Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Qingfei Zheng | -- | 1541 | 2023-05-01 20:46:03 | | | |
| 2 | Conner Chen | Meta information modification | 1541 | 2023-05-04 03:00:25 | | | | |
| 3 | Conner Chen | -1 word(s) | 1540 | 2023-05-04 10:02:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zheng, Q. Enzymatic Functions of DJ-1. Encyclopedia. Available online: https://encyclopedia.pub/entry/43662 (accessed on 08 February 2026).
Zheng Q. Enzymatic Functions of DJ-1. Encyclopedia. Available at: https://encyclopedia.pub/entry/43662. Accessed February 08, 2026.
Zheng, Qingfei. "Enzymatic Functions of DJ-1" Encyclopedia, https://encyclopedia.pub/entry/43662 (accessed February 08, 2026).
Zheng, Q. (2023, May 01). Enzymatic Functions of DJ-1. In Encyclopedia. https://encyclopedia.pub/entry/43662
Zheng, Qingfei. "Enzymatic Functions of DJ-1." Encyclopedia. Web. 01 May, 2023.
Copy Citation
DJ-1 (also known as PARK7) is a multifunctional enzyme in human beings that is highly conserved and that has also been discovered in diverse species (ranging from prokaryotes to eukaryotes). Its complex enzymatic and non-enzymatic activities (such as anti-oxidation, anti-glycation, and protein quality control), as well as its role as a transcriptional coactivator, enable DJ-1 to serve as an essential regulator in multiple cellular processes (e.g., epigenetic regulations) and make it a promising therapeutic target for diverse diseases (especially cancer and Parkinson’s disease).
DJ-1/PARK7
multifunctional enzyme
cancer
Parkinson's disease
therapeutic target
1. Introduction
The human gene DJ-1 (PARK7), which contains eight exons, locates at the chromosome band 1p36.23 and encodes a protein (named as DJ-1 or PARK7) composed of 189 amino acids, with seven β-strands and nine α-helices, and which belongs to the peptidase C56 family [1]. Biochemical and structural evidence has shown that DJ-1 functions as a homodimer [2][3][4][5]. Homologous genes of human DJ-1 have been variously discovered from microorganisms, plants, and other animals [6][7]. For example, DJ-1 is evolutionarily conserved within Escherichia coli chaperones (i.e., Hsp31, YhbO, and YajL) and archaea proteases [8][9]. Biochemical and structural studies have been conducted on DJ-1 variants from different organisms [10][11], which revealed its diverse physiological and pathological functions [1].
It has long been known that DJ-1 demonstrates an essential antioxidant activity in human cells, as a key protector against cellular oxidative stress, which is attributed to its three redox-active cysteine (Cys) residues (i.e., Cys43, Cys54, and Cys106) [12]. The redox activity of DJ-1 enables it to act as an important regulator in cells, against oxidative stress [1]. Recent research advances from one lab [13][14][15][16][17][18][19] and other groups [20][21][22][23][24][25][26][27][28][29] have indicated that DJ-1 is a scavenger enzyme, not only for quenching reactive oxygen species (ROS) [12], but also for reactive carbonyl species (RCS), which include methylglyoxal (MGO) and glyoxal (GO). Moreover, DJ-1 exhibits other enzymatic activities, such as glyoxalase [29][30], deglycase [20][25], peptidase/protease [31][32][33], and esterase [30][34] functions, which are important in maintaining cellular protein homeostasis [1]. Besides these enzymatic activities, DJ-1 also acts as a transcriptional coactivator, regulating gene transcription, without directly binding any promoters [35]. This non-enzymatic activity of DJ-1 relies on its non-covalent interactions with other nuclear proteins, such as p54nrb and pyrimidine tract-binding protein-associated splicing factor (PSF) [36].
Given its diverse activities, DJ-1 is believed to be involved in a number of physiological and pathological processes [1][37][38][39] and can serve as a druggable target for many types of disease [1][40][41][42]. For instance, DJ-1 has been found to be overexpressed in multiple types of cancer (especially those with high malignancy grades), and early in 1997 DJ-1 was identified as a novel oncoprotein that could transform cells in corporation with activated Ras [43]. Previous studies have also shown that loss of DJ-1 function leads to neurodegeneration, such as autosomal recessive early-onset parkinsonism [44]. Notably, mutations of the DJ-1 gene, including both deletion and substitution mutations, have been found in Parkinson’s disease (PD) patients [45], suggesting that DJ-1 plays significant roles in the brain neuronal maintenance and pathogenesis of PD. Taken together, DJ-1 is a noteworthy target for developing novel therapeutic and diagnostic strategies in both biomedical (e.g., cancer treatment) and psychological (e.g., early diagnosis of PD) research, leading to the establishment of high-throughput screening assays for identifying DJ-1 agonists and inhibitors [46].
2. Enzymatic Functions of DJ-1
DJ-1 is a small protein (~20 kDa) that belongs to the large multi-clade DJ-1 (also named as DJ-1/PfpI, ThiJ/PfpI, or DJ-1/ThiJ/PfpI) superfamily, which includes many known chaperones, proteases, and stress-response proteins [47]. The diverse enzymatic activities of DJ-1 (Figure 1) are attributed to its key catalytic residue, Cys106. Previous studies have shown that DJ-1 is structurally conserved within cysteine proteases (such as PfpI), while in vitro biochemical assays failed to detect any protease activity for purified full-length DJ-1 [2]. Further research indicated that DJ-1 could convert from a zymogen to an active protease, through carboxyl-terminal cleavage of a 15-amino acid peptide, and that its catalytic dyad contains Cys106 and His126 [31]. Notably, C-terminally cleaved DJ-1 with cysteine protease activity exhibits enhanced cytoprotective action against oxidative stress-induced apoptosis, while this cytoprotective function of DJ-1 is abolished by C106A or H126A mutations [31]. Furthermore, the protease activity of DJ-1 lacking a C-terminal α-helix (i.e., DJ-1ΔH9) was shown to be remarkable, and the most susceptible sequence digested by DJ-1ΔH9 was valine-lysine-valine-alanine (VKVA), while divalent ions (especially Cu2+) significantly inhibited DJ-1’s protease activity [33]. In addition to protein substrates, DJ-1 is capable of recognizing ester substrates and hydrolyzing them into acids and alcohols/phenols [34]. The esterase activity of DJ-1 has been applied for high-throughput screening of its inhibitors, where the oxyester compounds are utilized as substrates (Figure 1) [46].
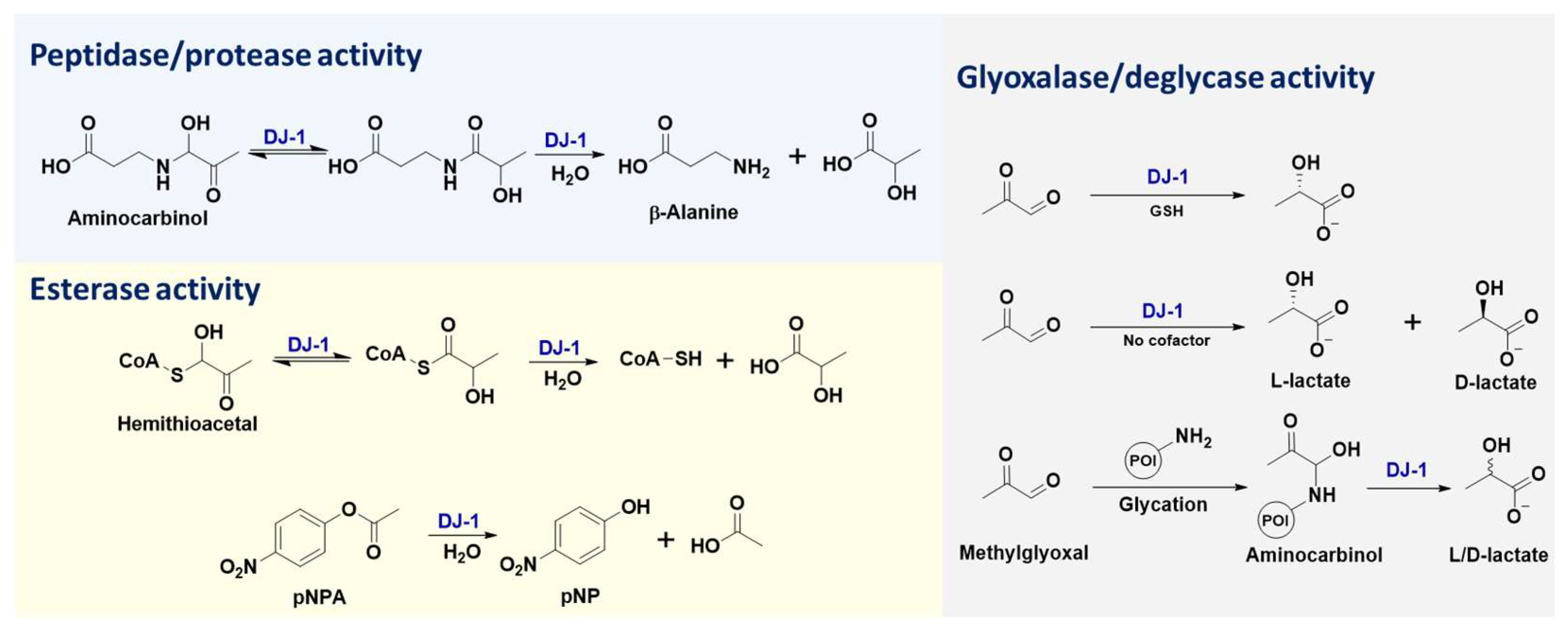
The extension of DJ-1’s esterase activity enables its glyoxalase activity, where the substrate is the thioester formed by glutathione (GSH) and dicarbonyl compounds (e.g., MGO and GO). In this enzymatic reaction, GSH is believed to serve as a cofactor of DJ-1 and activate the aldehyde substrates (Figure 1) [30]. Distinct from the glyoxalase activity of the GLO1–GLO2 system, the product of DJ-1 in the presence of GSH is L-lactate rather than D-lactate [19][30], which is attributed to the different enzyme microenvironments for catalytic stereoselectivity. Based on the same biochemical mechanism and esterase activity of DJ-1, other small molecule thiols (e.g., coenzyme A) can also act as the cofactor in this enzymatic process [30]. When the MGO activator is replaced, from thiols to primary amines (such as β-alanine), the resulting intermediates (i.e., aminocarbinal and imine) can also be enzymatically converted into L-lactate by DJ-1, due to its peptidase/protease activity (Figure 1). Similarly, the extension of DJ-1’s peptidase/protease activity enables its robust deglycase function [9][13][18][19][20][21][22][23][24][25][26][27], where DJ-1 removes MGO from the modified amino acid or nucleotide residues (such as lysine, arginine, cysteine, guanine, etc.) of the target biomacromolecules (including histones, DJ-1 itself, DNA, RNA, etc.) and transforms them into both L- and D-lactate (Figure 1) [19]. Even though a debate over DJ-1’s mechanism of glycation repair has emerged recently [48], characterization of the absolute configurations of its by-products (i.e., L- and D-lactate) [48] provided direct chemical evidence to support the fact that DJ-1 exhibits both glyoxalase and deglycase activities [19][48]. More intriguingly, the C106-based “scavenger activity” of DJ-1 was also reported to prevent the metabolite and protein damage caused by a glycolytic metabolite, 1,3-bisphosphoglycerate (1,3-BPG) [49]. This novel scavenger activity of DJ-1 might be another extension of its esterase or deglycase activity. Therefore, deglycase-activity oriented high-throughput screening has been conducted to identify DJ-1 inhibitors [46]. Taken together, all the aforementioned enzymatic functions of DJ-1 are attributed to the nucleophilicity of its catalytic residue, Cys106, resulting in its intrinsic redox-sensitivity to the cellular microenvironment (such as the concentration of ROS).
Regardless of the substrate size being small or large [48], it is a fact that DJ-1 enzymatically converts MGO and GO into L/D-lactate [19] and glycolate [18], respectively. The biosynthetic pathways mediated by DJ-1 for producing lactate and glycolate from reducing sugars enable DJ-1 to perform a critical metabolic role in regulating cellular functions. For example, lactate is highly enriched in the tumor microenvironment and acts as a pH regulator and signaling molecule [50]. Even though lactate has long been considered merely a dead-end waste product of glycolysis [51], it has been shown to play a constructive role in regulating basic cellular functions, though serving as a donor for protein post-translational modifications (PTMs) [52][53]. This newly identified epigenetic marker (named lactylation), which occurs in histone lysine residues, is a reversible and dynamic process, regulating gene expression [52]. Previous works showed that histone lactylation could be induced by p300 and removed by a series of histone deacetylases (HDACs) [54]. The generation of lactate by DJ-1 from MGO represents upper stream pathway regulating protein lactylation. MGO is a significant by-product of glycolysis via the spontaneous dephosphorylation of glyceraldehyde-3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP) [30]. Moreover, the generation of glycolate from GO by DJ-1 provides a novel pathway linking the metabolism of carbohydrates, ascorbate, proteins, and lipids. Notably, endogenous GO is generated from the autoxidation of carbohydrates and ascorbate, degradation of glycated proteins, and lipid peroxidation [55]. As one of the major precursors of GO, glucose can be either directly converted to GO through the retro-aldol cleavage reaction or indirectly transformed into GO via a glycoaldehyde intermediate that undergoes autoxidation [56]. The conversion from GO into glycolate by DJ-1 is believed to serve as a detoxification pathway and an important source for producing endogenous glyoxylate and oxalate [57]. In addition, the MGO and GO in human bodies can also originate from food intake (such as beer, wine, tea, coffee, yogurt, bread, rice, soybean paste, soy sauce, honey, and oil) and environmental sources (including cigarette smoke, smoke from residential log fires, vehicle exhaust, smog, fog, and some household cleaning products) [57]. Notably, fermented, roasted, baked, and fried foods are a particularly rich source of GO. The consumption of toxic dicarbonyl compounds (i.e., MGO and GO) in multiple ways represents a major role of DJ-1 in cellular metabolism. Another interesting metabolic function of DJ-1 is consuming 1,3-BPG [49], which is an important intermediate in both glycolysis during respiration and the Calvin cycle during photosynthesis. The enzymatic activity of DJ-1 against 1,3-BPG provides a unique feedback mechanism regulating glycolysis, which can further reduce the amount of MGO in cells.
References
- Ariga, H.; Iguchi-Ariga, S.M. DJ-1/PARK7 Protein: Parkinson’s Disease, Cancer and Oxidative Stress-Induced Diseases; Springer Nature Singapore Pte Ltd.: Singapore, 2017; Volume 1037.
- Wilson, M.A.; Collins, J.L.; Hod, Y.; Ringe, D.; Petsko, G.A. The 1.1-Å resolution crystal structure of DJ-1, the protein mutated in autosomal recessive early onset Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 9256–9261.
- Honbou, K.; Suzuki, N.N.; Horiuchi, M.; Niki, T.; Taira, T.; Ariga, H.; Inagaki, F. The crystal structure of DJ-1, a protein related to male fertility and Parkinson’s disease. J. Biol. Chem. 2003, 278, 31380–31384.
- Tao, X.; Tong, L. Crystal structure of human DJ-1, a protein associated with early onset Parkinson’s disease. J. Biol. Chem. 2003, 278, 31372–31379.
- Huai, Q.; Sun, Y.; Wang, H.; Chin, L.S.; Li, L.; Robinson, H.; Ke, H. Crystal structure of DJ-1/RS and implication on familial Parkinson’s disease. FEBS Lett. 2003, 549, 171–175.
- Culleton, B.A.; Lall, P.; Kinsella, G.K.; Doyle, S.; McCaffrey, J.; Fitzpatrick, D.A.; Burnell, A.M. A role for the Parkinson’s disease protein DJ-1 as a chaperone and antioxidant in the anhydrobiotic nematode Panagrolaimus superbus. Cell Stress Chaperones 2015, 20, 121–137.
- Lin, J.; Nazarenus, T.J.; Frey, J.L.; Liang, X.; Wilson, M.A.; Stone, J.M. A plant DJ-1 homolog is essential for Arabidopsis thaliana chloroplast development. PLoS ONE 2011, 6, e23731.
- Lee, S.J.; Kim, S.J.; Kim, I.K.; Ko, J.; Jeong, C.S.; Kim, G.H.; Park, C.; Kang, S.O.; Suh, P.G.; Lee, H.S.; et al. Crystal structures of human DJ-1 and Escherichia coli Hsp31, which share an evolutionarily conserved domain. J. Biol. Chem. 2003, 278, 44552–44559.
- Richarme, G.; Abdallah, J.; Mathas, N.; Gautier, V.; Dairou, J. Further characterization of the Maillard deglycase DJ-1 and its prokaryotic homologs, deglycase 1/Hsp31, deglycase 2/YhbO, and deglycase 3/YajL. Biochem. Biophys. Res. Commun. 2018, 503, 703–709.
- Hall, C.I.; Reese, M.L.; Weerapana, E.; Child, M.A.; Bowyer, P.W.; Albrow, V.E.; Haraldsen, J.D.; Phillips, M.R.; Sandoval, E.D.; Ward, G.E.; et al. Chemical genetic screen identifies Toxoplasma DJ-1 as a regulator of parasite secretion, attachment, and invasion. Proc. Natl. Acad. Sci. USA 2011, 108, 10568–10573.
- Child, M.A.; Garland, M.; Foe, I.; Madzelan, P.; Treeck, M.; van der Linden, W.A.; Oresic Bender, K.; Weerapana, E.; Wilson, M.A.; Boothroyd, J.C.; et al. Toxoplasma DJ-1 regulates organelle secretion by a direct interaction with calcium-dependent protein kinase 1. mBio 2017, 8, e02189-16.
- Kahle, P.J.; Waak, J.; Gasser, T. DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic. Biol. Med. 2009, 47, 1354–1361.
- Zheng, Q.; Omans, N.D.; Leicher, R.; Osunsade, A.; Agustinus, A.S.; Finkin-Groner, E.; D’Ambrosio, H.; Liu, B.; Chandarlapaty, S.; Liu, S.; et al. Reversible histone glycation is associated with disease-related changes in chromatin architecture. Nat. Commun. 2019, 10, 1289.
- Zheng, Q.; Prescott, N.A.; Maksimovic, I.; David, Y. (De)Toxifying the epigenetic code. Chem. Res. Toxicol. 2019, 32, 796–807.
- Zheng, Q.; Maksimovic, I.; Upad, A.; Guber, D.; David, Y. Synthesis of an alkynyl methylglyoxal probe to investigate nonenzymatic histone glycation. J. Org. Chem. 2020, 85, 1691–1697.
- Zheng, Q.; Maksimovic, I.; Upad, A.; David, Y. Non-enzymatic covalent modifications: A new link between metabolism and epigenetics. Protein Cell 2020, 11, 401–416.
- Zheng, Q.; Osunsade, A.; David, Y. Protein arginine deiminase 4 antagonizes methylglyoxal-induced histone glycation. Nat. Commun. 2020, 11, 3241.
- Ray, D.M.; Jennings, E.Q.; Maksimovic, I.; Chai, X.; Galligan, J.J.; David, Y.; Zheng, Q. Chemical labeling and enrichment of histone glyoxal adducts. ACS Chem. Biol. 2022, 17, 756–761.
- Zhou, X.; Zhang, N.; Hossain, F.; Kandalai, S.; Tian, H.; Zheng, Q. Biosynthesis of D/L-lactate from methylglyoxal. Tetrahedron 2022, 127, 133087.
- Richarme, G.; Mihoub, M.; Dairou, J.; Bui, L.C.; Leger, T.; Lamouri, A. Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues. J. Biol. Chem. 2015, 290, 1885–1897.
- Mihoub, M.; Abdallah, J.; Gontero, B.; Dairou, J.; Richarme, G. The DJ-1 superfamily member Hsp31 repairs proteins from glycation by methylglyoxal and glyoxal. Biochem. Biophys. Res. Commun. 2015, 463, 1305–1310.
- Abdallah, J.; Mihoub, M.; Gautier, V.; Richarme, G. The DJ-1 superfamily members YhbO and YajL from Escherichia coli repair proteins from glycation by methylglyoxal and glyoxal. Biochem. Biophys. Res. Commun. 2016, 470, 282–286.
- Advedissian, T.; Deshayes, F.; Poirier, F.; Viguier, M.; Richarme, G. The Parkinsonism-associated protein DJ-1/Park7 prevents glycation damage in human keratinocyte. Biochem. Biophys. Res. Commun. 2016, 473, 87–91.
- Richarme, G.; Marguet, E.; Forterre, P.; Ishino, S.; Ishino, Y. DJ-1 family Maillard deglycases prevent acrylamide formation. Biochem. Biophys. Res. Commun. 2016, 478, 1111–1116.
- Richarme, G.; Dairou, J. Parkinsonism-associated protein DJ-1 is a bona fide deglycase. Biochem. Biophys. Res. Commun. 2017, 483, 387–391.
- Richarme, G.; Liu, C.; Mihoub, M.; Abdallah, J.; Leger, T.; Joly, N.; Liebart, J.C.; Jurkunas, U.V.; Nadal, M.; Bouloc, P.; et al. Guanine glycation repair by DJ-1/Park7 and its bacterial homologs. Science 2017, 357, 208–211.
- Mihoub, M.; Abdallah, J.; Richarme, G. Protein repair from glycation by glyoxals by the DJ-1 family Maillard deglycases. Adv. Exp. Med. Biol. 2017, 1037, 133–147.
- Mencke, P.; Boussaad, I.; Romano, C.D.; Kitami, T.; Linster, C.L.; Krüger, R. The role of DJ-1 in cellular metabolism and pathophysiological implications for Parkinson’s disease. Cells 2021, 10, 347.
- Lee, J.-y.; Song, J.; Kwon, K.; Jang, S.; Kim, C.; Baek, K.; Kim, J.; Park, C. Human DJ-1 and its homologs are novel glyoxalases. Hum. Mol. Genet. 2012, 21, 3215–3225.
- Matsuda, N.; Kimura, M.; Queliconi, B.B.; Kojima, W.; Mishima, M.; Takagi, K.; Koyano, F.; Yamano, K.; Mizushima, T.; Ito, Y.; et al. Parkinson’s disease-related DJ-1 functions in thiol quality control against aldehyde attack in vitro. Sci. Rep. 2017, 7, 12816.
- Chen, J.; Li, L.; Chin, L.-S. Parkinson disease protein DJ-1 converts from a zymogen to a protease by carboxyl-terminal cleavage. Hum. Mol. Genet. 2010, 19, 2395–2408.
- Kern, U.; Fröhlich, K.; Bedacht, J.; Schmidt, N.; Biniossek, M.L.; Gensch, N.; Baerenfaller, K.; Schilling, O. Impact of DJ-1 and helix 8 on the proteome and degradome of neuron-like cells. Cells 2021, 10, 404.
- Mitsugi, H.; Niki, T.; Takahashi-Niki, K.; Tanimura, K.; Yoshizawa-Kumagaye, K.; Tsunemi, M.; Iguchi-Ariga, S.M.; Ariga, H. Identification of the recognition sequence and target proteins for DJ-1 protease. FEBS Lett. 2013, 587, 2493–2499.
- Vázquez-Mayorga, E.; Díaz-Sánchez, Á.G.; Dagda, R.K.; Domínguez-Solís, C.A.; Dagda, R.Y.; Coronado-Ramírez, C.K.; Martínez-Martínez, A. Novel redox-dependent esterase activity (EC 3.1.1.2) for DJ-1: Implications for Parkinson’s disease. Int. J. Mol. Sci. 2016, 17, 1346.
- Takahashi-Niki, K.; Niki, T.; Iguchi-Ariga, S.M.; Ariga, H. Transcriptional regulation of DJ-1. Adv. Exp. Med. Biol. 2017, 1037, 89–95.
- Xu, J.; Zhong, N.; Wang, H.; Elias, J.E.; Kim, C.Y.; Woldman, I.; Pifl, C.; Gygi, S.P.; Geula, C.; Yankner, B.A. The Parkinson’s disease-associated DJ-1 protein is a transcriptional co-activator that protects against neuronal apoptosis. Hum. Mol. Genet. 2005, 14, 1231–1241.
- Scumaci, D.; Olivo, E.; Fiumara, C.V.; La Chimia, M.; De Angelis, M.T.; Mauro, S.; Costa, G.; Ambrosio, F.A.; Alcaro, S.; Agosti, V.; et al. DJ-1 proteoforms in breast cancer cells: The escape of metabolic epigenetic misregulation. Cells 2020, 9, 1968.
- Olivo, E.; La Chimia, M.; Ceramella, J.; Catalano, A.; Chiaradonna, F.; Sinicropi, M.S.; Cuda, G.; Iacopetta, D.; Scumaci, D. Moving beyond the tip of the iceberg: DJ-1 implications in cancer metabolism. Cells 2022, 11, 1432.
- Cao, J.; Chen, X.; Jiang, L.; Lu, B.; Yuan, M.; Zhu, D.; Zhu, H.; He, Q.; Yang, B.; Ying, M. DJ-1 suppresses ferroptosis through preserving the activity of S-adenosyl homocysteine hydrolase. Nat. Commun. 2020, 11, 1251.
- Cao, J.; Lou, S.; Ying, M.; Yang, B. DJ-1 as a human oncogene and potential therapeutic target. Biochem. Pharmacol. 2015, 93, 241–250.
- Cao, J.; Chen, X.; Ying, M.; He, Q.; Yang, B. DJ-1 as a therapeutic target against cancer. Adv. Exp. Med. Biol. 2017, 1037, 203–222.
- Zhang, L.; Wang, J.; Wang, J.; Yang, B.; He, Q.; Weng, Q. Role of DJ-1 in immune and inflammatory diseases. Front. Immunol. 2020, 11, 994.
- Nagakubo, D.; Taira, T.; Kitaura, H.; Ikeda, M.; Tamai, K.; Iguchi-Ariga, S.M.; Ariga, H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem. Biophys. Res. Commun. 1997, 231, 509–513.
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003, 299, 256–259.
- Repici1, M.; Giorgini, F. DJ-1 in Parkinson’s disease: Clinical insights and therapeutic perspectives. J. Clin. Med. 2019, 8, 1377.
- Maksimovic, I.; Finkin-Groner, E.; Fukase, Y.; Zheng, Q.; Sun, S.; Michino, M.; Huggins, D.J.; Myers, R.W.; David, Y. Deglycase-activity oriented screening to identify DJ-1 inhibitors. RSC Med. Chem. 2021, 12, 1232–1238.
- Smith, N.; Wilson, M.A. Structural biology of the DJ-1 superfamily. Adv. Exp. Med. Biol. 2017, 1037, 5–24.
- Jun, Y.W.; Kool, E.T. Small substrate or large? Debate over the mechanism of glycation adduct repair by DJ-1. Cell Chem. Biol. 2020, 27, 1117–1123.
- Heremans, I.P.; Caligiore, F.; Gerin, I.; Bury, M.; Lutz, M.; Graff, J.; Stroobant, V.; Vertommen, D.; Teleman, A.A.; Van Schaftingen, E.; et al. Parkinson’s disease protein PARK7 prevents metabolite and protein damage caused by a glycolytic metabolite. Proc. Natl. Acad. Sci. USA 2022, 119, e2111338119.
- Pérez-Tomás, R.; Pérez-Guillén, I. Lactate in the tumor microenvironment: An essential molecule in cancer progression and treatment. Cancers 2020, 12, 3244.
- Rogatzki, M.J.; Ferguson, B.S.; Goodwin, M.L.; Gladden, L.B. Lactate is always the end product of glycolysis. Front. Neurosci. 2015, 9, 22.
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580.
- Yang, Z.; Yan, C.; Ma, J.; Peng, P.; Ren, X.; Cai, S.; Shen, X.; Wu, Y.; Zhang, S.; Wang, X.; et al. Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat. Metab. 2023, 5, 61–79.
- Moreno-Yruela, C.; Zhang, D.; Wei, W.; Bæk, M.; Liu, W.; Gao, J.; Danková, D.; Nielsen, A.L.; Bolding, J.E.; Yang, L.; et al. Class I histone deacetylases (HDAC1-3) are histone lysine delactylases. Sci. Adv. 2022, 8, eabi6696.
- O’Brien, P.J.; Siraki, A.G.; Shangari, N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005, 35, 609–662.
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344, 109–116.
- Lange, J.N.; Wood, K.D.; Knight, J.; Assimos, D.G.; Holmes, R.P. Glyoxal formation and its role in endogenous oxalate synthesis. Adv. Urol. 2012, 2012, 819202.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
637
Revisions:
3 times
(View History)
Update Date:
04 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




