Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Qingfei Zheng and Version 3 by Conner Chen.
DJ-1 (also known as PARK7) is a multifunctional enzyme in human beings that is highly conserved and that has also been discovered in diverse species (ranging from prokaryotes to eukaryotes). Its complex enzymatic and non-enzymatic activities (such as anti-oxidation, anti-glycation, and protein quality control), as well as its role as a transcriptional coactivator, enable DJ-1 to serve as an essential regulator in multiple cellular processes (e.g., epigenetic regulations) and make it a promising therapeutic target for diverse diseases (especially cancer and Parkinson’s disease).
- DJ-1/PARK7
- multifunctional enzyme
- cancer
- Parkinson's disease
- therapeutic target
1. Introduction
The human gene DJ-1 (PARK7), which contains eight exons, locates at the chromosome band 1p36.23 and encodes a protein (named as DJ-1 or PARK7) composed of 189 amino acids, with seven β-strands and nine α-helices, and which belongs to the peptidase C56 family [1]. Biochemical and structural evidence has shown that DJ-1 functions as a homodimer [2][3][4][5][2,3,4,5]. Homologous genes of human DJ-1 have been variously discovered from microorganisms, plants, and other animals [6][7][6,7]. For example, DJ-1 is evolutionarily conserved within Escherichia coli chaperones (i.e., Hsp31, YhbO, and YajL) and archaea proteases [8][9][8,9]. Biochemical and structural studies have been conducted on DJ-1 variants from different organisms [10][11][10,11], which revealed its diverse physiological and pathological functions [1].
It has long been known that DJ-1 demonstrates an essential antioxidant activity in human cells, as a key protector against cellular oxidative stress, which is attributed to its three redox-active cysteine (Cys) residues (i.e., Cys43, Cys54, and Cys106) [12]. The redox activity of DJ-1 enables it to act as an important regulator in cells, against oxidative stress [1]. Recent research advances from our owne lab [13][14][15][16][17][18][19][13,14,15,16,17,18,19] and other groups [20][21][22][23][24][25][26][27][28][29][20,21,22,23,24,25,26,27,28,29] have indicated that DJ-1 is a scavenger enzyme, not only for quenching reactive oxygen species (ROS) [12], but also for reactive carbonyl species (RCS), which include methylglyoxal (MGO) and glyoxal (GO). Moreover, DJ-1 exhibits other enzymatic activities, such as glyoxalase [29][30][29,30], deglycase [20][25][20,25], peptidase/protease [31][32][33][31,32,33], and esterase [30][34][30,34] functions, which are important in maintaining cellular protein homeostasis [1]. Besides these enzymatic activities, DJ-1 also acts as a transcriptional coactivator, regulating gene transcription, without directly binding any promoters [35]. This non-enzymatic activity of DJ-1 relies on its non-covalent interactions with other nuclear proteins, such as p54nrb and pyrimidine tract-binding protein-associated splicing factor (PSF) [36].
Given its diverse activities, DJ-1 is believed to be involved in a number of physiological and pathological processes [1][37][38][39][1,37,38,39] and can serve as a druggable target for many types of disease [1][40][41][42][1,40,41,42]. For instance, DJ-1 has been found to be overexpressed in multiple types of cancer (especially those with high malignancy grades), and early in 1997 DJ-1 was identified as a novel oncoprotein that could transform cells in corporation with activated Ras [43]. Previous studies have also shown that loss of DJ-1 function leads to neurodegeneration, such as autosomal recessive early-onset parkinsonism [44]. Notably, mutations of the DJ-1 gene, including both deletion and substitution mutations, have been found in Parkinson’s disease (PD) patients [45], suggesting that DJ-1 plays significant roles in the brain neuronal maintenance and pathogenesis of PD. Taken together, DJ-1 is a noteworthy target for developing novel therapeutic and diagnostic strategies in both biomedical (e.g., cancer treatment) and psychological (e.g., early diagnosis of PD) research, leading to the establishment of high-throughput screening assays for identifying DJ-1 agonists and inhibitors [46].
2. Enzymatic Functions of DJ-1
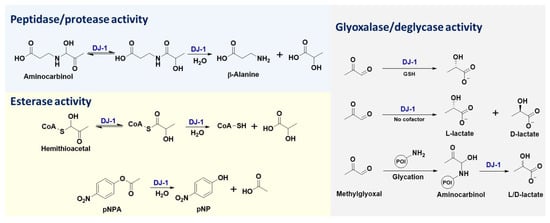
DJ-1 is a small protein (~20 kDa) that belongs to the large multi-clade DJ-1 (also named as DJ-1/PfpI, ThiJ/PfpI, or DJ-1/ThiJ/PfpI) superfamily, which includes many known chaperones, proteases, and stress-response proteins [47]. The diverse enzymatic activities of DJ-1 (Figure 12) are attributed to its key catalytic residue, Cys106. Previous studies have shown that DJ-1 is structurally conserved within cysteine proteases (such as PfpI), while in vitro biochemical assays failed to detect any protease activity for purified full-length DJ-1 [2]. Further research indicated that DJ-1 could convert from a zymogen to an active protease, through carboxyl-terminal cleavage of a 15-amino acid peptide, and that its catalytic dyad contains Cys106 and His126 [31]. Notably, C-terminally cleaved DJ-1 with cysteine protease activity exhibits enhanced cytoprotective action against oxidative stress-induced apoptosis, while this cytoprotective function of DJ-1 is abolished by C106A or H126A mutations [31]. Furthermore, the protease activity of DJ-1 lacking a C-terminal α-helix (i.e., DJ-1ΔH9) was shown to be remarkable, and the most susceptible sequence digested by DJ-1ΔH9 was valine-lysine-valine-alanine (VKVA), while divalent ions (especially Cu2+) significantly inhibited DJ-1’s protease activity [33]. In addition to protein substrates, DJ-1 is capable of recognizing ester substrates and hydrolyzing them into acids and alcohols/phenols [34]. The esterase activity of DJ-1 has been applied for high-throughput screening of its inhibitors, where the oxyester compounds are utilized as substrates (Figure 12) [46].