Chemotherapy of skin cancer can be either oral, parenteral, or topical. In the first case, only a limited amount of the drug reaches the target site, while the rest reaches other organs, tissues, and cells, and can cause harmful side effects. The case is quite similar in parenteral application together with its invasiveness. Therefore, the local application on different skin cancer forms can gain in therapeutic efficacy and safety. The current article discusses the various types of gel formulations applied topically in the treatment of skin cancer was discussed. The most common gelling agents, their concentration and mechanism of action is also provided. The possibility to combine nanotechnology for improvement of drug loading and delivery by incorporation of nanoparticles into hydrogels is also evaluated. The maireview summarizes as well the main methods for gel characterization in the light of topical delivery of chemotherapeutics were summarized.
1. Introduction
Skin is the largest organ in the human body, performing various functions ranging from protection to metabolism. The abnormal growth of the skin cells is referred to as skin cancer and this is the most common cancer type worldwide
[1]. There are various types of skin cancer, including benign and malignant variants such as melanoma, squamous cell carcinoma and basal cell carcinoma
[2]. Even though skin cancer is lethal in only 2% of cases in general, the malignant forms result in death in more than 80% of cases if not caught early
[1]. The management of skin cancer includes surgical excision, radiotherapy, topical drug delivery, and oral therapy
[2]. Although surgical treatment is considered the first line approach, topical therapy also has its role
[3]. The most commonly prescribed medications are 5-fluorouracil
[4][5][6][4,5,6] and imiquimod
[5][7][8][5,7,8]. Other active pharmaceutical ingredients (APIs) are either repurposed or subjected to investigation and evaluation because of their topical chemotherapeutic potential in various forms of skin cancer. Some examples are doxorubicin
[9], vismodegib
[10], sonidegib
[11], and metformin
[12]. Substances of natural origin (curcumin
[13][14][13,14], brucine
[15], silymarin
[16], chrysin
[17], daidzein
[18], and others) are also considered suitable in the topical treatment of skin cancer.
2. Skin Structure
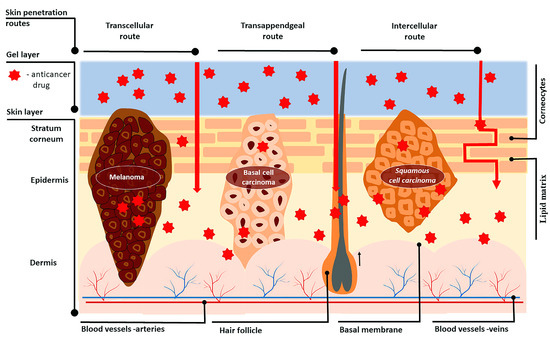
Skin is a complex organ with the main function to “maintain the insides in and outside out”, acting as a barrier
[19][31]. It consists of three layers—the epidermis, dermis, and hypo-dermis—each having a different composition and properties
[20][32] (
Figure 12). The inner hypodermis consists of adipose tissue and rarely plays an important role for drug delivery
[19][31]. The dermis (around 2–4 mm)
[21][33] is built up by a collagen and elastin network in a mucopolysaccharide gel which resembles a hydrogel structure. The vascularity of the dermis enables the transport of the most transdermally delivered drug molecules into the blood, maintaining a high concentration gradient. This layer also includes appendages—hair follicles, sebaceous and sweat glands—which have an impact only on the initial diffusion through the skin
[19][31]. The outermost skin layer—the epidermis—consists mainly of cells, called keratinocytes, which play a lead role in topical drug delivery
[22][34]. The epidermis has two avascular and hydrophobic sections, the viable epidermis and the stratum corneum (SC). The viable epidermis consists of four layers of keratinocytes at different stages of differentiation, melanocytes, Merkel cells, and Langerhans cells. The differentiation of the epidermis starts from inside to outside, and results in the formation of corneocytes—dead, anuclear, flattened, and keratin-rich cells
[21][33]. The corneocytes are surround by lipid matrix composed of triglycerides, cholesterol, free fatty acids and ceramides, and these structures act as the prime barrier to the entry of macro and micro anticancer molecules across the skin, limiting drug delivery
[23][24][25][35,36,37]. Normal melanin cells are characterized with physiological pH while tumor cells have a pH in the range 5–6.5
[26][38]. In the case of melanoma, the acidic pH is responsible for the invasion of surrounding tissues and the malignant cells have higher metastatic capacity
[27][39]
Figure 12.
Skin structure and routes of drug transport through cancerous skin after topical anticancer treatment.
Moreover, skin cancer studies revealed there are higher levels of keratin and lipids in cancer cells compared to healthy cells, resulting in a thicker SC layer and consequently a stronger barrier to drug entry, which makes it even more difficult for anticancer molecules to reach the tumor site
[22][28][34,40]. This is why transdermal drug dosage forms, employed against actinic keratosis (AK) and basal cell carcinoma (BCC), require proper design to reach the deeper epidermal layers
[20][29][30][32,41,42]. The optimal particle size needed to achieve transdermal delivery lies in the range 200–400 nm
[31][43]. Nanoparticles with sizes of about 300 nm can reach deeper skin layers through the transappendgeal route
[32][44].
3. Skin Penetration Routes and Factors Influencing Skin Penetration
The penetration of drugs through the skin can happen by three possible routes depending on the physicochemical properties of the active substance: intracellular (through the stratum corneum), intercellular (through the lipid matrix), and through the skin appendages (sweat glands or hair follicles)
[19][31] (
Figure 12). The transport via skin appendages (shunt route) is more suitable for hydrophilic molecules, but since the fractional area of appendages is relatively small, this shunt route is not as important for drug delivery
[33][45]. On the other hand, highly lipophilic drug molecules can easily pass through the skin intercellularly via the lipid matrix. The intercellular (transcellular) route is the most complicated one because the drug molecule should repeatedly pass through different media of the “brick wall”
[34][46]. First, the permeant should partition into keratin-filled corneocytes (hydrophilic environment), and after that it should diffuse through the corneocytes, followed by partitioning into the intercellular lipid matrix (lipophilic environment)
[35][47]. Since crossing the lipid bilayers is involved in both transcellular and intercellular routes, diffusion through these lipid media is highly important. Therefore, lipophilic drugs are preferred candidates for transdermal delivery
[19][31]. However, when passing through the stratum corneum, molecules reach the more hydrophilic lower epidermal layers (viable epidermis), and in the capillaries of the epidermal–dermal junction, they can be cleared, entering the systemic circulation
[29][41]. Thus, high lipophilicity hinders the clearance. Ideally, the permeant should possess moderate hydrophilic–lipophilic properties, expressed as a logarithm partition coefficient (log P
water/octanol) in the range 1–3
[20][33][32,45].
Unfortunately, not all anticancer medications possess this desired hydrophilic–lipophilic balance. For example, 5-fluorouracil, approved for actinic keratosis (AK) and superficial basal cell carcinoma (sBCC), is a highly hydrophilic molecule (log P: −0.89)
[36][48]. This hydrophilicity restricts penetration through the hydrophobic stratum corneum and is reflected in low treatment efficacy for deeper laying lesions
[36][48]. Furthermore, the insufficient skin penetration of 5-FU requires frequent and higher administration doses, which leads to side effects such as skin inflammation
[37][38][39][49,50,51]. The situation with imiquimod, used for the treatment of BCC, is similar. Due to its low water solubility, permeability within the hydrophilic dermis media is difficult. Moreover, there is an interaction between the amine groups of the drug molecule with the anionic components of the skin, limiting additional imiquimod permeation, and resulting in reduced therapeutic effect
[40][41][52,53].
Another factor that influences skin penetration is the molecular weight of a permeant as the transport of molecules via the skin happens by passive diffusion under a concentration gradient, following Fick’s law. According to the Stokes–Einstein equation, the diffusion coefficient of a molecule increases with the increase in its approximate radius. Therefore, a higher molecular weight is related to a higher approximate radius, so that the diffusion coefficient is generally smaller and thus the diffusion is hindered. For transdermal delivery, the drug’s molecular weight (MW) should be less than 500 Da
[19][20][31,32], making the penetration of anticancer drugs with higher molecular weight difficult.
Another problem arising in cancer therapy is multidrug resistance (MDR). The interaction between the drug and the tumor media is a complex phenomenon and cancers can exhibit significant resistance to various molecules. Multidrug resistance can be defined as the decrease in the efficacy and potency of a drug to produce a therapeutic effect and is a major problem that reduces the chemotherapies’ effectiveness
[22][42][34,54]. Drug resistance in skin cancers can be primary (intrinsic) or acquired. Primary resistance appears without prior exposure to anticancer agents, and thus the initial response to treatment is poor
[43][55]. Acquired resistance is developed during the application of the cytostatic drug, and it is associated with devastating results after initially good ones
[42][44][54,56].
Different mechanisms are associated with intrinsic resistance, such as changes in drug transport and efflux pump, alteration in enzyme activation and DNA repair, modulation of the apoptotic pathway, etc.
[44][45][46][47][56,57,58,59]. Acquired drug resistance is affected mainly by genetic or environmental factors that enable the progress of drug-resistant cancer cell lines or induce enzyme mutations
[42][43][48][54,55,60]. Therefore, understanding the modifications in molecular processes involved in drug resistance can trigger the development of new therapeutic strategies against skin cancers.
One of the potential factors leading to the sensitivity of drugs is the limited amount of drug reaching the tumor cells. This is why the determination of the “maximum tolerated dose” (MTD)—the highest single dose of an agent that does not cause significant or intolerable toxicity effects—is of great importance
[42][49][54,61]. Therefore, any methods for increasing the penetration of the anticancer drug through the skin, and thus increasing the amount reaching the tumor cells, can lead to improved therapeutic outcomes.
4. Opportunities for Increasing Skin Penetration
Considering all the issues discussed and the factors involved in managing the transport of anticancer drugs through the skin, the most important challenge of therapy is the improvement in the drug uptake, allowing the drug to pass through the deeper layers of skin, inside cancerous cells
[20][32].
One of the techniques to augment penetration is the utilization of “penetration enhancers”
[20][50][32,62], such as ethanol, Azones, fatty alcohols, glycols, and DMSO. These substances mainly disrupt the lipid bilayer packing, interacting with intercellular proteins
[19][31]. It is important to know that water possesses enhancer properties, as the drug diffusion is higher through hydrated skin; thus, occlusion is necessary for improved therapy
[50][62].
Many dosage forms are used for topical delivery of skin anticancer medication, such as powders, aerosols, emulsions, and creams. However, hydrogels have superior properties
[20][51][32,63]. Their structure allows them to be controlled at a molecular scale, which can be used to modify the properties such as degradation rate, long-time release, tunable pore size, and chemical and biological response to stimuli such as pH, enzymes, and temperature, in accordance to desired values
[52][64]. Moreover, hydrogel-based drug dosage forms exhibit improved chemotherapy outcomes by increasing drug half-life, enabling controlled drug release, and reducing nontargeted exposure
[53][54][55][65,66,67].
The combination of gel formation technology and nanotechnology leads to the creation of advanced drug delivery systems such as nanogels, liposomes, ethosomes, niosomes, and transferosomes, improving the skin penetration and bioavailability, and can be potentially used for topical skin cancer therapy
[28][37][56][40,49,68].