C.; Edlich, A.; Giulbudagian, M.; Schumacher, F.; Zhang, N.; Said, A.; Yealland, G.; Lohan, S.B.; Neumann, F.; Meinke, M.C.; et al. Biocompatibility and Characterization of Polyglycerol-Based Thermoresponsive Nanogels Designed as Novel Drug-Delivery Systems and Their Intracellular Localization in Keratinocytes. Nanotoxicology 2017, 11, 267–277.




