Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jonathan Vigne | -- | 2226 | 2023-05-04 09:35:21 | | | |
| 2 | Catherine Yang | -6 word(s) | 2220 | 2023-05-04 10:14:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ladrière, T.; Faudemer, J.; Levigoureux, E.; Peyronnet, D.; Desmonts, C.; Vigne, J. Implementation of Lutetium-177 Therapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/43740 (accessed on 07 February 2026).
Ladrière T, Faudemer J, Levigoureux E, Peyronnet D, Desmonts C, Vigne J. Implementation of Lutetium-177 Therapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/43740. Accessed February 07, 2026.
Ladrière, Typhanie, Julie Faudemer, Elise Levigoureux, Damien Peyronnet, Cédric Desmonts, Jonathan Vigne. "Implementation of Lutetium-177 Therapy" Encyclopedia, https://encyclopedia.pub/entry/43740 (accessed February 07, 2026).
Ladrière, T., Faudemer, J., Levigoureux, E., Peyronnet, D., Desmonts, C., & Vigne, J. (2023, May 04). Implementation of Lutetium-177 Therapy. In Encyclopedia. https://encyclopedia.pub/entry/43740
Ladrière, Typhanie, et al. "Implementation of Lutetium-177 Therapy." Encyclopedia. Web. 04 May, 2023.
Copy Citation
Peptide receptor radionuclide therapy (PRRT) using Lutetium-177 (177Lu) based radiopharmaceuticals has emerged as a therapeutic area in the field of nuclear medicine and oncology, allowing for personalized medicine. Since the first market authorization in 2018 of [¹⁷⁷Lu]Lu-DOTATATE (Lutathera®) targeting somatostatin receptor type 2 in the treatment of gastroenteropancreatic neuroendocrine tumors, intensive research has led to transfer innovative 177Lu containing pharmaceuticals to the clinic.
radiopharmaceuticals
Lutetium-177
nuclear medicine
peptide receptor radionuclide therapy
1. Patient Eligibility
1.1. [177Lu]Lu-DOTATATE (Lutathera®)
Patients with advanced midgut neuroendocrine tumors who have had disease progression during first-line somatostatin analog therapy have limited therapeutic options [1][2][3]. With the exception of everolimus for the treatment of nonfunctional neuroendocrine tumors, no standard second-line systemic treatment options are currently available [4][5]. NETTER-1, a phase 3 trial that was carried out at 41 centers in eight countries across Europe and the USA, allowed for the evaluation of the place of Lutathera® in neuroendocrine tumors in patients aged 18 years and older with advanced, inoperable, well-differentiated (Ki67 index ≤20%) midgut NETs with positive uptake on ¹¹¹In -DTPA octreotide scintigraphy (OctreoScan®) or on [68Ga]Ga-DOTATATE/DOTATOC PET-CT on all target lesions and centrally confirmed disease progression on CT or MRI (as per the Response Evaluation Criteria in Solid Tumors [RECIST], version 1.1) while taking a fixed dose of long-acting octreotide 20−30 mg every 3–4 weeks for at least 12 weeks before randomization. Patients had to have a Karnofsky performance status score of at least 60. Previous PRRT was not allowed, nor was any surgery, transarterial therapy, or chemotherapy within 12 weeks of randomization [6][7]. The indication retained in the marketing authorization is the treatment of inoperable, metastatic, progressive, well-differentiated (G1 and G2) gastroenteropancreatic neuroendocrine tumors (GEP-NETs) expressing somatostatin receptors [8]. Lutathera® is a second-line treatment after disease progression with octreotide in metastatic, progressive, well-differentiated, somatostatin receptor-expressing intestinal NETs or a third-line treatment after everolimus failure [9][10][11][12][13][14][15][16]. Eligible patients must have had positive imaging with [68Ga]Ga -DOTATATE or [68Ga]Ga-DOTATOC to confirm somatostatin receptor overexpression. Patient eligibility should also take into account other treatment options as well as factors that may affect the response to treatment (tumor burden, primary tumor site or previous treatments) [17][18][19][20][21][22][23]. The guidelines published since 2013 [13][15] retain the need for a multidisciplinary meeting (oncologists, nuclear physicians, radiologists, radiopharmacists, surgeons, gastroenterologists, and anatomopathologists) to validate the patient’s eligibility for treatment with [¹⁷⁷Lu]Lu-DOTATATE [18][22][24][25]. Contraindications to treatment are pregnancy, hypersensitivity to the active substance or to one of the excipients, severe associated disease (severe cardiac, hepatic, or hematological disorders), and severe psychiatric disorders. [177Lu]Lu-DOTATATE is contraindicated in patients with severe renal impairment and creatinine clearance <30 mL/min. It is not recommended in patients with a creatinine clearance at initiation <40 mL/min [8][15][18]. The ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms recommend that patients have a creatinine clearance ≥50mL/min [13][18].
1.2. [177Lu]Lu-vipivotide Tetraxetan (Pluvicto®)
Pluvicto® is indicated for the treatment of adult patients with progressive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen channel blocking hormone therapy and taxane-based chemotherapy [26].
Pluvicto® was studied as a targeted radioligand therapy (RLT) in an international phase 3 trial named VISION. The VISION trial was a randomized, multicenter, active-control study comparing the best standard of care with or without Pluvicto® [27].
The study enrolled men with mCRPC that were in progression after one or more approved androgen–receptor-pathway inhibitor therapies and with either one or two taxane regimens. Eligible patients had PSMA-positive metastatic castration-resistant prostate cancer, in other words, at least one PSMA-positive metastatic lesion (PSMA-positive status was determined with the use of Gallium-68 (68Ga)–labeled PSMA-11 ([68Ga]Ga-PSMA-11) PET–CT imaging at baseline) [28]. The presence of PSMA-positive lesions was defined in the protocol as a [68Ga]Ga-PSMA-11 uptake greater than that of liver parenchyma in one or more metastatic lesions of any size in any organ system. A performance-status score of 0 through 2 (on a scale from 0 to 5, with higher numbers indicating greater disability) [29], a life expectancy of at least 6 months, and adequate organ and bone marrow function were also required [30][31][32][33][34].
2. Procedure of Treatment
2.1. [¹⁷⁷Lu]Lu-DOTATATE (Lutathera®)
On the day of injection, the patient is placed in a radiation-protected room sealed by the medical staff (nurse, nursing assistant, or nuclear medicine technician). The floor, especially in the bathroom, should be chosen to facilitate cleaning in the case of vomiting or splashes. It is also possible to cover the floor with absorbent sheets in the case of vomiting. Zoning of the room (i.e., a delimitation and signposting of the restricted zones, allowing the danger of exposure to ionizing radiation to be identified) must be carried out by a competent person in charge of radiation protection (Figure 1) [35]. Patients do not need to fast before treatment.
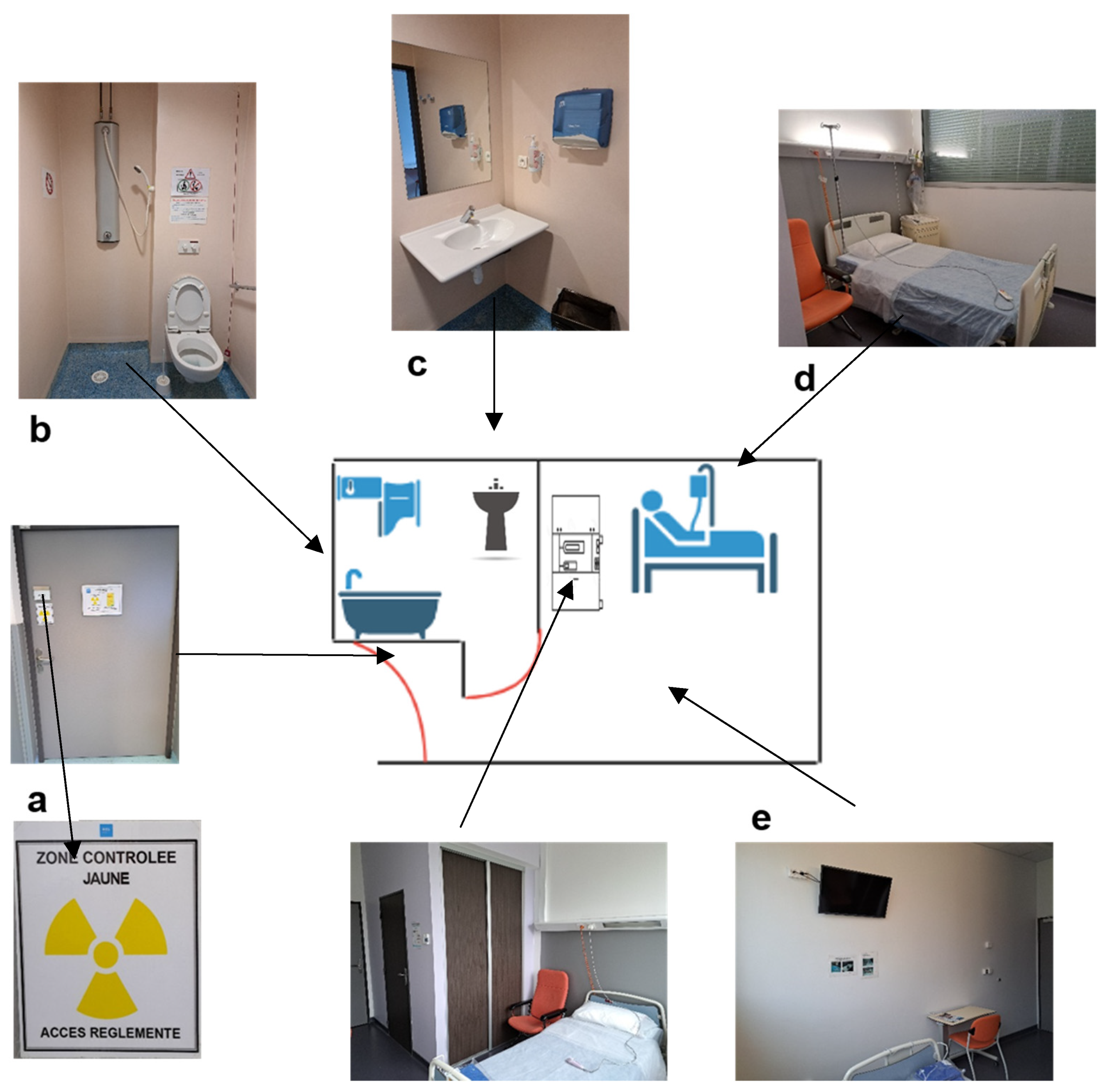
Figure 1. Scheme and photos of a radioprotected room in the therapy department of the Hospices Civils de Lyon, France. (a) Shielded door with zoning of the room (according to the different activities performed, here, the yellow controlled zone in the room was due to Iodine-131 therapy activity). (b) Shower with toilet connected to decay tanks for waste disposal. (c) Wash basin (connected to the decay tanks only due to Iodine-131 therapy activity). (d) Bed with single-use sheets. (e) Posting in the room and bathroom of the radiation protection rules for patients.
A fixed dose of 7.4 GBq (200 mCi) of [¹⁷⁷Lu]Lu-DOTATATE is infused intravenously over a period of 30 min. Patients receive four infusions approximately every 8 weeks (cumulative radioactivity, 29.6 GBq [800 mCi]). Reassessment of the clinical and biological status should be routinely undertaken between treatments [6][18][36]. Some patients may continue to receive supportive care with long-acting octreotide, administered intramuscularly at a dose of 30 mg approximately 24 h after each infusion of [¹⁷⁷Lu]Lu-DOTATATE, and then monthly after the completion of four courses [6][18][36] (Figure 2). A post-therapy whole body scan can be performed 24 h after the injection to check the tumor targeting and to obtain an initial idea of the therapeutic response [36].
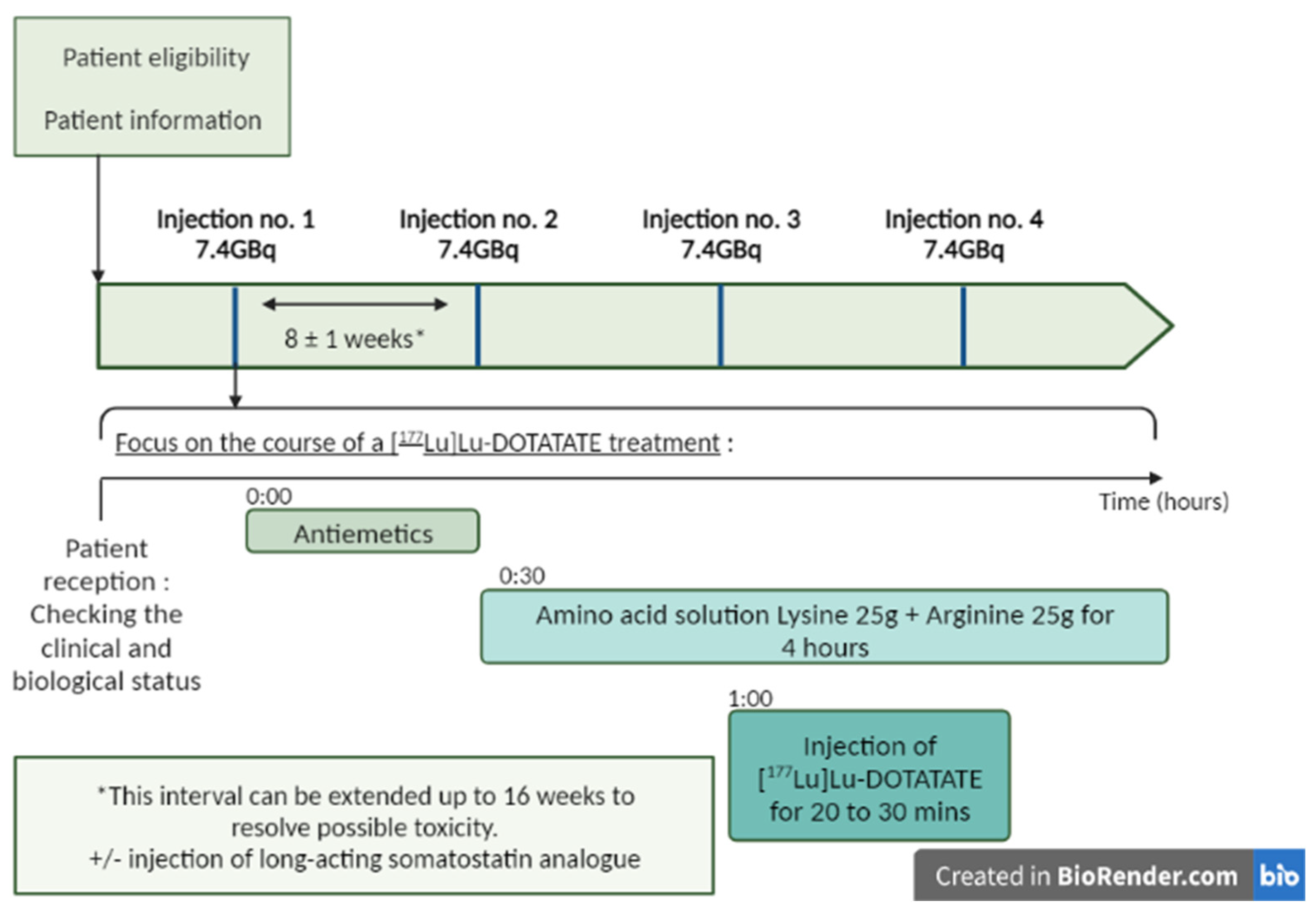
Figure 2. Treatment process for Lutathera®: four infusions of [177Lu]Lu-DOTATATE every 8 weeks (±1 week). Lutathera® administration should be preceded by an infusion of antiemetics and amino acids. The antiemetics were infused before the amino acids. The amino acids should be administered 30 min before [177Lu]Lu-DOTATATE and continue during treatment. For some patients, a long-acting somatostatin analog (Sandostatin LAR) may be administered intramuscularly (i.m.) every 3 to 4 weeks. A short-acting somatostatin analog may be used but should not be administered for 24 h prior to treatment with [177Lu]Lu-DOTATATE. Created in Biorender.com.
For patients with clinical symptoms (diarrhea and flushing) and/or adverse events (defined as Grade 2 platelet count toxicity, Grades 3 or 4 hematological toxicity other than lymphocytopenia, a 40% increase in serum creatinine from the baseline with a concomitant decrease of more than 40% in creatinine clearance, or any other Grades 3 or 4 toxicity that may be related to the study drug), infusions may be extended for up to 16 weeks to allow for the resolution of acute toxicity. After the resolution of adverse events, 50% of the standard treatment dose (i.e., 3.7 GBq–100 mCi) can be prescribed [6][8] (Table 1).
Table 1. Dose modification schemes for Lutathera®.Adapted from [8].
| Toxicity | Action | Result | Next Step |
|---|---|---|---|
| Grade 2 toxicity platelet count 40% increase in serum creatinine with a 40% decrease in clearance |
Postpone treatment up to 16 weeks interval and monitor every 2 weeks | If resolution: re-treat with decrease of 50% radioactivity dose | No toxicity: full dose |
| If persisting toxicity: stop treatment | Toxicity: stop treatment | ||
| Any Grade 3 (except pre-existing serum liver enzyme abnormalities) | Postpone treatment up to 16 weeks interval and monitor every 2 weeks | If resolution: re-treat with decrease of 50% radioactivity dose | No toxicity: full dose |
| If persisting toxicity: stop treatment | Toxicity: stop treatment | ||
| Any Grade 4 | Postpone treatment up to 16 weeks interval and monitor frequently. Act as necessary |
If resolution: re-treat with decrease of 50% radioactivity dose | No toxicity: full dose |
| If persisting toxicity: stop treatment | Toxicity: stop treatment |
Short-acting octreotide is not allowed for 24 h before the administration of [177Lu]Lu-DOTATATE, unless this is clinically impossible. Short-acting octreotide can only be continued if the tumor uptake on SSTR imaging during continued somatostatin analog medication is superior to the liver uptake.
2.2. [177Lu]Lu-Vipivotide Tetraxetan (Pluvicto®)
As with Lutathera®, the patient is placed, in the morning, in a radiation protected room or dedicated room with a toilet connected to the radiation decay tanks by medical staff (nurse, nursing assistant or nuclear medicine technician) (Figure 2). Patients do not need to fast before treatment.
The patient receives intravenous infusions of [177Lu]Lu-PSMA-617 at a dose of 7.4 GBq (200 mCi) once every 6 weeks (± 1 week) up to a maximum of six cycles, unless there is disease progression or unacceptable toxicity [26][27][30][31][37]. In the case of a patient with a serious adverse event, Pluvicto® may be withheld until improvement or the dose may be reduced by 20% to 5.9 GBq (160 mCi). If these effects persist, treatment may be discontinued (Table 2) [26][34][37].
Table 2. Examples of dosage adjustments for Pluvicto® in the case of adverse events. Adapted from [26][38].
| Adverse events | Severity | Action |
|---|---|---|
| Myelosuppression | Grade 2 | Withhold Pluvicto® until improvement to Grade 1 or the baseline. |
| Grade ≥3 | Withhold Pluvicto® until improvement to Grade 1 or the baseline and reduce the Pluvicto® dose by 20%to 5.9 GBq (160 mCi). | |
| Recurrent Grade ≥3 after one dose reduction | Permanently discontinue Pluvicto®. | |
| Renal toxicity | Confirmed serum creatinine increase (Grade ≥2) confirmed CLcr <30 mL/min | Withhold Pluvicto® until improvement. |
| Confirmed ≥40% increase from baseline serum creatinine confirmed >40% decrease from baseline CLcr | Withhold Pluvicto® until improvement or a return to baseline. Reduce Pluvicto® dose by 20% to 5.9 GBq (160 mCi). | |
| Grade ≥3 renal toxicity and recurrent renal toxicity after one dose reduction | Permanently discontinue Pluvicto®. | |
| Gastrointestinal toxicity | Grade ≥3 | Withhold Pluvicto® until improvement to Grade 2 or the baseline. Reduce the Pluvicto® dose by 20% to 5.9 GBq (160 mCi). |
| Recurrent Grade ≥3 gastrointestinal toxicity after one dose reduction | Permanently discontinue Pluvicto®. | |
| Dry mouth | Grade 2 | Withhold Pluvicto® until improvement or a return to the baseline. Consider reducing the Pluvicto® dose by 20% to 5.9 GBq (160 mCi). |
| Grade 3 | Withhold Pluvicto® until improvement or a return to the baseline. Reduce the Pluvicto® dose by 20% to 5.9 GBq (160 mCi). |
|
| Recurrent Grade 3 dry mouth after one dose reduction | Permanently discontinue Pluvicto®. | |
| Fatigue | Grade ≥3 | Withhold Pluvicto® until improvement to Grade 2 or the baseline. |
Before any injection of Pluvicto® and between doses (approximately every three weeks), a biological and clinical check-up should be performed [26][30][34][37]. In addition, in non-surgically castrated patients, chemical castration with a gonadotropin-releasing hormone analog should be maintained [26] Patients do not need to fast before treatment.
Monitoring of the PSA biomarker should be carried out throughout the treatment period. A post-injection scan should be performed to confirm the radiopharmaceutical uptake and therefore the tumor uptake [30][34].
3. Premedication and Administration
3.1. [¹⁷⁷Lu]Lu-DOTATATE (Lutathera®)
For renal protection, the most commonly used intravenous amino acid solution is one containing 25 g arginine and 25 g lysine, diluted in 1 l of 0.9% NaCl. This infusion is started about 30 min before the Lutathera® infusion and continues for 4 h [23][36][39][40] (Figure 2). Other infusion protocols exist as well as other commercial solutions of amino acids, but are often more osmolar and therefore more at risk of adverse effects such as nausea and vomiting [23][40][41].
To prevent the onset of nausea or vomiting, premedication with antiemetic drugs is administered 30 min before the infusion of the amino acid solution by an intravenous bolus of granisetron (3 mg), ondansetron (8 mg and most commonly used), or tropisetron (5 mg) [23][36][40].
The administration of Lutathera and its associated pre-medication requires collaboration between different health care professionals, (e.g., nurses, nursing assistants, and nuclear medicine technicians).
Different methods of administration are reported and the three main ones are presented herein (Figure 3) [40]. Variations exist but the researchers will not dwell on them here. For gravity administration, during the infusion, the flow of the 9 mg/mL (0.9%) sodium chloride infusion solution increases the pressure in the Lutathera® vial, which facilitates the flow of Lutathera® into the catheter inserted in the patient’s peripheral vein. The amino acid solution is passed through another catheter in the patient’s other arm. For the pump and vial method, the pump is set to deliver the volume of [¹⁷⁷Lu]Lu-DOTATATE over 25–30 min. The [¹⁷⁷Lu]Lu-DOTATATE is delivered in 20–25 mL of volume, resulting in an administration rate of 0.8–0.9 mL/min. Then, there is the pump method with a syringe that will be inserted into a syringe guard. At the end of the infusion, the therapy bottle is rinsed with sterile saline solution [8][23][40][42][43]
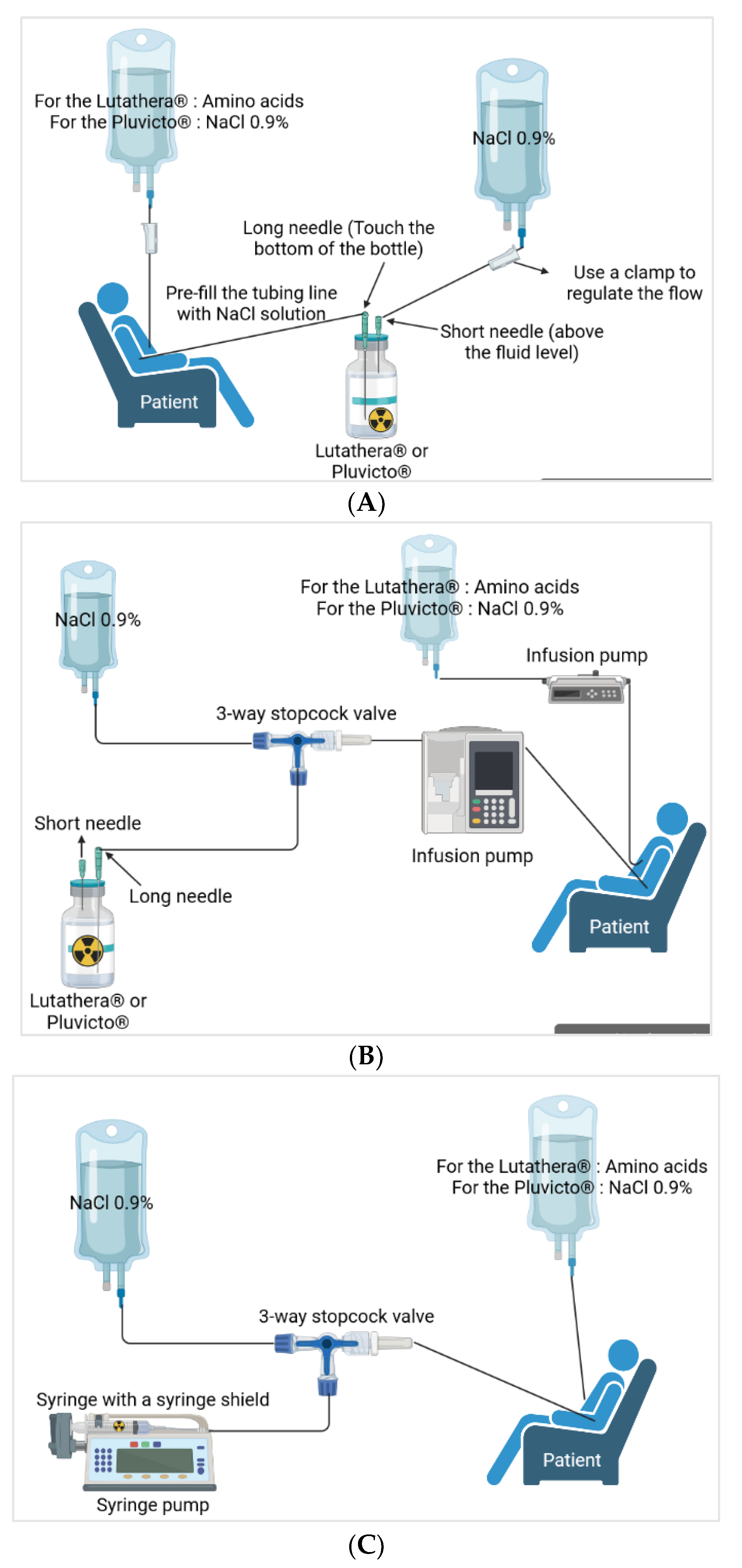
3.2. [177Lu]Lu-Vipivotide Tetraxetan (Pluvicto®)
Prophylactic treatments may include antiemetics (e.g., ondansetron) and corticosteroids (such as dexamethasone). Optional treatments include allopurinol in patients with gout or high tumor burden, cooling of the salivary glands before and/or during administration, and the infusion of 500 mL of 0.9% saline after administration [26][30][34][38].
[177Lu]Lu-PSMA-617 is administered slowly by the intravenous route. The infusion is preceded and followed by a saline flush with ≥10 mL of sterile 0.9% sodium chloride administered to ensure patency of the IV line and to minimize the risk of extravasation. Vital signs are monitored 15 min before administration and 30 and 60 min after administration. Patients are encouraged to maintain adequate fluid intake to reduce radiation exposure to the bladder [26][30][34].
Pluvicto® is administered intravenously by syringe with a syringe shield (with or without a syringe pump), by gravity infusion (with or without an infusion pump), or by vial infusion (peristaltic infusion pump) (Figure 3). Pluvicto® should not be injected directly into another intravenous solution. If a reduced dose of [177Lu]Lu-PSMA-617 is available, it should be administered by the syringe or vial method with a peristaltic pump. The gravity method is not recommended as the volume administered may be incorrect [26][38].
References
- Rinke, A.; Müller, H.-H.; Schade-Brittinger, C.; Klose, K.-J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.-F.; Bläker, M.; et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 4656–4663.
- Kvols, L.K.; Moertel, C.G.; O’Connell, M.J.; Schutt, A.J.; Rubin, J.; Hahn, R.G. Treatment of the Malignant Carcinoid Syndrome. N. Engl. J. Med. 1986, 315, 663–666.
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233.
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, nonfunctional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet Lond. Engl. 2016, 387, 968–977.
- Kulke, M.H.; Siu, L.L.; Tepper, J.E.; Fisher, G.; Jaffe, D.; Haller, D.G.; Ellis, L.M.; Benedetti, J.K.; Bergsland, E.K.; Hobday, T.J.; et al. Future Directions in the Treatment of Neuroendocrine Tumors: Consensus Report of the National Cancer Institute Neuroendocrine Tumor Clinical Trials Planning Meeting. J. Clin. Oncol. 2011, 29, 934–943.
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135.
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763.
- EMA Annexe I—Résumé Des Caractéristiques Du Produit—Lutathera 370MBq/mL Solution Pour Perfusion . Available online: https://www.ema.europa.eu/en/documents/product-information/lutathera-epar-product-information_fr.pdf (accessed on 11 January 2023).
- Haute Autorité de Santé Avis de la Commission de la Transparence Du 11 Juillet 2018 Concernant le 177Lutécium-Oxodotréotide. Available online: https://www.has-sante.fr/upload/docs/evamed/CT-16606_LUTATHERA_PIC_INS_Avis3_CT16606.pdf (accessed on 27 January 2023).
- Société Savante Des Maladies Et Cancers de L’appareil Digestif Néoplasies Neuroendocrines (NNe) Digestives—Thésaurus National de Cancérologie Digestive. Available online: https://www.snfge.org/sites/default/files/SNFGE/TNCD/tncd_chap-11_nne_2020_03_17.pdf (accessed on 1 February 2023).
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Klöppel, G.; et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171.
- Pavel, M.; O’’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.-F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185.
- Hicks, R.J.; Kwekkeboom, D.J.; Krenning, E.; Bodei, L.; Grozinsky-Glasberg, S.; Arnold, R.; Borbath, I.; Cwikla, J.; Toumpanakis, C.; Kaltsas, G.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms: Peptide Receptor Radionuclide Therapy with Radiolabelled Somatostatin Analogues. Neuroendocrinology 2017, 105, 295–309.
- Kwekkeboom, D.J.; Krenning, E.P.; Lebtahi, R.; Komminoth, P.; Kos-Kudła, B.; de Herder, W.W.; Plöckinger, U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Peptide Receptor Radionuclide Therapy with Radiolabeled Somatostatin Analogs. Neuroendocrinology 2009, 90, 220–226.
- Hope, T.A.; Bodei, L.; Chan, J.A.; El-Haddad, G.; Fidelman, N.; Kunz, P.L.; Mailman, J.; Menda, Y.; Metz, D.C.; Mittra, E.S.; et al. NANETS/SNMMI Consensus Statement on Patient Selection and Appropriate Use of 177Lu-DOTATATE Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2020, 61, 222–227.
- Hendifar, A.E.; Mehr, S.H.; McHaffie, D.R. Best Practices for the Coordinated Care of Patients with Neuroendocrine Tumors Undergoing Peptide Receptor Radionuclide Therapy. Pancreas 2022, 51, 213–218.
- Apostolidis, L.; Dal Buono, A.; Merola, E.; Jann, H.; Jäger, D.; Wiedenmann, B.; Winkler, E.C.; Pavel, M. Multicenter Analysis of Treatment Outcomes for Systemic Therapy in Well Differentiated Grade 3 Neuroendocrine Tumors (NET G3). Cancers 2021, 13, 1936.
- Ambrosini, V.; Zanoni, L.; Filice, A.; Lamberti, G.; Argalia, G.; Fortunati, E.; Campana, D.; Versari, A.; Fanti, S. Radiolabeled Somatostatin Analogues for Diagnosis and Treatment of Neuroendocrine Tumors. Cancers 2022, 14, 1055.
- Ezziddin, S.; Attassi, M.; Yong-Hing, C.J.; Ahmadzadehfar, H.; Willinek, W.; Grünwald, F.; Guhlke, S.; Biersack, H.-J.; Sabet, A. Predictors of Long-Term Outcome in Patients with Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors After Peptide Receptor Radionuclide Therapy with 177Lu-Octreotate. J. Nucl. Med. 2014, 55, 183–190.
- Sansovini, M.; Severi, S.; Ianniello, A.; Nicolini, S.; Fantini, L.; Mezzenga, E.; Ferroni, F.; Scarpi, E.; Monti, M.; Bongiovanni, A.; et al. Long-term follow-up and role of FDG PET in advanced pancreatic neuroendocrine patients treated with 177Lu-D OTATATE. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 490–499.
- Aalbersberg, E.A.; Huizing, D.M.V.; Walraven, I.; de Wit-van der Veen, B.J.; Kulkarni, H.R.; Singh, A.; Stokkel, M.P.M.; Baum, R.P. Parameters to Predict Progression-Free and Overall Survival After Peptide Receptor Radionuclide Therapy: A Multivariate Analysis in 782 Patients. J. Nucl. Med. 2019, 60, 1259–1265.
- Mittra, E.S. Neuroendocrine Tumor Therapy: 177Lu-DOTATATE. Am. J. Roentgenol. 2018, 211, 278–285.
- Zaknun, J.J.; Bodei, L.; Mueller-Brand, J.; Pavel, M.E.; Baum, R.P.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisiol, T.M.; Howe, J.R.; Cremonesi, M.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816.
- Médicaments Radiopharmaceutiques: Du Diagnostic Au Thranostique Des Tumeurs Neuroendocrines. Available online: https://reader.elsevier.com/reader/sd/pii/S092812582100019X?token=5AD3413788D8F96FBA939BD43322BB1B2E50F75604042F460F0D77C4202FEAABA18EDBAA394289780F60C4F92ACD6BC8&originRegion=eu-west-1&originCreation=20230111143928 (accessed on 11 January 2023).
- Endocan—Renaten. GTE—Groupes d’étude des Tumeurs Endocrines. Available online: https://www.reseau-gte.org/renaten/ (accessed on 27 January 2023).
- EMA Annexe I—Résumé Des Caractéristiques Du Produit—Pluvicto 1000Mbq/mL Solution Injectable/Pour Perfusion. Available online: https://www.ema.europa.eu/en/documents/product-information/pluvicto-epar-product-information_fr.pdf (accessed on 11 January 2023).
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103.
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Ceci, F.; Cho, S.; Giesel, F.; Haberkorn, U.; Hope, T.A.; Kopka, K.; et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1014–1024.
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655.
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Delgado Bolton, R.C.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544.
- AlSadi, R.; Bouhali, O.; Dewji, S.; Djekidel, M. 177Lu-PSMA Therapy for Metastatic Castration-Resistant Prostate Cancer: A Mini-Review of State-of-the-Art. The Oncologist 2022, 27, e957–e966.
- Hennrich, U.; Kopka, K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114.
- Fallah, J.; Agrawal, S.; Gittleman, H.; Fiero, M.H.; Subramaniam, S.; John, C.; Chen, W.; Ricks, T.K.; Niu, G.; Fotenos, A.; et al. FDA Approval Summary: Lutetium Lu 177 vipivotide tetraxetan for patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, CCR-22-2875.
- Sanli, Y.; Simsek, D.H.; Sanli, O.; Subramaniam, R.M.; Kendi, A.T. 177Lu-PSMA Therapy in Metastatic Castration-Resistant Prostate Cancer. Biomedicines 2021, 9, 430.
- Radecki, J.-J.; Aubert, B.; Cordier, G.; Fracas, P. La mise en œuvre de l’arrêté zonage. Radioprotection 2007, 42, 463–476.
- Bournaud, C.; Lombard-Bohas, C.; Habouzit, V.; Carlier, T.; Hindié, E.; Ansquer, C. La radiothérapie interne vectorisée par les analogues de la somatostatine, en pratique, en 2019. Médecine Nucl. 2019, 43, 251–266.
- Hennrich, U.; Eder, M. Lu-PSMA-617 (PluvictoTM): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer. Pharmaceuticals 2022, 15, 1292.
- Advanced Accelerator Applications Dosing and Administration Guide—Pluvicto®. Available online: https://www.hcp.novartis.com/siteassets/vilupsa/dosing/184981-pluvicto-branded-hcp-dosing-guide-digital_3.29.22.pdf (accessed on 10 January 2023).
- Das, S.; Al-Toubah, T.; El-Haddad, G.; Strosberg, J. 177Lu-DOTATATE for the treatment of gastroenteropancreatic neuroendocrine tumors. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1023–1031.
- Hope, T.A.; Abbott, A.; Colucci, K.; Bushnell, D.L.; Gardner, L.; Graham, W.S.; Lindsay, S.; Metz, D.C.; Pryma, D.A.; Stabin, M.G.; et al. NANETS/SNMMI Procedure Standard for Somatostatin Receptor–Based Peptide Receptor Radionuclide Therapy with 177 Lu-DOTATATE. J. Nucl. Med. 2019, 60, 937–943.
- Courault, P.; Deville, A.; Habouzit, V.; Gervais, F.; Bolot, C.; Bournaud, C.; Levigoureux, E. Amino Acid Solutions for 177Lu-Oxodotreotide Premedication: A Tolerance Study. Cancers 2022, 14, 5212.
- Love, C.; Desai, N.B.; Abraham, T.; Banks, K.P.; Bodei, L.; Boike, T.; Brown, R.K.J.; Bushnell, D.L.; DeBlanche, L.E.; Dominello, M.M.; et al. ACR-ACNM-ASTRO-SNMMI Practice Parameter for Lutetium-177 (Lu-177) DOTATATE Therapy. Clin. Nucl. Med. 2022, 47, 503–511.
- Advanced Accelerator Applications Monographie de Produit—Lutathera®. Available online: https://canm-acmn.ca/resources/Documents/Lutathera%20Product%20Monograph_FR.pdf (accessed on 31 January 2023).
- Advanced Accelerator Applications. Dosing and Administration—LUTATHERA®. Available online: https://www.hcp.novartis.com/products/lutathera/gep-nets/dosing-and-administration/ (accessed on 1 February 2023).
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.9K
Entry Collection:
Peptides for Health Benefits
Revisions:
2 times
(View History)
Update Date:
04 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




