You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nikolaos Machairas | -- | 2058 | 2023-05-04 00:53:51 | | | |
| 2 | Catherine Yang | Meta information modification | 2058 | 2023-05-04 02:53:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Papadakos, S.P.; Dedes, N.; Gkolemi, N.; Machairas, N.; Theocharis, S. The Role of EPH/Ephrin System in the Pancreas. Encyclopedia. Available online: https://encyclopedia.pub/entry/43719 (accessed on 22 December 2025).
Papadakos SP, Dedes N, Gkolemi N, Machairas N, Theocharis S. The Role of EPH/Ephrin System in the Pancreas. Encyclopedia. Available at: https://encyclopedia.pub/entry/43719. Accessed December 22, 2025.
Papadakos, Stavros P., Nikolaos Dedes, Nikolina Gkolemi, Nikolaos Machairas, Stamatios Theocharis. "The Role of EPH/Ephrin System in the Pancreas" Encyclopedia, https://encyclopedia.pub/entry/43719 (accessed December 22, 2025).
Papadakos, S.P., Dedes, N., Gkolemi, N., Machairas, N., & Theocharis, S. (2023, May 03). The Role of EPH/Ephrin System in the Pancreas. In Encyclopedia. https://encyclopedia.pub/entry/43719
Papadakos, Stavros P., et al. "The Role of EPH/Ephrin System in the Pancreas." Encyclopedia. Web. 03 May, 2023.
Copy Citation
Pancreatic ductal adenocarcinoma (PDAC) is a major concern for health care systems worldwide, since its mortality remains unaltered despite the surge in cutting-edge science. The EPH/ephrin signaling system was first investigated in the 1980s. EPH/ephrins have been shown to exert bidirectional signaling and cell-to-cell communication, influencing cellular morphology, adhesion, migration and invasion.
EPH/eprin
signaling pathway
PDAC
pancreatic cancer
1. The EPH/Ephrin System in Pancreatic Embryology and Physiology
The EPH/ephrin system has been associated with numerous processes involving the embryologic integration of the pancreatic parenchyma and the positioning of the islets of Langerhans [1], which represent a major endocrine component in the regulation of insulin secretion [2]. Transcriptomic analyses of the main exocrine and endocrine pancreatic cells demonstrated that the sophistication of the system is determined by compounded heterotypic cellular interactions. The EPH/ephrin system, in tandem with 7-Transmembrane receptors (7-TM receptors) and ligands from the TGF-b class, compose the main regulators of the heterotypic synergy among the aforementioned cellular compartments [3]. The alpha and beta endocrine cellular populations are characterized by the overexpression of EFNA5 and EFNB3, while EFNA1 and EFNB2 predominate in the small and large ducts and acinar cells. A more in-depth presentation of those interactions has been given elsewhere, and this goes beyond the scope of our manuscript [3]. Class B of the EPH/ephrins orchestrate the pancreatic morphogenesis. They appear earlier than class A molecules, at embryonic day 12.5, and regulate the alignment of the pancreatic epithelium, branching and lumen formation. The interplay among the epithelium-expressed EPHB2 and EPHB3 and their concomitant ligands in the pancreatic arteries and mesenchyme [2][3][4] mediates the expression of several cell adherence molecules, such as junctional b-catenin and E-cadherin [5]. Roughly, EPHB3 comprises the only EPH/ephrin molecule that is expressed in mesoderm. Its interplay with the endodermal ephrin-B1 guides the formation of the extrahepatic bile duct, the gallbladder and the common bile duct. The EPHB3-EPHB4 interaction contributes to gallbladder formation, the EPHB3-ephrinB2 regulates the development of the gallbladder and common bile duct and the EPHB3-EPHB3 in the endoderm directs the composition of the extrapancreatic duct [6]. Since pancreatic morphogenesis emerges as a summation of consecutive processes, e.g., the arrangement of the epithelium into distinct layers, the periodic loss of apical–basal polarity and epithelial tubule reconstruction, it is highly dependent on EPHB signaling. Contrarily, several findings have suggested that the class A EPH/ephrins comprise a central regulator of insulin secretion. It is well-established that the metabolism of glucose in β-cells stimulates basal insulin secretion [7], while the interactions between β-cells shape insulin secretion in response to glucose [8]. This process is essential to achieving the suppression of insulin secretion during starvation and adequate amounts of insulin during feeding [9]. Presently, it is common knowledge that blood glucose levels regulate insulin secretion, exerting their effects on class A EPH/ephrins. At high glucose levels, the dephosphorylation of EPHA5 by protein tyrosine phosphatases (PTPs) suppresses the EPH forward signaling. The unopposed ephrin-A5 backward signaling results in insulin secretion. On the other hand, at low glucose concentrations, the forward signaling outweighs the reverse signaling, inhibiting the insulin secretion. Insulin secretion could result either from the suppression of EPHA signaling or from the enhancement of ephrin-A reverse signaling [9]. Analogously, in α-cells, the enhancement of EPHA4 forward signaling suppresses the glucagon secretion [10]. All of the above indicate that the shaping of pancreatic morphology and physiology are interconnected and the EPH/ephrin system exerts major influence on their configuration.
2. The EPH/Ephrin System in PDAC—Preclinical Data
EPHA2 and EPHA4 are the most important targets in the field of PDAC translational research [11]. EPHA2 has attracted the attention of the research community due to its involvement in tumor capillary formation [12]. Despite the fact that the initial attempts to target EPHA2 in order to enhance the specificity of adenoviral vectors were not fruitful [13], the development of EPHA2-specific antibody agonists and ephrinA1 antagonists suppressed tumor growth and metastatic disease, inhibiting angiogenesis in mice with orthotopical transplantation of MiaPaCa2 cells [14]. In their groundbreaking study, Markosyan et al. analytically investigated the role of EPHA2 in PDAC [15]. The CRIPSR-Cas9-mediated generation of Epha2-KO congenic mice by 6419c5 and 6694c2 cell lines, which have low T-cell infiltration levels, exhibited substantially modified immune microenvironment in comparison with the wild-type cell lines. They documented, in Epha2-KO tumors, enhanced infiltrates of CD4 + and CD8 + T-cells, with diminished presence of myeloid and myeloid-derived suppressor cells (MDSCs) and unaltered numbers of antigen-presenting cells, such as macrophages and dendritic cells. The therapeutic combination of gemcitabine, nab-paclitaxel, anti-CD40 agonists, anti–CTLA-4 and anti–PD1-1 in EPHA2-KO tumors achieved results that were more efficacious than those for EPHA2-wild-type tumors, but comparable with those for high T-cell-infiltrating ones. Altogether, the above strongly suggest that EPHA2 exerts modifying properties over the immune tumor microenvironment (TME) [15]. A series of sophisticated experiments unfolded the existence of the EPHA2/TGF-β/PTGS2 pathway. The prostaglandin endoperoxide synthase 2 (PTGS2) gene encodes the cyclooxygenase-2 (COX-2). The tumor-accelerating properties of PTGS2 are owed to its efficacy in activating downstream signaling pathways such as the RAS [16], PI3K/AKT [17] and ERK [18]. The identification of this pathway could offer novel therapeutic avenues in the medical management of PDAC, since the COX-2 inhibition could sensitize PDAC to immunotherapy [19]. Finally, there is an auspicious perspective that the utilization of EPHA2 as a surface marker to increase the sensitivity of exosomal collection and assortment will provide an invaluable source of clinical data [20]. Recent evidence implicated ephrin-A5 in the development of fibrotic stroma [21]. Nakajima et al. documented a significant reduction in collagen density (type I, III and IV collagen) upon exposure to neoadjuvant therapy (NAT). In human-derived PDAC cell cultures, it was evident that ephrin-A5 signaling regulated the expression of several genes implicated in collagen synthesis. Collectively, NAT inhibited the expression of CAFs, shaping the PDAC microenvironment, and indirectly inhibited PDAC cells, reducing the fibrotic stroma through EFNA5 downregulation [21].
EPHB4/ephrin-B2 is signaling pathway which has been the studied in the most depth with regard to the class B EPH/ephrin system [22]. Initial studies reported that soluble EPHB4 blockers, inhibiting the ephrin-B2 forward signaling in venous endothelial cells and the backward signaling in the arterial endothelium, diminish tumor growth. This became more evident with additional Dll4/Notch inhibition [23]. Aside from its effects on angiogenesis, ephrinB2 signaling influences cellular proliferation and migration, exerting its impact on the cell cycle and epithelial–mesenchymal transition (EMT). In more detail, Zhu et al. demonstrated that the EFNB2 knockdown upregulates p53, inducing a fixation in the G0/G1 phase and cell cycle arrest. In parallel, an upward trend occurred in E-cadherin expression with a concomitant downregulation of vimentin, which are decidedly suggestive of an influence of ephrin-B2 signaling on cellular invasion through EMT regulation [24]. EPHB4 suggests an attractive cytotoxic target in PDAC. In vivo data from orthotopic xenografts showed enhanced tumor growth retardation with the addition of EPHB4 inhibition in combination with gemcitabine [22]. Furthermore, data associating the EPHB4/ephrinB2 signaling with the modulation of PDAC ΤΜΕ have begun to emerge [25][26]. Radiotherapy (RT) induces immune infiltration, attracting both anti-tumorigenic (effector T-cells and interferon I signaling activation) and pro-tumorigenic (regulatory T-cells (Tregs), tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) cellular populations [25]. Lennon et al. documented, both in vitro and in vivo, that the inhibition of EPHB4/ephrin-B2 signaling in conjunction with RT shifts the balance towards the anti-tumor responses, reducing PDAC tumor growth and limiting the fibrotic response [26]. This could have major clinical applications in the therapeutic management of PDAC. The above are briefly illustrated in Figure 1.
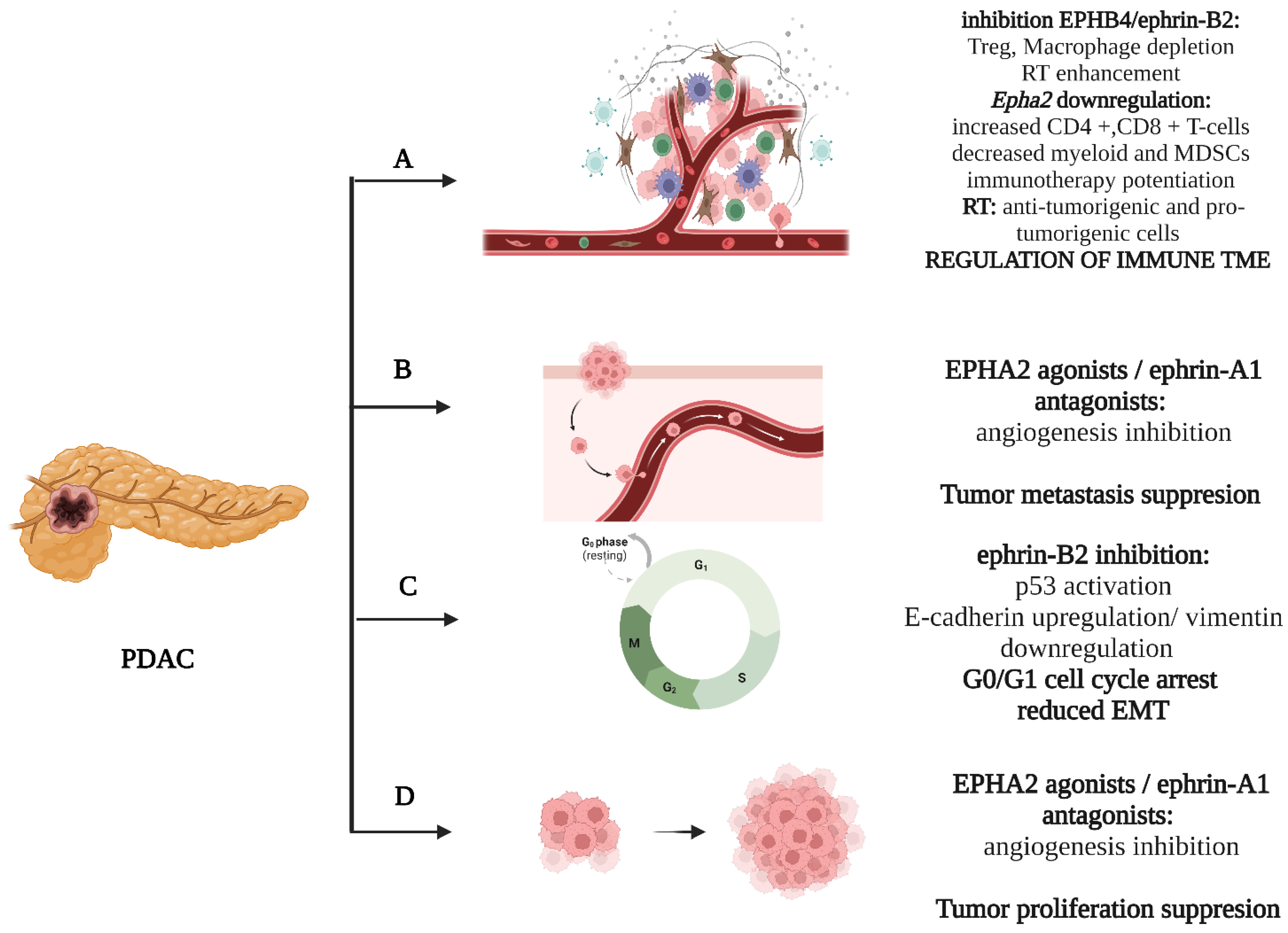
Figure 1. Basic mechanisms influenced by the EPH/ephrin system: (A) RT, EPHA2 and EPHB4/ephrin-B2 shape the immune cellular population of TME. (B) EPHA2/ephrin-A1 influences the metastatic potential of cancer cells. (C) Ephrin-B2 regulates G0/G1 cell cycle transition. (D) EPHA2/ephrin-A1 induce tumor growth. Created with BioRender.com.
3. The EPH/Ephrin System in PDAC—Clinical Data
The significance of the EPH/ephrin system for PDAC became conceivable due to its overexpression in a multitude of studies [15][22][27][28]. EPHA2 is the most clinically relevant member of class A EPH/ephrin signaling. Despite the fact that the earliest references in the literature were restrained regarding its role in PDAC carcinogenesis, only documenting association with patients’ age [27], data concerning its actual impact have begun to emerge [15][28]. Van den Broecket et al. reported that EPHA2 has been overexpressed in PDAC, with unfavorable clinical outcomes [27]. The above finding is in accordance with a clinical study by Nakajima et al., which documented that EPHA2 was expressed in the vast majority of PDAC cases with variable density. EPHA2 was stained principally in the cancer cells and, to a lesser extent, in CAFs. An association with a more invasive tumor phenotype was also documented [20]. Markosyan et al. confirmed via human clinical samples that the EPH/ephrin system is one of the most ubiquitously expressed signaling pathways in T cell noninflamed PDAC, with EPHA2 being the principally expressed gene. The expression of CD8A, CD3, PRF1 and GZMB mRNA levels exhibits a negative association with EPHA2, which collectively suggests that the EPHA2 possesses immune-modifying properties [15].
EPHA2 also displays clinical usefulness as a biomarker; Koshikawa et al. documented an 89.0% sensitivity and 90.0% specificity of soluble EPHA2 fragments in PDAC diagnosis, in opposition with the respective 88.9% and 72.0% of the Ca19-9 [29]. Wei et al. suggested that the combination of serum exosomal EPHA2 with Ca19-9 could potently distinguish early-stage pancreatic cancer (stage I, II) from benign pancreatic disease [30]. The above could reshape the diagnostic management of pancreatic cancer, constituting useful alternatives for population screenings. Finally, monoclonal antibodies against EPHA2 are under clinical investigation without evidences of dose-limiting toxicity or adverse events [31].
Regarding the class B EPH/ephrin system, its importance has been also recognized in human clinical studies. The EPHB4 and ephrin-B2 overexpression shape, in conjunction with several other genes, a more malignant clinical phenotype [28], which is partially parallel to the fact that ephrin-B2′s expression correlates with the TNM Classification of Malignant Tumors (TNM) staging [24]. Ephrin-B2 seems to possess a predictive capacity for patients with a PDAC prognosis who respond to therapy [32]. Analogously, Lu et al. demonstrated that the overexpression of EPHB2 and ephrin-B2 clinically correlated with more aggressive PDAC behavior, as well as with abdominal and back pain [33]. In a recent phosphoproteomics analysis, which mirrored the activation of a multitude of signaling pathways, Renuse et al. reported the existence of 709 proteins with, overall, 1199 loci. EPHB4 in parallel with EPHA2 were identified as the molecules with the most kinase-regulating sites among the EPH/ephrin system [22]. The above points are summarized in Table 1.
Table 1. Published clinical data regarding the EPH/ephrin signaling system.
| EPH/Ephrin | Study Material | Result | References |
|---|---|---|---|
| EPHA1/A2/A4/A5/A7 | Neoplastic tissue | EPHA1 staining intensity was significantly associated with
|
[27] |
| EPHA2 | Neoplastic tissue | EPHA2 was associated with poor outcome and aggressive disease | [21][28] |
| EPHA2 | Soluble EPHA2 fragments | May be applicable as a diagnostic biomarker | [29] |
| EPHA2 | Neoplastic tissue | The expression of EPHA2 was inversely correlated with the degree of T cell infiltration in PDAC | [15] |
| EPHA2 | PC patients | Dasatinib (inhibition of EPHA2) did not show clinical activity in metastatic PDAC | [34] |
| EPHA4 | Neoplastic tissue | EPHA4 positivity was associated with lower overall survival | [35] |
| EPHB2/ephrin-B2 | Neoplastic tissue | Overexpression of EPHB2 and ephrin-B2 was associated with:
|
[33] |
| ephrin-B2 | Neoplastic tissue | High ephrin-B2 expression correlated with:
|
[32] |
| ephrin-B2 | Neoplastic tissue | Lower expression of ephrin-B2 and ADAM10 after neo-adjuvant therapy was associated with better:
|
[36] |
| EPHB4 | PDAC patients | Significant expression of EPHB4 in >70% of patients with PDAC | [17] |
Despite the fact that the above findings may not be urgently transferable to clinical practice, several clinical trials in humans have begun to emerge [31][37][38]. To date, EPHA2 has been the only targeted molecule. Shitara et al. utilized DS-8895a, a humanized IgG1 EPHA2-targeting antibody with the capacity to augment antibody-dependent cellular cytotoxicity, in a phase I study. They demonstrated its safety at the doses utilized, as well as the activation of NK cells [37]. Regardless of the limited drug uptake from normal tissue, further clinical studies did not succeed due to poor biodistribution results [31]. Two more Phase I clinical studies including PDAC patients are currently recruiting. Weston et al. are currently investigating the effects of siRNA-EPHA2 in tumor metabolism and perfusion, utilizing diffusion weighted MRI and 18FDG-PET [39], while Huang et al. explored the efficacy of EPHA2-specific taxane-loaded immunoliposomes [38].
References
- Bartolomé, A.; Suda, N.; Yu, J.; Zhu, C.; Son, J.; Ding, H.; Califano, A.; Accili, D.; Pajvani, U.B. Notch-mediated Ephrin signaling disrupts islet architecture and β cell function. JCI Insight 2022, 7, e157694.
- Pan, F.C.; Wright, C. Pancreas organogenesis: From bud to plexus to gland. Dev. Dyn. 2011, 240, 530–565.
- Dorrell, C.; Schug, J.; Lin, C.F.; Canaday, P.S.; Fox, A.J.; Smirnova, O.; Bonnah, R.; Streeter, P.R.; Stoeckert, C.J.; Kaestner, K.H.; et al. Transcriptomes of the major human pancreatic cell types. Diabetologia 2011, 54, 2832.
- Villasenor, A.; Chong, D.C.; Henkemeyer, M.; Cleaver, O. Epithelial dynamics of pancreatic branching morphogenesis. Development 2010, 137, 4295–4305.
- van Eyll, J.M.; Passante, L.; Pierreux, C.E.; Lemaigre, F.P.; Vanderhaeghen, P.; Rousseau, G.G. Eph receptors and their ephrin ligands are expressed in developing mouse pancreas. Gene Expr. Patterns 2006, 6, 353–359.
- Thestrup, M.I.; Caviglia, S.; Cayuso, J.; Heyne, R.L.S.; Ahmad, R.; Hofmeister, W.; Satriano, L.; Wilkinson, D.G.; Andersen, J.B.; Ober, E.A. A morphogenetic EphB/EphrinB code controls hepatopancreatic duct formation. Nat. Commun. 2019, 10, 5220.
- Maechler, P.; Wollheim, C.B. Mitochondrial function in normal and diabetic β-cells. Nature 2001, 414, 807–812.
- Luther, M.J.; Hauge-Evans, A.; Souza, K.L.A.; Jörns, A.; Lenzen, S.; Persaud, S.J.; Jones, P.M. MIN6 β-cell-β-cell interactions influence insulin secretory responses to nutrients and non-nutrients. Biochem. Biophys. Res. Commun. 2006, 343, 99–104.
- Konstantinova, I.; Nikolova, G.; Ohara-Imaizumi, M.; Meda, P.; Kučera, T.; Zarbalis, K.; Wurst, W.; Nagamatsu, S.; Lammert, E. EphA-Ephrin-A-Mediated β Cell Communication Regulates Insulin Secretion from Pancreatic Islets. Cell 2007, 129, 359–370.
- Hutchens, T.; Piston, D.W. EphA4 receptor forward signaling inhibits glucagon secretion from α-cells. Diabetes 2015, 64, 3839–3851.
- Xiao, T.; Xiao, Y.; Wang, W.; Tang, Y.Y.; Xiao, Z.; Su, M. Targeting EphA2 in cancer. J. Hematol. Oncol. 2020, 13, 114.
- Ogawa, K.; Pasqualini, R.; Lindberg, R.A.; Kain, R.; Freeman, A.L.; Pasquale, E.B. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene 2000, 19, 6043–6052.
- van Geer, M.A.; Bakker, C.T.; Koizumi, N.; Mizuguchi, H.; Wesseling, J.G.; Elferink, R.P.J.O.; Bosma, P.J. Ephrin A2 receptor targeting does not increase adenoviral pancreatic cancer transduction in vivo. World J. Gastroenterol. 2009, 15, 2754–2762.
- Vitelli, A.; Ansuini, H.; Meola, A.; Gunes, Z.; Paradisi, V.; Pezzanera, M.; Acali, S.; Santini, C.; Luzzago, A.; Mori, F.; et al. Anti-EphA2 antibodies with distinct in vitro properties have equal in vivo efficacy in pancreatic cancer. J. Oncol. 2009, 2009, 951917.
- Markosyan, N.; Li, J.; Sun, Y.H.; Richman, L.P.; Lin, J.H.; Yan, F.; Quinones, L.; Sela, Y.; Yamazoe, T.; Gordon, N.; et al. Tumor cell-intrinsic EPHA2 suppresses antitumor immunity by regulating PTGS2 (COX-2). J. Clin. Investig. 2019, 129, 3594–3609.
- Müller-Decker, K.; Fürstenberger, G.; Annan, N.; Kucher, D.; Pohl-Arnold, A.; Steinbauer, B.; Esposito, I.; Chiblak, S.; Friess, H.; Schirmacher, P.; et al. Preinvasive Duct-Derived Neoplasms in Pancreas of Keratin 5–Promoter Cyclooxygenase-2 Transgenic Mice. Gastroenterology 2006, 130, 2165–2178.
- Hill, R.; Li, Y.; Tran, L.M.; Dry, S.; Calvopina, J.H.; Garcia, A.; Kim, C.; Wang, Y.; Donahue, T.R.; Herschman, H.R.; et al. Cell intrinsic role of COX-2 in pancreatic cancer development. Mol. Cancer Ther. 2012, 11, 2127–2137.
- Philip, B.; Roland, C.L.; Daniluk, J.; Liu, Y.; Chatterjee, D.; Gomez, S.B.; Ji, B.; Huang, H.; Wang, H.; Fleming, J.B.; et al. A High-Fat Diet Activates Oncogenic Kras and COX2 to Induce Development of Pancreatic Ductal Adenocarcinoma in Mice. Gastroenterology 2013, 145, 1449–1458.
- Conejo-Garcia, J.R. Breaking barriers for T cells by targeting the EPHA2/ TGF-β/COX-2 axis in pancreatic cancer. J. Clin. Investig. 2019, 129, 3521–3523.
- Zhou, S.; Hu, T.; Han, G.; Wu, Y.; Hua, X.; Su, J.; Jin, W.; Mou, Y.; Mou, X.; Li, Q.; et al. Accurate Cancer Diagnosis and Stage Monitoring Enabled by Comprehensive Profiling of Different Types of Exosomal Biomarkers: Surface Proteins and miRNAs. Small 2020, 16, e2004492.
- Nakajima, K.; Ino, Y.; Naito, C.; Nara, S.; Shimasaki, M.; Ishimoto, U.; Iwasaki, T.; Doi, N.; Esaki, M.; Kishi, Y.; et al. Neoadjuvant therapy alters the collagen architecture of pancreatic cancer tissue via Ephrin-A5. Br. J. Cancer 2022, 126, 628–639.
- Renuse, S.; Madamsetty, V.S.; Mun, D.G.; Madugundu, A.K.; Singh, S.; Udainiya, S.; Mangalaparthi, K.K.; Kim, M.S.; Liu, R.; Kumar, S.R.; et al. Tyrosine phosphoproteomics of patient-derived xenografts reveals ephrin type-b receptor 4 tyrosine kinase as a therapeutic target in pancreatic cancer. Cancers 2021, 13, 3404.
- Djokovic, D.; Trindade, A.; Gigante, J.; Badenes, M.; Silva, L.; Liu, R.; Li, X.; Gong, M.; Krasnoperov, V.; Gill, P.S.; et al. Combination of Dll4/Notch and Ephrin-B2/EphB4 targeted therapy is highly effective in disrupting tumor angiogenesis. BMC Cancer 2010, 10, 641.
- Zhu, F.; Dai, S.N.; Xu, D.L.; Hou, C.Q.; Liu, T.T.; Chen, Q.Y.; Wu, J.L.; Miao, Y. EFNB2 facilitates cell proliferation, migration, and invasion in pancreatic ductal adenocarcinoma via the p53/p21 pathway and EMT. Biomed. Pharmacother. 2020, 125, 109972.
- Demaria, S.; Coleman, C.N.; Formenti, S.C. Radiotherapy: Changing the Game in Immunotherapy. Trends Cancer 2016, 2, 286–294.
- Lennon, S.; Oweida, A.; Milner, D.; Phan, A.V.; Bhatia, S.; Van Court, B.; Darragh, L.; Mueller, A.C.; Raben, D.; Martínez-Torrecuadrada, J.L.; et al. Pancreatic tumor microenvironment modulation by EphB4-ephrinB2 inhibition and radiation combination. Clin. Cancer Res. 2019, 25, 3352–3365.
- Giaginis, C.; Tsourouflis, G.; Zizi-Serbetzoglou, A.; Kouraklis, G.; Chatzopoulou, E.; Dimakopoulou, K.; Theocharis, S.E. Clinical Significance of Ephrin (Eph)-A1, -A2, -A4, -A5 and -A7 Receptors in Pancreatic Ductal Adenocarcinoma. Pathol. Oncol. Res. 2010, 16, 267–276.
- Van Den Broeck, A.; Vankelecom, H.; Van Eijsden, R.; Govaere, O.; Topal, B. Molecular markers associated with outcome and metastasis in human pancreatic cancer. J. Exp. Clin. Cancer Res. 2012, 31, 68.
- Koshikawa, N.; Minegishi, T.; Kiyokawa, H.; Seiki, M. Specific detection of soluble EphA2 fragments in blood as a new biomarker for pancreatic cancer. Cell Death Dis. 2017, 8, e3134.
- Wei, Q.; Zhang, J.; Li, Z.; Wei, L.; Ren, L. Serum Exo-EphA2 as a Potential Diagnostic Biomarker for Pancreatic Cancer. Pancreas 2020, 49, 1213–1219.
- Gan, H.K.; Parakh, S.; Lee, F.T.; Tebbutt, N.C.; Ameratunga, M.; Lee, S.T.; O’Keefe, G.J.; Gong, S.J.; Vanrenen, C.; Caine, J.; et al. A phase 1 safety and bioimaging trial of antibody DS-8895a against EphA2 in patients with advanced or metastatic EphA2 positive cancers. Investig. New Drugs 2022, 40, 747–755.
- Oweida, A.; Bhatia, S.; Hirsch, K.; Calame, D.; Griego, A.; Keysar, S.; Pitts, T.; Sharma, J.; Eckhardt, G.; Jimeno, A.; et al. Ephrin-B2 overexpression predicts for poor prognosis and response to therapy in solid tumors. Mol. Carcinog. 2017, 56, 1189–1196.
- Lu, Z.; Zhang, Y.; Li, Z.; Yu, S.; Zhao, G.; Li, M.; Wang, Z.; Wang, Q.; Yang, Y. Overexpression of the B-type Eph and ephrin genes correlates with progression and pain in human pancreatic cancer. Oncol. Lett. 2012, 3, 1207–1212.
- Chee, C.E.; Krishnamurthi, S.; Nock, C.J.; Meropol, N.J.; Gibbons, J.; Fu, P.; Bokar, J.; Teston, L.; O’Brien, T.; Gudena, V.; et al. Phase II Study of Dasatinib (BMS-354825) in Patients With Metastatic Adenocarcinoma of the Pancreas. Oncologist 2013, 18, 1091–1092.
- Takano, H.; Nakamura, T.; Tsuchikawa, T.; Kushibiki, T.; Hontani, K.; Inoko, K.; Takahashi, M.; Sato, S.; Abe, H.; Takeuchi, S.; et al. Inhibition of Eph receptor A4 by 2,5-dimethylpyrrolyl benzoic acid suppresses human pancreatic cancer growing orthotopically in nude mice. Oncotarget 2015, 6, 41063–41076.
- Mueller, A.C.; Piper, M.; Goodspeed, A.; Bhuvane, S.; Williams, J.S.; Bhatia, S.; Phan, A.V.; Van Court, B.; Zolman, K.L.; Peña, B.; et al. Induction of ADAM10 by Radiation Therapy Drives Fibrosis, Resistance, and Epithelial-to-Mesenchyal Transition in Pancreatic Cancer. Cancer Res. 2021, 81, 3255–3269.
- Shitara, K.; Satoh, T.; Iwasa, S.; Yamaguchi, K.; Muro, K.; Komatsu, Y.; Nishina, T.; Esaki, T.; Hasegawa, J.; Kakurai, Y.; et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the afucosylated, humanized anti-EPHA2 antibody DS-8895a: A first-in-human phase i dose escalation and dose expansion study in patients with advanced solid tumors. J. Immunother. Cancer 2019, 7, 219.
- Huang, Z.R.; Tipparaju, S.K.; Kirpotin, D.B.; Pien, C.; Kornaga, T.; Noble, C.O.; Koshkaryev, A.; Tran, J.; Kamoun, W.S.; Drummond, D.C. Formulation optimization of an ephrin A2 targeted immunoliposome encapsulating reversibly modified taxane prodrugs. J. Control. Release 2019, 310, 47–57.
- Wagner, M.J.; Mitra, R.; Mcarthur, M.J.; Baze, W.; Barnhart, K.; Wu, S.Y.; Rodriguez-Aguayo, C.; Zhang, X.; Coleman, R.L.; Lopez-Berestein, G.; et al. Preclinical Mammalian Safety Studies of EPHARNA (DOPC Nanoliposomal EphA2-Targeted siRNA). Mol. Cancer Ther. 2017, 16, 1114–1123.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
977
Entry Collection:
Gastrointestinal Disease
Revisions:
2 times
(View History)
Update Date:
04 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




