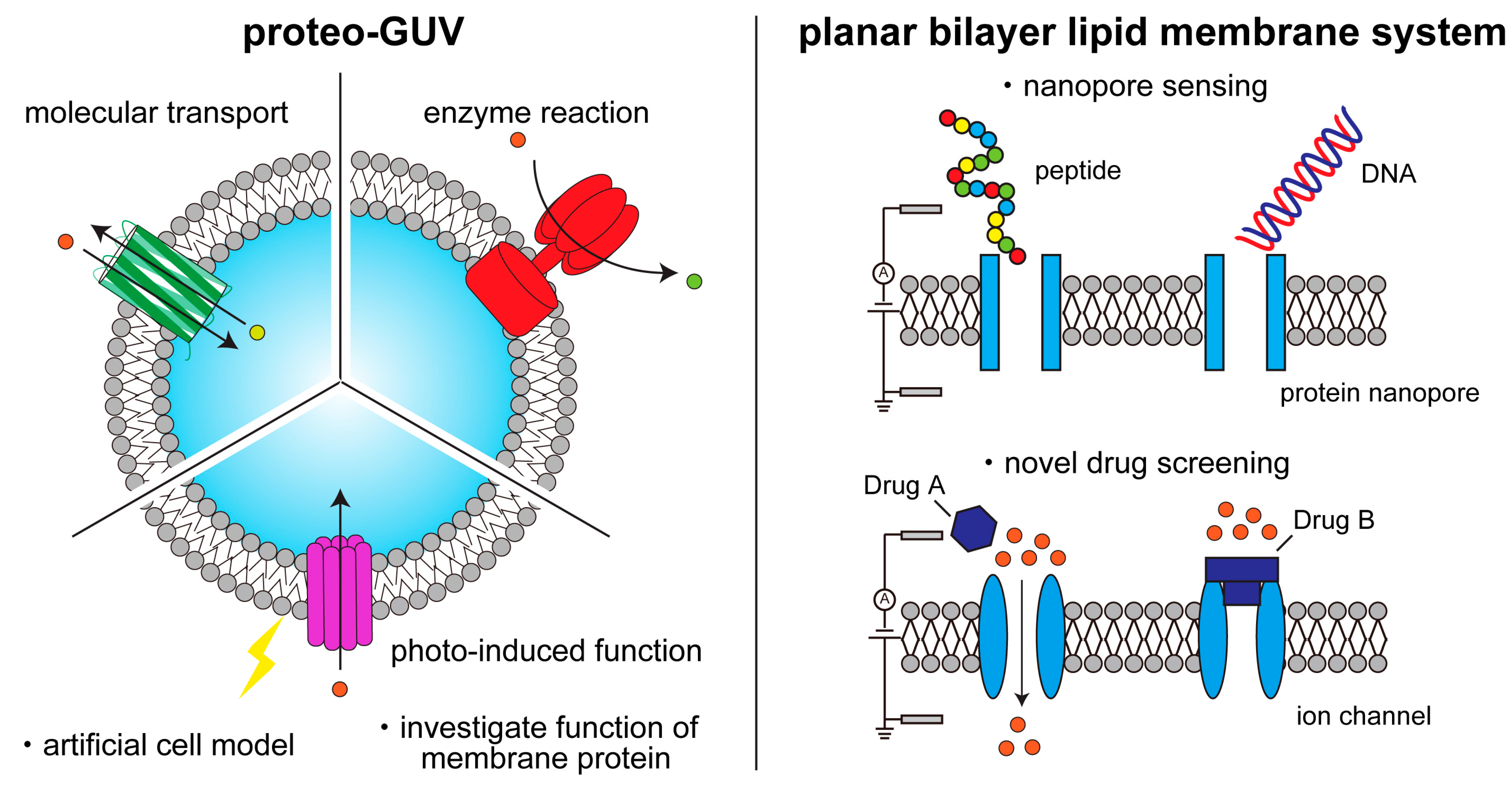
Membrane proteins play an important role in key cellular functions, such as signal transduction, apoptosis, and metabolism. Therefore, structural and functional studies of these proteins are essential in fields such as fundamental biology, medical science, pharmacology, biotechnology, and bioengineering.
1. Introduction
Membrane proteins are a vital component of the cell membrane, which is composed of a phospholipid bilayer. They play a critical role in various cellular functions, such as molecular transportation, conversion, production, and transduction, including signal transduction, apoptosis, and metabolism
[1][2][3][4][5][6][7][8][9][10][11]. Different types of membrane proteins on the cell membrane interact to activate these functions. G protein-coupled receptors (GPCR) and ion channels, which are types of membrane proteins, are important targets for drugs used to treat diseases such as cancer, neurodegenerative disease, metabolic disease and cardiovascular disease
[12][13][14][15][16][17]. However, investigating the precise elemental reaction and structure of a membrane protein in complex cell reactions activated by several types of membrane proteins can be challenging. To overcome this issue, methodologies for investigating the function of membrane proteins purified from biological cells have been developed. In the early 1970s, proteoliposomes were first developed by reconstituting the Ca
2+-dependent ATPase of the sarcoplasmic reticulum into nano-sized liposomes
[18][19]. In the late 1980s, the proteoliposomes with K
+-channels of the sarcoplasmic reticulum were generated using the cell-sized liposomes with diameters of approximately 10 μm, due to the measurement of single ion channel current
[20]. Recently, cell-sized proteoliposomes with two or three types of membrane proteins were formed to connect each membrane protein reaction
[21][22][23]. Purified membrane proteins have been crystallized to reveal their structures, and they have been reconstituted into artificial cell membranes, such as liposomes, planar lipid bilayers, and bicelles, to observe their functions
[24][25][26]. These artificial cell membranes consist of phospholipid bilayers, and the structure and function of the membrane proteins reconstituted into them have been identified using various techniques, such as NMR, fluorescence activated cell sorter, cryo-electron microscopy (cryo-EM), fluorescence spectrophotometry, patch clamp method (for nanopores or ion channels), and optical microscopy, including fluorescence microscopy, and confocal microscopy
[25][27][28][29][30][31][32][33][34]. These investigations have not only revealed the functions of membrane proteins but also led to the development of biological sensors using membrane proteins because of their highly specific interactions with ligands and membrane proteins
[35][36][37]. Thus, studies of membrane proteins can have a wide range of applications (
Figure 1).
Figure 1. Schematic illustration of functional studies and applications of membrane proteins on artificial lipid membranes.
2. Formation of Cell-Sized Lipid Vesicles
Membrane proteins are typically reconstituted into artificial cell membranes such as lipid vesicles, liposomes, and bicelles to maintain their structure and function. In this section, the researchers introduce methods for forming lipid vesicles and liposomes, from conventional techniques to recent microfluidic approaches. Liposomes and lipid vesicles, discovered by Bangham in the 1960s, consist of a phospholipid bilayer
[38]. Conventional methods for liposome formation include gentle hydration and electroformation
[39][40][41][42][43]. With the gentle hydration method, phospholipids dissolved in chloroform are added to a glass microtube, and the films are formed by evaporating the chloroform under flowing argon or nitrogen gas. The lipid films are then hydrated with an aqueous solution (pure water or buffer solution), forming giant liposomes with diameters of 1–100 μm. Nano-sized liposomes are obtained by vortexing or sonication of the lipid films
[1]. With the electroformation method, a lipid film is formed on the surface of an indium tin oxide (ITO)-coated glass. An alternating current (AC) electric field is applied to the lipid films containing a hydrated solution (pure water or a buffer solution with low salt concentration) to enhance lipid hydration. Although the giant liposomes formed by the electroformation method have more unilamellar membranes than those formed by the gentle hydration method, the former has lower liposome production than the latter. Methods have been developed to improve the drawbacks of both the gentle hydration and electroformation methods. These conventional liposome formation methods have been used for studying membrane dynamics, functional properties of membrane proteins, and encapsulation of biological reactions.
The gentle hydration and electroformation methods are limited in their ability to produce liposomes with uniform sizes, in their high encapsulation efficiencies, and in their asymmetric lipid distributions. Microfluidic technologies have been integrated into liposome formation to overcome these limitations and achieve a uniform size and high encapsulation efficiency. The droplet emulsion transfer method, developed by Pautot et al.
[44][45], is the basis for many of the liposome formation techniques using microfluidic technologies. The liposome formation procedure with the droplet emulsion transfer method is as follows: First, water-in-oil (w/o) emulsions are generated by vortexing or sonication of buffer solution and phospholipid solution dissolved in an organic solvent such as mineral oil or
n-decane. The w/o emulsions are then added to the oil phase in a microtube. When the w/o emulsions are transferred to the lipid monolayer between the oil phase and the aqueous phase, liposomes are generated. Asymmetric lipid membranes between the outer leaflet and the inner leaflet can be generated by changing the lipid compositions of the w/o emulsions and the lipid monolayer. By applying the droplet emulsion transfer method, many high-throughput methods for forming liposomes using microfluidic devices have been developed
[46][47][48][49][50][51][52][53]. These methods integrate the system of w/o emulsion generation and emulsion transfer from the oil phase to the aqueous phase. Like other methods of liposome formation using microfluidic devices, a new approach has been developed that creates single or multicompartment liposomes by utilizing the de-wetting phenomena of a water-in-oil-in-water double emulsion using a coaxial microcapillary fluidic device
[54][55][56][57][58][59][60][61][62].
Another method for forming asymmetric lipid vesicles without an organic solvent (
n-decane) between the lipid bilayer is by applying a pulsed jet flow against a planar lipid bilayer in a double-well device (called the pulsed-jet flow method)
[63][64]. The lipid bilayer of liposomes formed by the microfluidic technology method is normally contaminated with an organic solvent, which can cause instability of the liposomes. In contrast, liposomes formed by the pulsed-jet flow method have long-term stability (up to one week). The flip-flop phenomenon and membrane protein reconstitution have been investigated on the asymmetric lipid vesicles formed by this method
[64][65]. Modification of the double-well device allows for the formation of nano-sized asymmetric lipid vesicles
[66], automatic formation of liposomes
[67][68], and the formation of vesicles-in-a-vesicle structures that mimic the asymmetric lipid composition of plasma membranes and intracellular vesicle membranes
[69]. Multiple enzyme reactions can be created within the liposomes formed by microfluidic devices
[70]. A complex of cell-penetrating peptides and water-soluble proteins can interact with asymmetric lipid vesicles containing negatively charged lipids on the inner leaflet, and the water-soluble proteins can be transferred into the asymmetric lipid vesicles
[71].
3. Methodology of Reconstitution of Membrane Proteins into Cell-Sized Artificial Membrane
Giant unilamellar vesicles (GUVs) are widely used as models of artificial cells
[72][73]. To understand complex biological phenomena in cells, membrane protein function has been studied by reconstituting membrane proteins into GUVs. Various methods have been developed for this, including rehydration, membrane fusion, detergent removal, and direct insertion (
Table 1)
[74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90].
Table 1. Selected examples of membrane proteins reconstitution in GUVs.