
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chaiyavat Chaiyasut | -- | 2579 | 2023-05-04 13:08:35 | | | |
| 2 | Conner Chen | -5 word(s) | 2574 | 2023-05-06 04:35:42 | | |
Video Upload Options
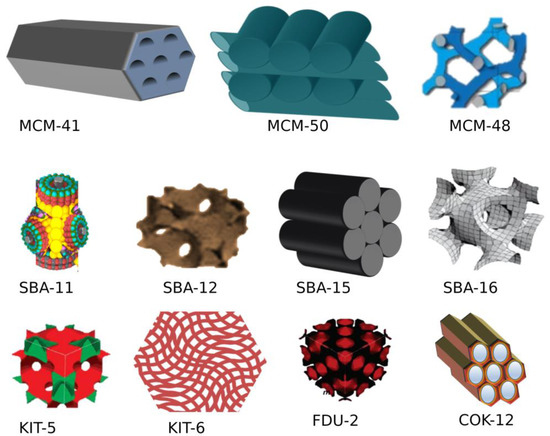
Mesoporous silica nanoparticles (MSNs) are a porous inorganic framework synthesized using the inorganic silica sources sodium silicates or silica tetraethyl orthosilicate as a precursor with quaternary ammonium salts as the surfactant. The size and porosity of MSNs are regulated by various factors such as surfactants, the source of silica, ion strength, aging duration, temperature, and pH. Highly ordered mesoporous silica came into the limelight after the fabrication of the silica-based material Mobile crystalline material 41 (MCM) by Kazuyuki Kuroda’s groups and scientists at Mobil Oil Corporation from aluminium silicate gel. MSNs have a unique porous solid framework with a large surface area facilitating functionalization with various functional groups for targeted drug delivery. MCM-41, MCM-48, and SBA-15 are the most common mesoporous silica materials with two-dimensional hexagonal and three-dimensional cubic structures possessing pore sizes of 2–10 nm.
1. Types of Mesoporous silica nanoparticles (MSNs)
2. Fabrication of MSNs
-
Cationic surfactants: Surfactants such as cetyl trimethyl ammonium chloride (CTAC), cetyl pyridinium chloride (CPyC), and cetyl tri methyl ammonium bromide (CTAB) with positively charged hydrophilic trimethyl ammonium/pyridinium groups as polar head and a sixteen-carbon hydrocarbon chain nonpolar tail act as a cationic surfactant.
-
Anionic surfactant: Low-cost, eco-friendly anionic surfactants such as sodium salts of alkyl carboxylic acids, phosphoric acid, and sulfonic acid with a negatively charged polar head and hydrocarbon nonpolar tail are widely used as an anionic surfactant.
-
Non-ionic surfactants: Non-ionic triblock copolymers such as alkyl poly (ethylene oxide) (PEO) oligomeric surfactants, poly (alkylene oxide) block copolymers, Triton X-100, polysorbate, and pluronic F 127 with a non-dissociable hydrophilic head which cannot ionize in aqueous solution act as a non-ionic surfactant.
-
Amphoteric/zwitterionic surfactants: Includes surfactants with a net neutral charge such as sodium dodecyl benzene sulfonate (SDBS), sodium dodecyl sulphate (SDS), phospholipids, betaines/sulfobetaine, amino acids, propyl ortho-silicate (TPOS), trimethoxy silane (TMS) and sodium metasilicate (Na2SiO3).
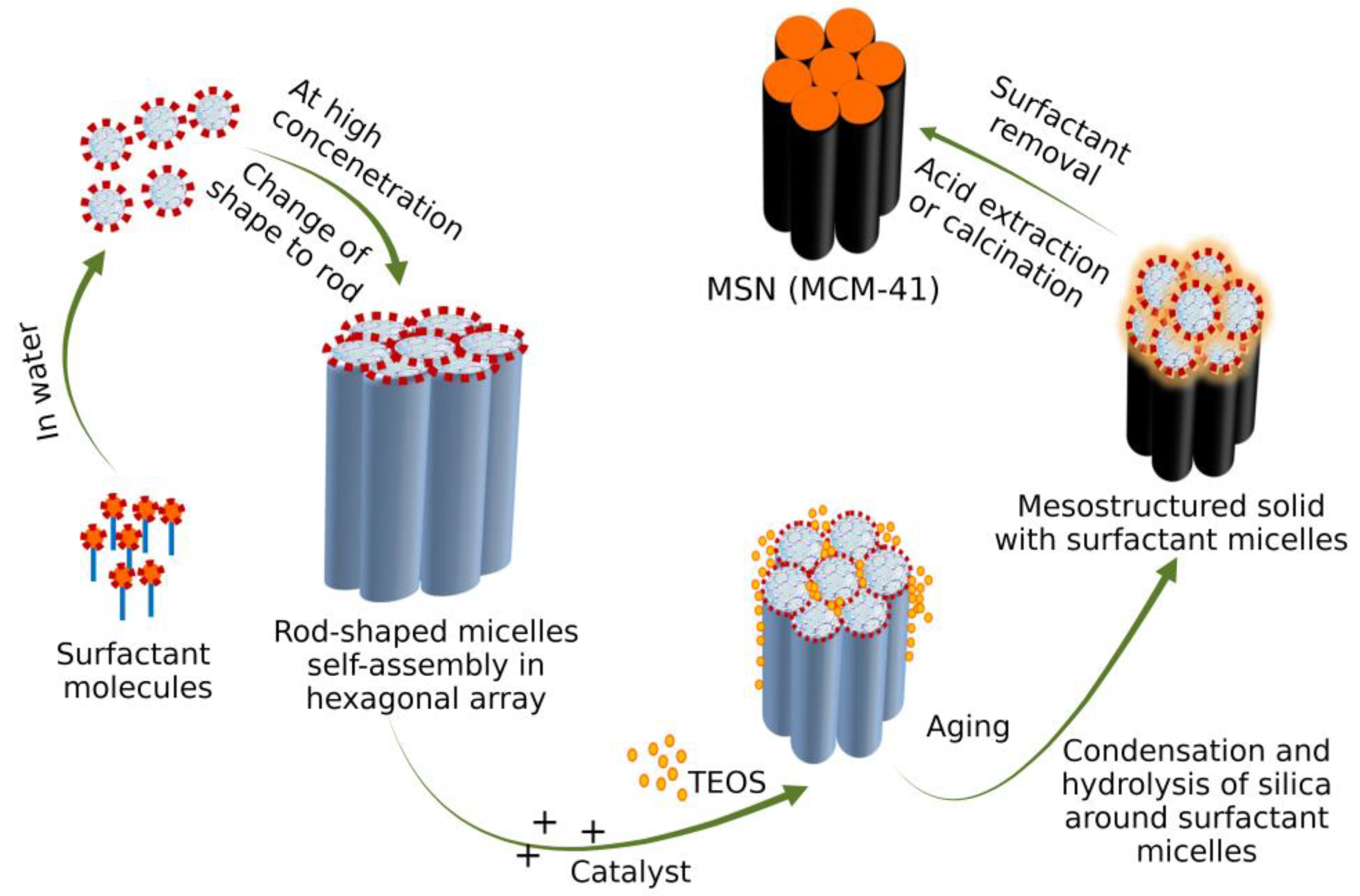
3. Mechanism of Formation of MSN
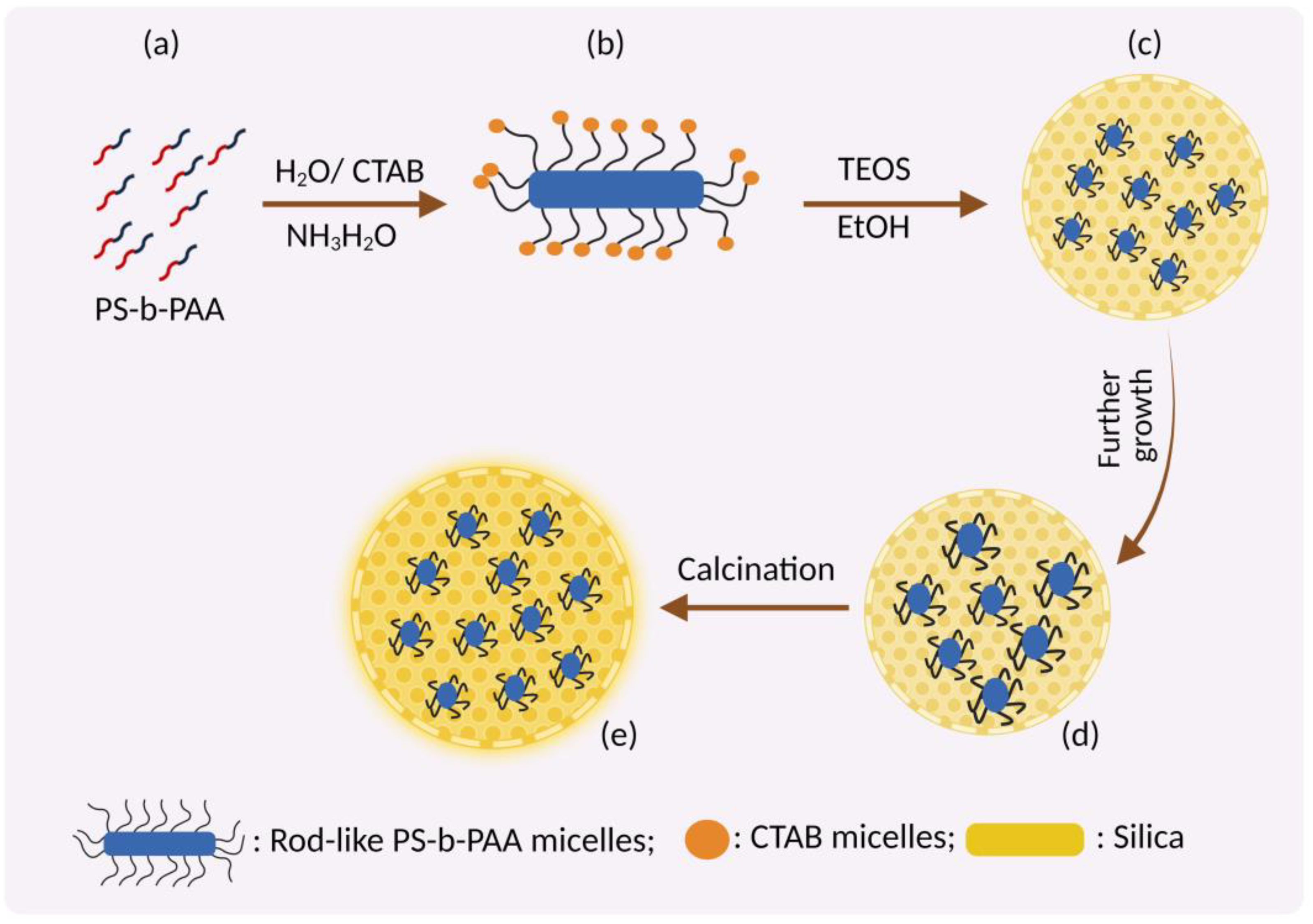
3.1. Swelling Shrinking Mechanism
3.2. Sol-Gel Method
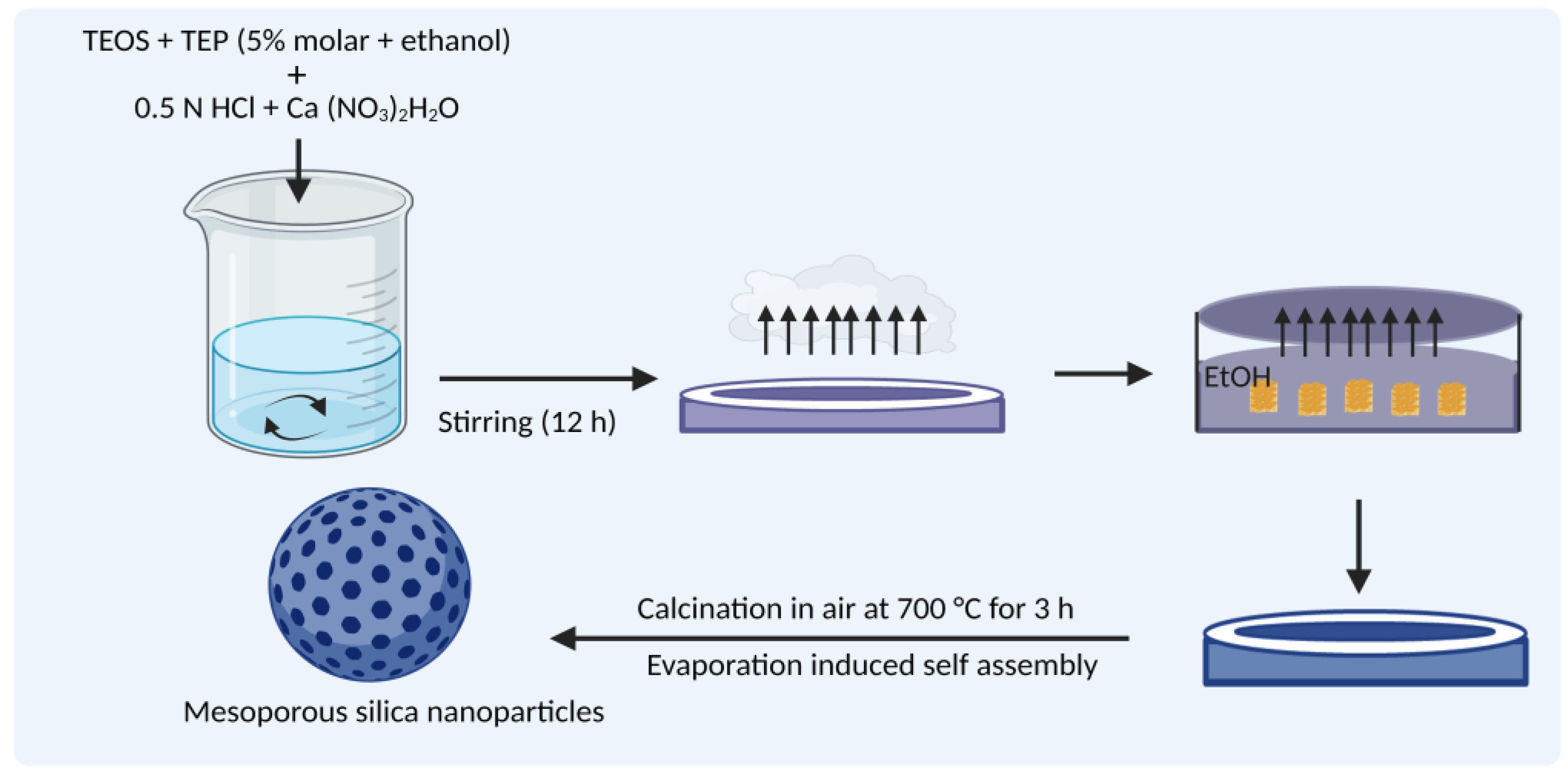
3.3. Evaporation-Induced Self-Assembly (EISA)
3.4. Removal of Surfactant after Synthesis
- For biomedical applications, the cytotoxic nature of surfactants affects living cells due to its interaction with the phospholipid layer of cells leading to lysis [13].
- Surfactants reduce the pore size and volume, affecting the drug-loading efficiency and release kinetics [14].
- The presence of surfactants affects surface functionalization.
3.5. Factors That Influence MSN Particle Synthesis
- (a)
-
Rate of silica interaction and condensation
- (b)
-
Assembly kinetics, nucleation, and growth rates
- (c)
-
Charge of silica: The rate of silane hydrolysis and condensation of the siloxane bond depends strongly on the charge states.
- (d)
-
pH of reaction mixture: The silica charge depends on the reaction mixture’s pH. Hydrolysis of the Si–OR bond in silanes occurs faster in an acidic/basic environment compared to a neutral solution. At a pH below the isoelectric point (IEP of silica—2.0), the silica is positively charged, and its charge density increases with a decrease in pH. In reaction mixtures with a pH above the IEP, the silica becomes negatively charged and the charge density increases with the pH. At pH 2–4, the negatively charged silicates interact with positively charged surfactants via electrostatic and hydrogen bond interactions. At pH 4–7, the negative charge density of silicate increases; hence interaction with the surfactant occurs only through the electrostatic force [20].
- (e)
-
Co-surfactants used: Alcohols such as ethanol and butanol influence the pore size, shape, and flexibility when their concentration increases. An increase in co-surfactant concentration disrupts the spherical shape of MSNs, forming amorphous particles with disordered pore sizes.
- (f)
-
Solvent used: Alcohols such as ethanol, propanol, butanol, and pentanol enhance the pore formation in mesopores. The channel rotations of mesoporous materials are also modified by alcohol. Removal of surfactants after the synthesis of MSNs is promoted by alcohol with a high boiling point which prevents aggregation of MSNs. Long-chain alcohols help MSNs transit from one phase to another.
- (g)
-
Silica sources: Sodium silicates, colloidal solutions, and organosilanes form mesoporous silicate structures more rapidly than other precursors [21].
- (h)
-
Temperature: The critical temperature for determining the final properties of MSNs is between 10 and 130 °C, within which 25 °C is the optimum temperature [22].
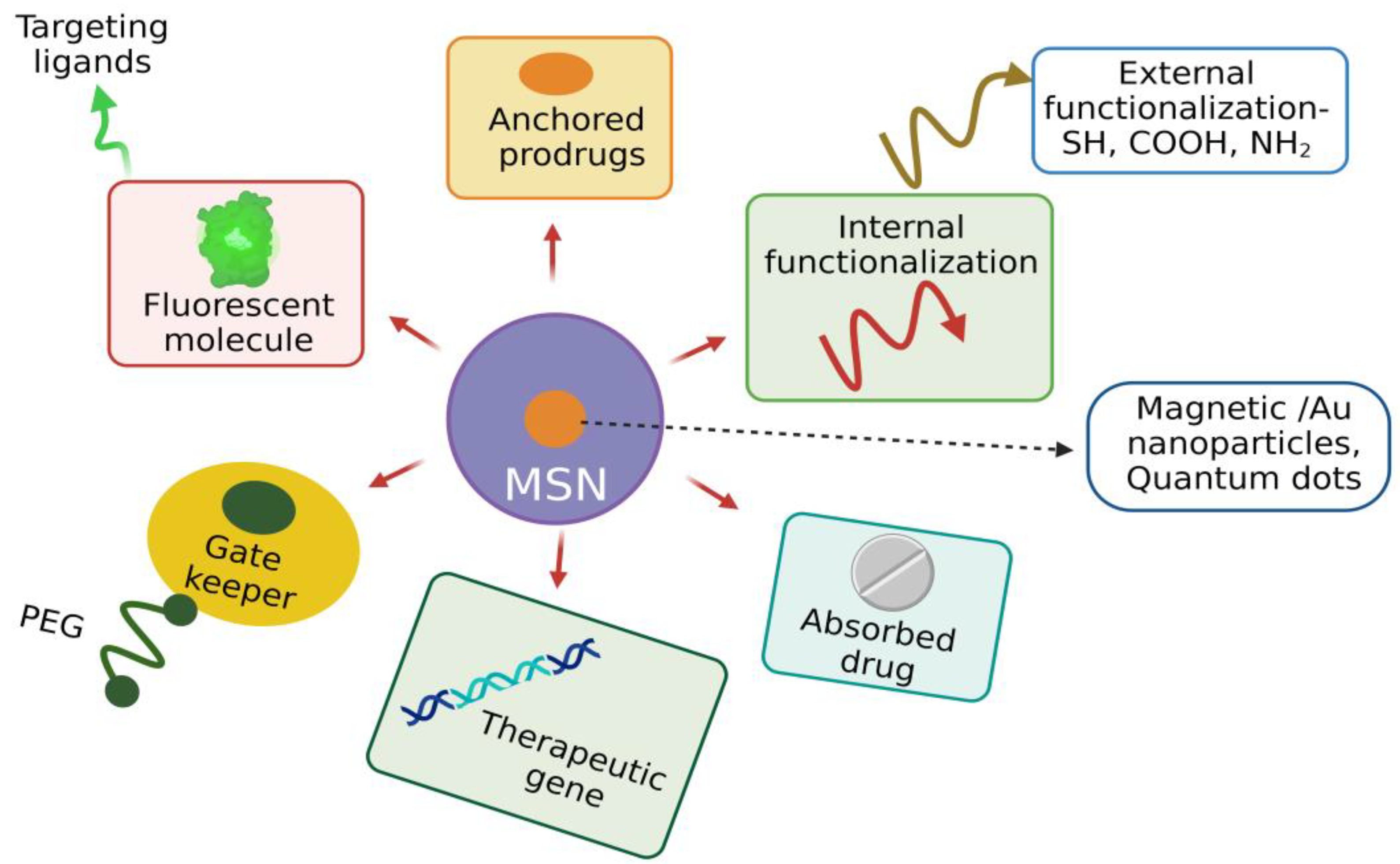
3.6. Functionalization of MSNs
References
- Zhang, J.; Luz, Z.; Goldfarb, D. EPR studies of the formation mechanism of the mesoporous materials MCM-41 and MCM-50. J. Phys. Chem. B. 1997, 101, 7087–7094.
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036.
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B. 2018, 8, 165–177.
- Grun, M.; Unger, K.K.; Matsumoto, A.; Tsutsumi, K. Novel Pathways for the Preparation of Mesoporous MCM-41 Materials: Control of Porosity and Morphology. Microporous Mesoporous Mater. 1999, 27, 207–216.
- Hu, Y.; Wang, J.; Zhi, Z.; Jiang, T.; Wang, S. Facile synthesis of 3D cubic mesoporous silica microspheres with a controllable pore size and their application for improved delivery of a water-insoluble drug. J. Colloid Interface Sci. 2011, 363, 410–417.
- Bonifaz, L.; Bonnyay, D.; Mahnke, K.; Rivera, M.; Nussenzweig, M.C.; Steinman, R.M. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 2002, 196, 1627–1638.
- Mirzaei, M.; Zarch, M.B.; Darroudi, M.; Sayyadi, K.; Keshavarz, S.T.; Sayyadi, J.; Fallah, A.; Maleki, H. Silica mesoporous structures: Effective nanocarriers in drug delivery and nanocatalysts. Appl. Sci. 2020, 10, 7533.
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica nanoparticles—a versatile tool for the treatment of bacterial infections. Front. Chem. 2020, 8, 602.
- Blin, J.L.; Impéror-Clerc, M. Mechanism of self-assembly in the synthesis of silica mesoporous materials: In situ studies by X-ray and neutron scattering. Chem. Soc. Rev. 2013, 42, 4071–4082.
- Yi, Z.; Dumée, L.F.; Garvey, C.J.; Feng, C.; She, F.; Rookes, J.E.; Mudie, S.; Cahill, D.M.; Kong, L. A new insight into growth mechanism and kinetics of mesoporous silica nanoparticles by in situ small angle X-ray scattering. Langmuir 2015, 31, 8478–8487.
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of “sol–gel” chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112.
- Fontecave, T.; Boissiere, C.; Baccile, N.; Plou, F.J.; Sanchez, C. Using evaporation-induced self-assembly for the direct drug templating of therapeutic vectors with high loading fractions, tunable drug release, and controlled degradation. Chem. Mater. 2013, 25, 4671–4678.
- Zhao, Q.; Wu, B.; Shang, Y.; Huang, X.; Dong, H.; Liu, H.; Chen, W.; Gui, R.; Li, J. Development of a nano-drug delivery system based on mesoporous silica and its anti-lymphoma activity. Appl. Nanosci. 2020, 10, 3431–3442.
- Hoffmann, F.; Fröba, M. Vitalising porous inorganic silica networks with organic functions—PMOs and related hybrid materials. Chem. Soc. Rev. 2011, 40, 608–620.
- Fuertes, A.B. Synthesis of ordered nanoporous carbons of tunable mesopore size by templating SBA-15 silica materials. Microporous Mesoporous Mater. 2004, 67, 273–281.
- Yang, L.M.; Wang, Y.J.; Luo, G.S.; Dai, Y.Y. Simultaneous removal of copolymer template from SBA-15 in the crystallization process. Microporous Mesoporous Mater. 2005, 81, 107–114.
- Büchel, G.; Denoyel, R.; Llewellyn, P.L.; Rouquerol, J. In situ surfactant removal from MCM-type mesostructures by ozone treatment. J. Mater. Chem. 2001, 11, 589–593.
- Manet, S.; Schmitt, J.; Impéror-Clerc, M.; Zholobenko, V.; Durand, D.; Oliveira, C.L.; Pedersen, J.S.; Gervais, C.; Baccile, N.; Babonneau, F.; et al. Kinetics of the formation of 2D-hexagonal silica nanostructured materials by nonionic block copolymer templating in solution. J. Phys. Chem. B 2011, 115, 11330–11344.
- Tella, J.O.; Adekoya, J.A.; Ajanaku, K.O. Mesoporous silica nanocarriers as drug delivery systems for anti-tubercular agents: A review. R. Soc. Open Sci. 2022, 9, 220013.
- Yu, J.; Shi, J.L.; Chen, H.R.; Yan, J.N.; Yan, D.S. Effect of inorganic salt addition during synthesis on pore structure and hydrothermal stability of mesoporous silica. Microporous Mesoporous Mater. 2001, 46, 153–162.
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133.
- Kleitz, F.; Marlow, F.; Stucky, G.D.; Schüth, F. Mesoporous silica fibers: Synthesis, internal structure, and growth kinetics. Chem. Mater. 2001, 13, 3587–3595.
- Natarajan, S.K.; Selvaraj, S. Mesoporous silica nanoparticles: Importance of surface modifications and its role in drug delivery. RSC Adv. 2014, 4, 14328–14334.
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic– inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251.
- Tang, Q.; Xu, Y.; Wu, D.; Sun, Y. A study of carboxylic-modified mesoporous silica in controlled delivery for drug famotidine. J. Solid State Chem. 2006, 179, 1513–1520.
- Hartono, S.B.; Gu, W.; Kleitz, F.; Liu, J.; He, L.; Middelberg, A.P.; Yu, C.; Lu, G.Q.; Qiao, S.Z. Poly-L-lysine functionalized large pore cubic mesostructured silica nanoparticles as biocompatible carriers for gene delivery. ACS Nano 2012, 6, 2104–2117.
- Tang, Q.; Chen, Y.; Chen, J.; Li, J.; Xu, Y.; Wu, D.; Sun, Y. Drug delivery from hydrophobic-modified mesoporous silicas: Control via modification level and site-selective modification. J. Solid State Chem. 2010, 183, 76–83.
- Slowing, I.I.; Vivero-Escoto, J.L.; Trewyn, B.G.; Lin, V.S.Y. Mesoporous silica nanoparticles: Structural design and applications. J. Mater. Chem. 2010, 20, 7924–7937.
- Lv, X.; Zhang, L.; Xing, F.; Lin, H. Controlled synthesis of monodispersed mesoporous silica nanoparticles: Particle size tuning and formation mechanism investigation. Microporous Mesoporous Mater. 2016, 225, 238–244.
- Radu, D.R.; Lai, C.Y.; Wiench, J.W.; Pruski, M.; Lin, V.S.Y. Gatekeeping layer effect: A poly (lactic acid)-coated mesoporous silica nanosphere-based fluorescence probe for detection of amino-containing neurotransmitters. J. Am. Chem. Soc. 2004, 126, 1640–1641.
- Ye, F.; Laurent, S.; Fornara, A.; Astolfi, L.; Qin, J.; Roch, A.; Martini, A.; Toprak, M.S.; Muller, R.N.; Muhammed, M. Uniform mesoporous silica coated iron oxide nanoparticles as a highly efficient, nontoxic MRI T (2) contrast agent with tunable proton relaxivities. Contrast Media Mol. Imaging 2012, 7, 460–468.
- Meng, H.; Mai, W.X.; Zhang, H.; Xue, M.; Xia, T.; Lin, S.; Wang, X.; Zhao, Y.; Ji, Z.; Zink, J.I.; et al. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano 2013, 7, 994–1005.
- Álvarez, E.; González, B.; Lozano, D.; Doadrio, A.L.; Colilla, M.; Izquierdo-Barba, I. Nanoantibiotics Based in Mesoporous Silica Nanoparticles: New Formulations for Bacterial Infection Treatment. Pharmaceutics 2021, 13, 2033.
- Frickenstein, A.N.; Hagood, J.M.; Britten, C.N.; Abbott, B.S.; McNally, M.W.; Vopat, C.A.; Patterson, E.G.; MacCuaig, W.M.; Jain, A.; Walters, K.B.; et al. Mesoporous Silica Nanoparticles: Properties and Strategies for Enhancing Clinical Effect. Pharmaceutics 2021, 1, 570.
- Ahir, M.; Upadhyay, P.; Ghosh, A.; Sarker, S.; Bhattacharya, S.; Gupta, P.; Ghosh, S.; Chattopadhyay, S.; Adhikary, A. Delivery of dual miRNA through CD44-targeted mesoporous silica nanoparticles for enhanced and effective triple-negative breast cancer therapy. Biomater. Sci. 2020, 8, 2939–2954.
- Paris, J.L.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Co-Delivery of Drugs and Nucleic Acids in Oncology: A Review. Pharmaceutics 2020, 12, 526.




