Mesoporous silica nanoparticles (MSNs) are a porous inorganic framework synthesized using the inorganic silica sources sodium silicates or silica tetraethyl orthosilicate as a precursor with quaternary ammonium salts as the surfactant. The size and porosity of MSNs are regulated by various factors such as surfactants, the source of silica, ion strength, aging duration, temperature, and pH. Highly ordered mesoporous silica came into the limelight after the fabrication of the silica-based material Mobile crystalline material 41 (MCM) by Kazuyuki Kuroda’s groups and scientists at Mobil Oil Corporation from aluminium silicate gel. MSNs have a unique porous solid framework with a large surface area facilitating functionalization with various functional groups for targeted drug delivery. MCM-41, MCM-48, and SBA-15 are the most common mesoporous silica materials with two-dimensional hexagonal and three-dimensional cubic structures possessing pore sizes of 2–10 nm.
- nanoparticles
- mesoporous silica nanoparticles
- inflammatory diseases
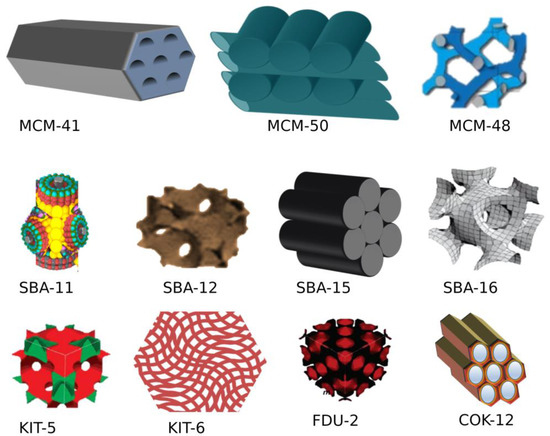
1. Types of MSNs
1. Types of Mesoporous silica nanoparticles (MSNs)

2. Fabrication of MSNs
2. Fabrication of MSNs
-
Cationic surfactants: Surfactants such as cetyl trimethyl ammonium chloride (CTAC), cetyl pyridinium chloride (CPyC), and cetyl tri methyl ammonium bromide (CTAB) with positively charged hydrophilic trimethyl ammonium/pyridinium groups as polar head and a sixteen-carbon hydrocarbon chain nonpolar tail act as a cationic surfactant.
-
Anionic surfactant: Low-cost, eco-friendly anionic surfactants such as sodium salts of alkyl carboxylic acids, phosphoric acid, and sulfonic acid with a negatively charged polar head and hydrocarbon nonpolar tail are widely used as an anionic surfactant.
-
Non-ionic surfactants: Non-ionic triblock copolymers such as alkyl poly (ethylene oxide) (PEO) oligomeric surfactants, poly (alkylene oxide) block copolymers, Triton X-100, polysorbate, and pluronic F 127 with a non-dissociable hydrophilic head which cannot ionize in aqueous solution act as a non-ionic surfactant.
-
Amphoteric/zwitterionic surfactants: Includes surfactants with a net neutral charge such as sodium dodecyl benzene sulfonate (SDBS), sodium dodecyl sulphate (SDS), phospholipids, betaines/sulfobetaine, amino acids, propyl ortho-silicate (TPOS), trimethoxy silane (TMS) and sodium metasilicate (Na2SiO3).
3. Mechanism of Formation of MSN
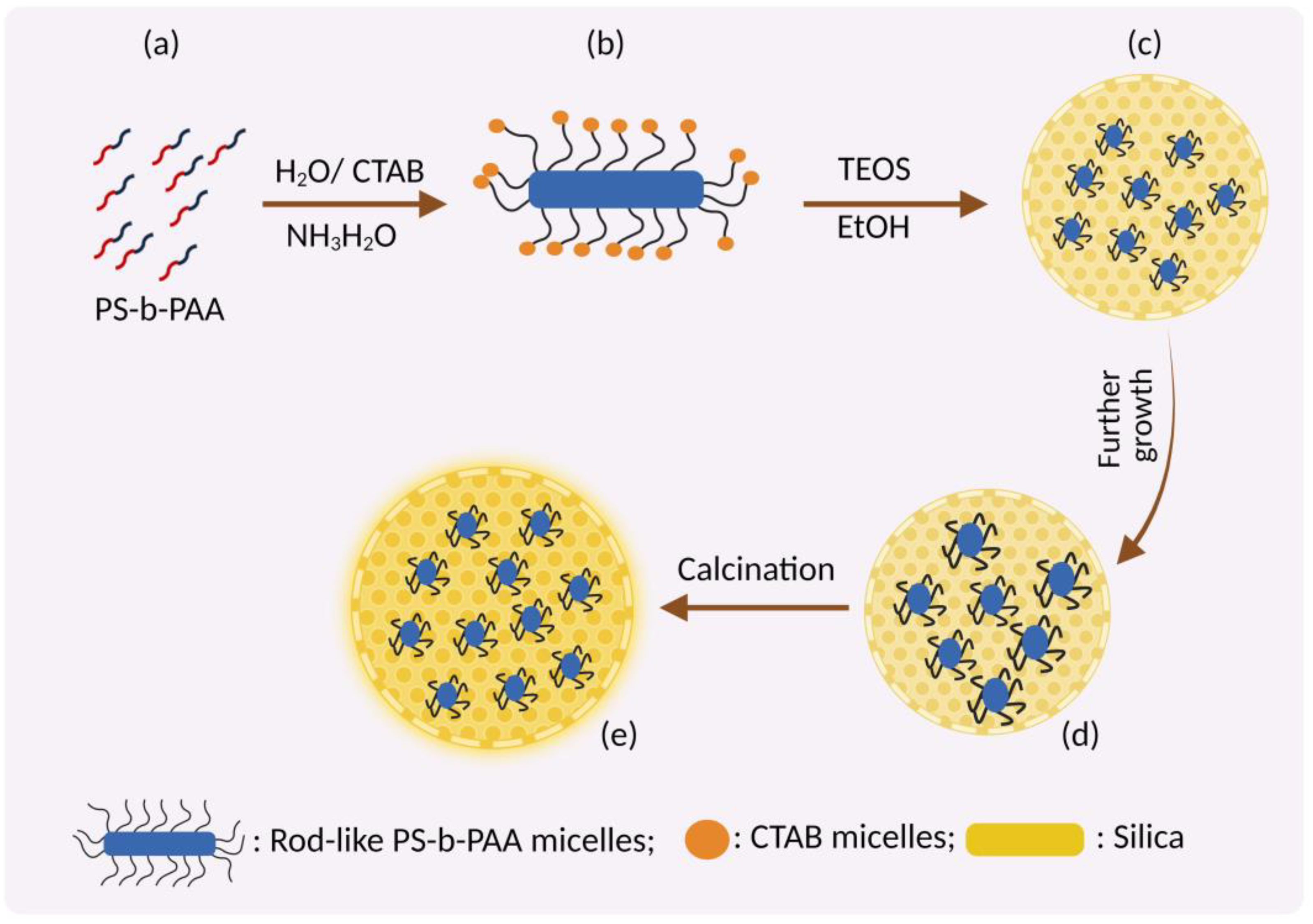
3.1. Swelling Shrinking Mechanism
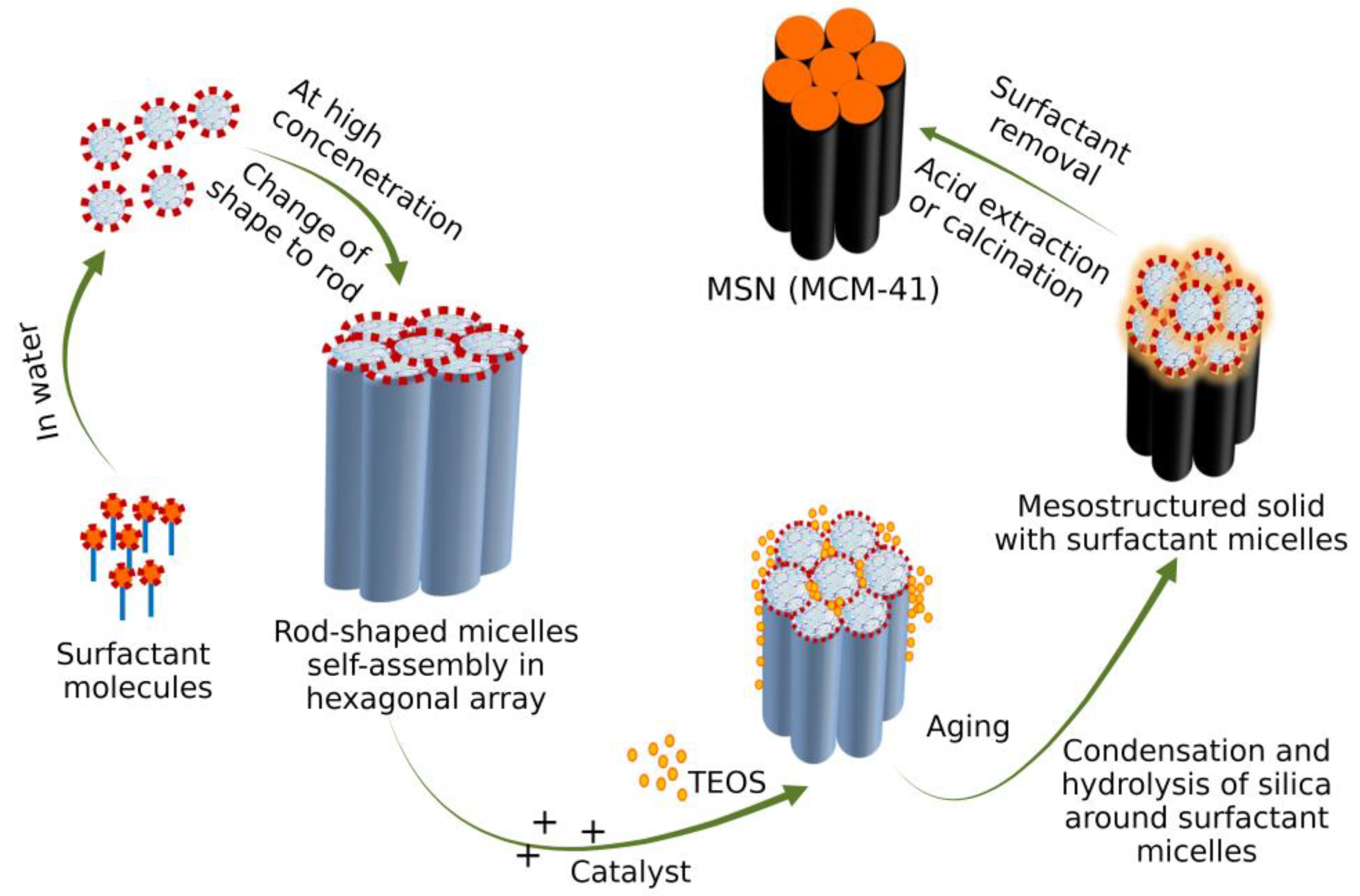
3.2. Sol-Gel Method
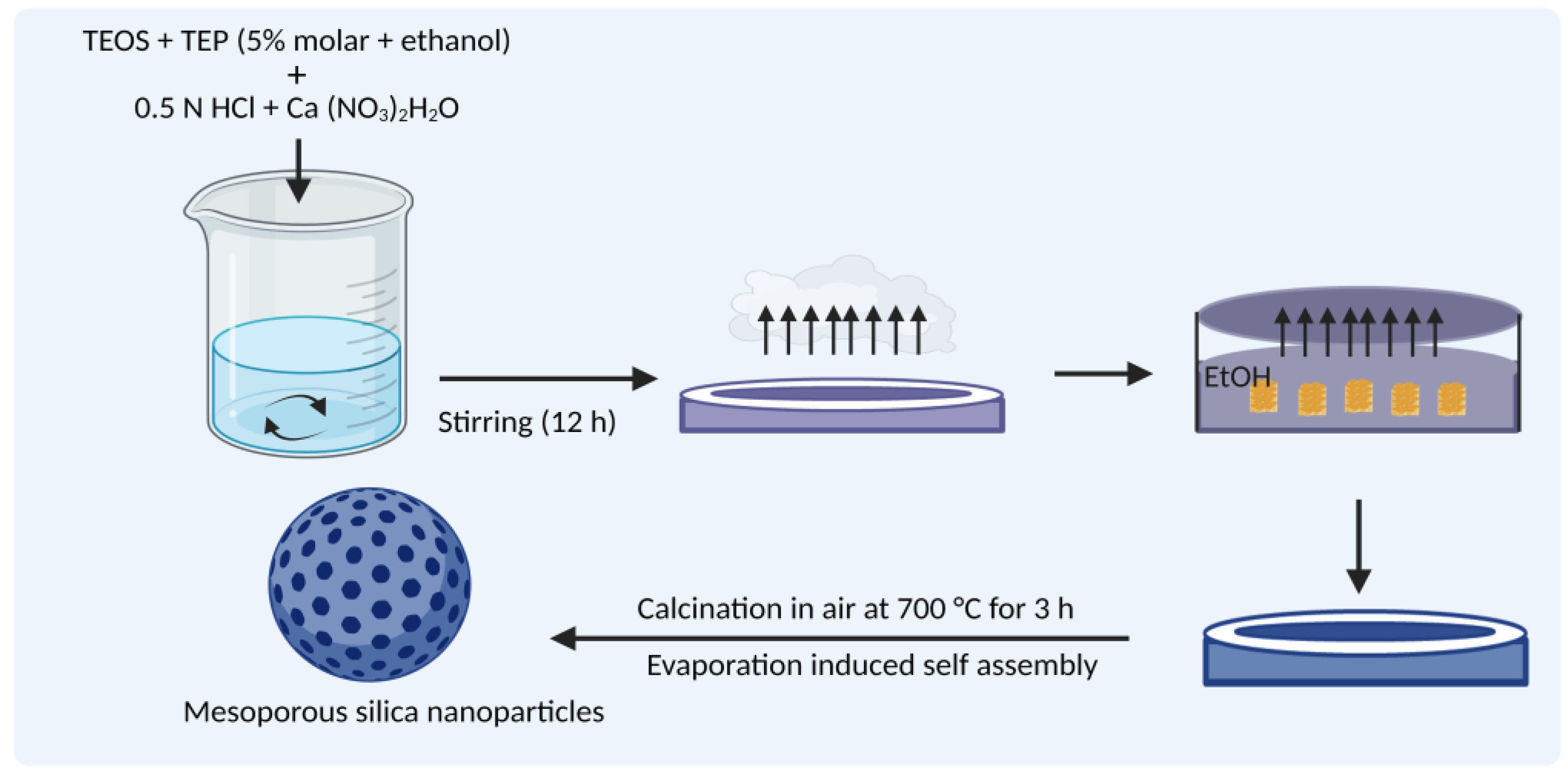
3.3. Evaporation-Induced Self-Assembly (EISA)
3.4. Removal of Surfactant after Synthesis
-
For biomedical applications, the cytotoxic nature of surfactants affects living cells due to its interaction with the phospholipid layer of cells leading to lysis [55].
- For biomedical applications, the cytotoxic nature of surfactants affects living cells due to its interaction with the phospholipid layer of cells leading to lysis [13].
- Surfactants reduce the pore size and volume, affecting the drug-loading efficiency and release kinetics [56].
- Surfactants reduce the pore size and volume, affecting the drug-loading efficiency and release kinetics [14]
- The presence of surfactants affects surface functionalization.
- .
- The presence of surfactants affects surface functionalization.
3.5. Factors That Influence MSN Particle Synthesis
The following factors influence MSNs particle synthesis [61][19].- (a)
-
Rate of silica interaction and condensation
- (b)
-
Assembly kinetics, nucleation, and growth rates
- (c)
-
Charge of silica: The rate of silane hydrolysis and condensation of the siloxane bond depends strongly on the charge states.
- (d)
-
pH of reaction mixture: The silica charge depends on the reaction mixture’s pH. Hydrolysis of the Si–OR bond in silanes occurs faster in an acidic/basic environment compared to a neutral solution. At a pH below the isoelectric point (IEP of silica—2.0), the silica is positively charged, and its charge density increases with a decrease in pH. In reaction mixtures with a pH above the IEP, the silica becomes negatively charged and the charge density increases with the pH. At pH 2–4, the negatively charged silicates interact with positively charged surfactants via electrostatic and hydrogen bond interactions. At pH 4–7, the negative charge density of silicate increases; hence interaction with the surfactant occurs only through the electrostatic force [62][20].≡Si-OH + HO-Si≡ ⇌ ≡Si-O-Si≡ + HOHwhere R represents methyl, ethyl, propyl, and butyl groups, etc. The condensation rate increases with an increase in the negative charge of silicate due to its nucleophilic attack.≡Si-OH + RO-Si≡ ⇌ ≡Si-O-Si≡ + ROH
- (e)
-
Co-surfactants used: Alcohols such as ethanol and butanol influence the pore size, shape, and flexibility when their concentration increases. An increase in co-surfactant concentration disrupts the spherical shape of MSNs, forming amorphous particles with disordered pore sizes.
- (f)
-
Solvent used: Alcohols such as ethanol, propanol, butanol, and pentanol enhance the pore formation in mesopores. The channel rotations of mesoporous materials are also modified by alcohol. Removal of surfactants after the synthesis of MSNs is promoted by alcohol with a high boiling point which prevents aggregation of MSNs. Long-chain alcohols help MSNs transit from one phase to another.
- (g)
-
Silica sources: Sodium silicates, colloidal solutions, and organosilanes form mesoporous silicate structures more rapidly than other precursors [63][21].
- (h)
-
Temperature: The critical temperature for determining the final properties of MSNs is between 10 and 130 °C, within which 25 °C is the optimum temperature [64][22].
3.6. Functionalization of MSNs
Despite its unique physiochemical properties, the functionalization of MSNs is required to improve their physical and chemical properties to enhance their drug adsorption and sustained release at the target site [65][23]. The unique architecture of MSNs facilitates functionalization, i.e., introducing various moieties both in the internal and external surface of MSN, which plays a crucial role in targeted drug delivery. MSNs have two functional surfaces: a cylindrical pore surface and an exterior particle surface. The surface-containing silanol group can be selectively functionalized for controlled drug loading and targeted cell-specific drug release [66][24]. The organic silanes vinyltriethoxy silane (VTES), 3-aminopropyl triethoxysilane (APTES), methoxy-PEG-silane, and 3-mercaptopropyl trimethoxysilane make the fabricated MSNs versatile and promising candidates to perform a specific task. Various surface-modifying functional groups include carboxyl groups, polymers such as PEG, phospholipids, polyethyleneimine (PEI), phospholipids, organic phosphates, and thiols. For the modification of the negatively charged surface, polymers such as 3-aminopropyltriethoxysilane, polylysines, and polyethyleneimine are used [67,68][25][26]. Diethoxydimethyl silane, trimethylchlorosilane, and polymethyl hydrosiloxane reduce the hydrophobicity of MSNs and improves the loading efficiency of hydrophobic drugs [69][27]. Generally, MSNs are functionalized in the internal/external surfaces, walls of pores, and their entry site. Entrapment of drugs with MSNs is based on various interactive forces such as covalent and hydrogen bonding, van der Waals interaction, and electrostatic forces depending on the functional group present at that site [70,71][28][29]. Functionalization influences the MSN’s behavior in biological fluids, such as solubility, dispersibility, drug loading, and drug-release kinetics. The surface modification of MSNs is carried out by co-condensation and post-synthetic modification. Co-condensation involves incorporating functional groups with silica precursors during the synthesis of MSNs. In contrast, the post-synthetic approach involves selectively attaching functional groups to the external and pore surfaces. Co-condensation has several advantages, including the wide range of organoalkoxysilanes that can be used, uniform homogenous distribution of functional groups, and high payload of the functional group without any adverse effect on the MSN structure [72][30]. Covalent attachment of ligands and imaging agents to MSNs will modify the characteristics of MSNs. For example, incorporating iron oxide into MSNs enhances the magnetic resonance imaging (MRI) properties and their therapeutic output [73][31]. For the delivery of hydrophobic drugs, the MSN surface is functionalized with hydrophobic groups, as these drugs cannot interact with the hydrophilic surface of MSNs. Delivery of nucleic acids is facilitated by functionalizing the surface of MSNs with the positively charged polymer PEI so that DNA/RNA can bind to the MSNs via the electrostatic force of attraction. This nonviral mode of gene delivery prevents enzymatic degradation of nucleic acids [74][32]. Functionalization of MSNs with a cationic polymer facilitates the delivery of antibiotics as MSNs interact with negatively charged bacterial cell walls and biofilms and quickly enter microbial cells [75][33]. MSNs can be conjugated with several ligands such as aptamers, growth factors, and vitamins, facilitating cellular entry by receptor-mediated endocytosis forming endosomes resulting in targeted drug delivery with reduced toxicity [76][34]. Incorporating photosensitizers into MSNs functionalized with specific ligands to cancer cells promotes photodynamic therapy for killing cancer cells more efficiently than radio- and chemotherapies [77][35]. Peripheral functionalization of MSNs facilitates the colloidal nature, chemical stability, specific targeting, and pore-gating capacity, improving the MSN’s biocompatibility and nontoxicity. In addition, surface functionalization promotes the co-delivery of cargo with different properties for the synergistic effect to enhance the therapeutic response of the drug. For example, MSNs coated with PEI and loaded with osteostatin functionalized with SOST siRNA effectively treat osteoporosis [78][36]. Surface functionalization of MSNs with various moieties based on different therapeutic strategies enhances its application efficiency (Figure 75).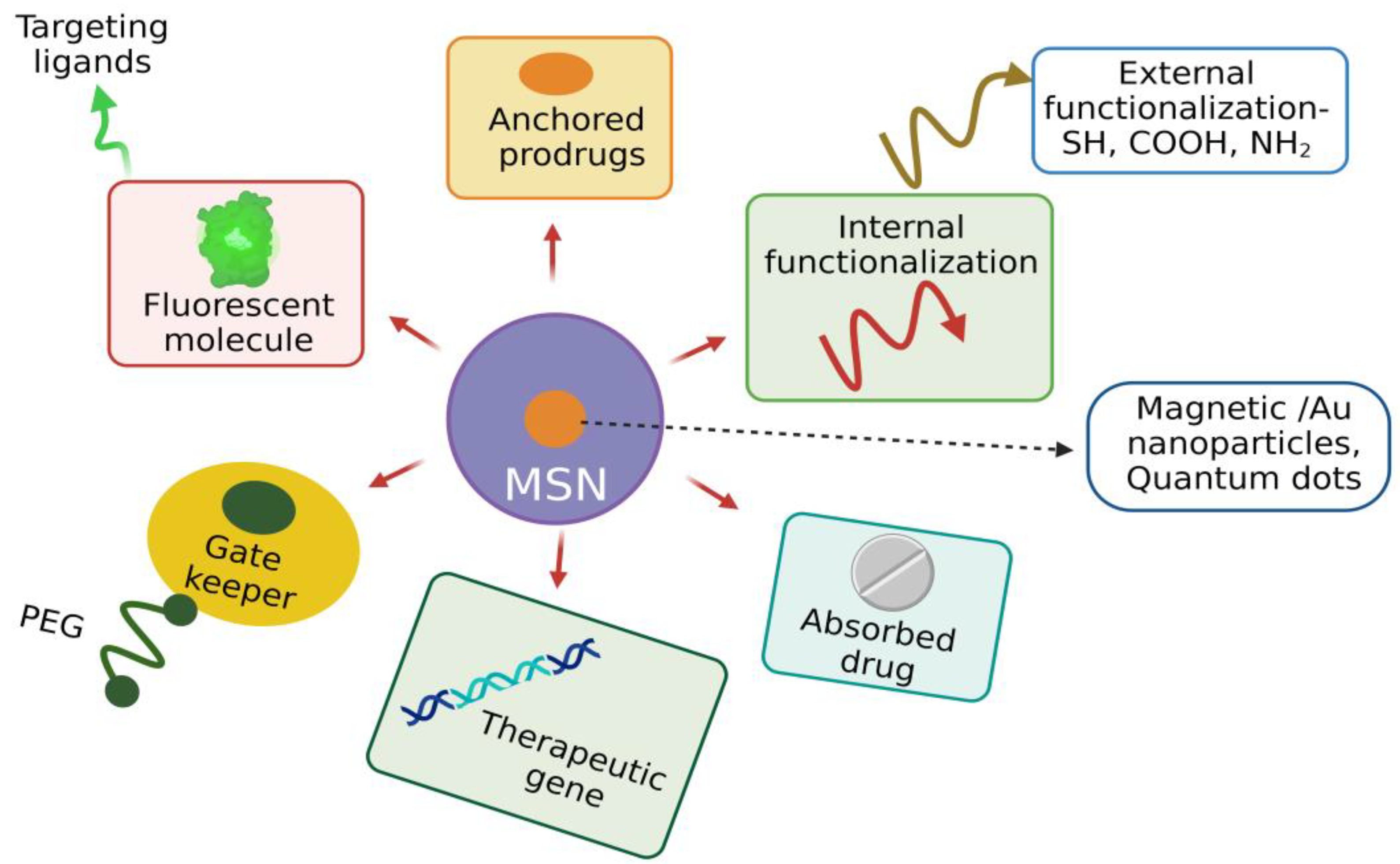 Figure 75. Functionalization of MSN as a promising theranostic agent enhancing its biocompatibility. (Figure created using BioRender.com; accessed on 2 December 2022).
Figure 75. Functionalization of MSN as a promising theranostic agent enhancing its biocompatibility. (Figure created using BioRender.com; accessed on 2 December 2022).
References
- Zhang, J.; Luz, Z.; Goldfarb, D. EPR studies of the formation mechanism of the mesoporous materials MCM-41 and MCM-50. J. Phys. Chem. B. 1997, 101, 7087–7094.
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036.
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B. 2018, 8, 165–177.
- Grun, M.; Unger, K.K.; Matsumoto, A.; Tsutsumi, K. Novel Pathways for the Preparation of Mesoporous MCM-41 Materials: Control of Porosity and Morphology. Microporous Mesoporous Mater. 1999, 27, 207–216.
- Hu, Y.; Wang, J.; Zhi, Z.; Jiang, T.; Wang, S. Facile synthesis of 3D cubic mesoporous silica microspheres with a controllable pore size and their application for improved delivery of a water-insoluble drug. J. Colloid Interface Sci. 2011, 363, 410–417.
- Bonifaz, L.; Bonnyay, D.; Mahnke, K.; Rivera, M.; Nussenzweig, M.C.; Steinman, R.M. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 2002, 196, 1627–1638.
- Mirzaei, M.; Zarch, M.B.; Darroudi, M.; Sayyadi, K.; Keshavarz, S.T.; Sayyadi, J.; Fallah, A.; Maleki, H. Silica mesoporous structures: Effective nanocarriers in drug delivery and nanocatalysts. Appl. Sci. 2020, 10, 7533.
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica nanoparticles—a versatile tool for the treatment of bacterial infections. Front. Chem. 2020, 8, 602.
- Blin, J.L.; Impéror-Clerc, M. Mechanism of self-assembly in the synthesis of silica mesoporous materials: In situ studies by X-ray and neutron scattering. Chem. Soc. Rev. 2013, 42, 4071–4082.
- Yi, Z.; Dumée, L.F.; Garvey, C.J.; Feng, C.; She, F.; Rookes, J.E.; Mudie, S.; Cahill, D.M.; Kong, L. A new insight into growth mechanism and kinetics of mesoporous silica nanoparticles by in situ small angle X-ray scattering. Langmuir 2015, 31, 8478–8487.
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of “sol–gel” chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112.
- Fontecave, T.; Boissiere, C.; Baccile, N.; Plou, F.J.; Sanchez, C. Using evaporation-induced self-assembly for the direct drug templating of therapeutic vectors with high loading fractions, tunable drug release, and controlled degradation. Chem. Mater. 2013, 25, 4671–4678.
- Zhao, Q.; Wu, B.; Shang, Y.; Huang, X.; Dong, H.; Liu, H.; Chen, W.; Gui, R.; Li, J. Development of a nano-drug delivery system based on mesoporous silica and its anti-lymphoma activity. Appl. Nanosci. 2020, 10, 3431–3442.
- Hoffmann, F.; Fröba, M. Vitalising porous inorganic silica networks with organic functions—PMOs and related hybrid materials. Chem. Soc. Rev. 2011, 40, 608–620.
- Fuertes, A.B. Synthesis of ordered nanoporous carbons of tunable mesopore size by templating SBA-15 silica materials. Microporous Mesoporous Mater. 2004, 67, 273–281.
- Yang, L.M.; Wang, Y.J.; Luo, G.S.; Dai, Y.Y. Simultaneous removal of copolymer template from SBA-15 in the crystallization process. Microporous Mesoporous Mater. 2005, 81, 107–114.
- Büchel, G.; Denoyel, R.; Llewellyn, P.L.; Rouquerol, J. In situ surfactant removal from MCM-type mesostructures by ozone treatment. J. Mater. Chem. 2001, 11, 589–593.
- Manet, S.; Schmitt, J.; Impéror-Clerc, M.; Zholobenko, V.; Durand, D.; Oliveira, C.L.; Pedersen, J.S.; Gervais, C.; Baccile, N.; Babonneau, F.; et al. Kinetics of the formation of 2D-hexagonal silica nanostructured materials by nonionic block copolymer templating in solution. J. Phys. Chem. B 2011, 115, 11330–11344.
- Tella, J.O.; Adekoya, J.A.; Ajanaku, K.O. Mesoporous silica nanocarriers as drug delivery systems for anti-tubercular agents: A review. R. Soc. Open Sci. 2022, 9, 220013.
- Yu, J.; Shi, J.L.; Chen, H.R.; Yan, J.N.; Yan, D.S. Effect of inorganic salt addition during synthesis on pore structure and hydrothermal stability of mesoporous silica. Microporous Mesoporous Mater. 2001, 46, 153–162.
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133.
- Kleitz, F.; Marlow, F.; Stucky, G.D.; Schüth, F. Mesoporous silica fibers: Synthesis, internal structure, and growth kinetics. Chem. Mater. 2001, 13, 3587–3595.
- Natarajan, S.K.; Selvaraj, S. Mesoporous silica nanoparticles: Importance of surface modifications and its role in drug delivery. RSC Adv. 2014, 4, 14328–14334.
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic– inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251.
- Tang, Q.; Xu, Y.; Wu, D.; Sun, Y. A study of carboxylic-modified mesoporous silica in controlled delivery for drug famotidine. J. Solid State Chem. 2006, 179, 1513–1520.
- Hartono, S.B.; Gu, W.; Kleitz, F.; Liu, J.; He, L.; Middelberg, A.P.; Yu, C.; Lu, G.Q.; Qiao, S.Z. Poly-L-lysine functionalized large pore cubic mesostructured silica nanoparticles as biocompatible carriers for gene delivery. ACS Nano 2012, 6, 2104–2117.
- Tang, Q.; Chen, Y.; Chen, J.; Li, J.; Xu, Y.; Wu, D.; Sun, Y. Drug delivery from hydrophobic-modified mesoporous silicas: Control via modification level and site-selective modification. J. Solid State Chem. 2010, 183, 76–83.
- Slowing, I.I.; Vivero-Escoto, J.L.; Trewyn, B.G.; Lin, V.S.Y. Mesoporous silica nanoparticles: Structural design and applications. J. Mater. Chem. 2010, 20, 7924–7937.
- Lv, X.; Zhang, L.; Xing, F.; Lin, H. Controlled synthesis of monodispersed mesoporous silica nanoparticles: Particle size tuning and formation mechanism investigation. Microporous Mesoporous Mater. 2016, 225, 238–244.
- Radu, D.R.; Lai, C.Y.; Wiench, J.W.; Pruski, M.; Lin, V.S.Y. Gatekeeping layer effect: A poly (lactic acid)-coated mesoporous silica nanosphere-based fluorescence probe for detection of amino-containing neurotransmitters. J. Am. Chem. Soc. 2004, 126, 1640–1641.
- Ye, F.; Laurent, S.; Fornara, A.; Astolfi, L.; Qin, J.; Roch, A.; Martini, A.; Toprak, M.S.; Muller, R.N.; Muhammed, M. Uniform mesoporous silica coated iron oxide nanoparticles as a highly efficient, nontoxic MRI T (2) contrast agent with tunable proton relaxivities. Contrast Media Mol. Imaging 2012, 7, 460–468.
- Meng, H.; Mai, W.X.; Zhang, H.; Xue, M.; Xia, T.; Lin, S.; Wang, X.; Zhao, Y.; Ji, Z.; Zink, J.I.; et al. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano 2013, 7, 994–1005.
- Álvarez, E.; González, B.; Lozano, D.; Doadrio, A.L.; Colilla, M.; Izquierdo-Barba, I. Nanoantibiotics Based in Mesoporous Silica Nanoparticles: New Formulations for Bacterial Infection Treatment. Pharmaceutics 2021, 13, 2033.
- Frickenstein, A.N.; Hagood, J.M.; Britten, C.N.; Abbott, B.S.; McNally, M.W.; Vopat, C.A.; Patterson, E.G.; MacCuaig, W.M.; Jain, A.; Walters, K.B.; et al. Mesoporous Silica Nanoparticles: Properties and Strategies for Enhancing Clinical Effect. Pharmaceutics 2021, 1, 570.
- Ahir, M.; Upadhyay, P.; Ghosh, A.; Sarker, S.; Bhattacharya, S.; Gupta, P.; Ghosh, S.; Chattopadhyay, S.; Adhikary, A. Delivery of dual miRNA through CD44-targeted mesoporous silica nanoparticles for enhanced and effective triple-negative breast cancer therapy. Biomater. Sci. 2020, 8, 2939–2954.
- Paris, J.L.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Co-Delivery of Drugs and Nucleic Acids in Oncology: A Review. Pharmaceutics 2020, 12, 526.
