Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Takshashila Tripathi | -- | 1956 | 2023-04-28 17:08:45 | | | |
| 2 | Conner Chen | Meta information modification | 1956 | 2023-05-04 02:29:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Anand, S.; Sowbhagya, R.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; Almalik, A.I.; Ahmad, W.; Tripathi, T.; Elderdery, A.Y. Classification of Polyphenols. Encyclopedia. Available online: https://encyclopedia.pub/entry/43637 (accessed on 01 March 2026).
Anand S, Sowbhagya R, Ansari MA, Alzohairy MA, Alomary MN, Almalik AI, et al. Classification of Polyphenols. Encyclopedia. Available at: https://encyclopedia.pub/entry/43637. Accessed March 01, 2026.
Anand, Santosh, Ramachandregowda Sowbhagya, Mohammad Azam Ansari, Mohammad A. Alzohairy, Mohammad N. Alomary, Asiyah I. Almalik, Wasim Ahmad, Takshashila Tripathi, Abozer Y. Elderdery. "Classification of Polyphenols" Encyclopedia, https://encyclopedia.pub/entry/43637 (accessed March 01, 2026).
Anand, S., Sowbhagya, R., Ansari, M.A., Alzohairy, M.A., Alomary, M.N., Almalik, A.I., Ahmad, W., Tripathi, T., & Elderdery, A.Y. (2023, April 28). Classification of Polyphenols. In Encyclopedia. https://encyclopedia.pub/entry/43637
Anand, Santosh, et al. "Classification of Polyphenols." Encyclopedia. Web. 28 April, 2023.
Copy Citation
Polyphenols are the secondary metabolites synthesized by the plants as a part of defense machinery. Owing to their antioxidant, anti-inflammatory, anticancerous, antineoplastic, and immunomodulatory effects, natural polyphenols have been used for a long time to prevent and treat a variety of diseases. Polyphenols are plant secondary metabolites, with over 8000 polyphenolic compounds discovered to date, and have a variety of complicated structures. The most common and abundant polyphenols are phenolic acids, phenolic alcohols, flavonoids, lignans, and stiblins, which are available in the plant kingdom.
polyphenols
bioavailability
nanoformulations
human health
nanonization
1. Classification of Polyphenols
Polyphenols are molecules with at least a single hydroxyl group present on an aromatic ring structure, and their backbone can range from a simple moiety to a complex polymer with a high molecular mass. The most widely accepted classification divides phenolics into two classes: flavonoids and non-flavonoid polyphenols [1]. The non-flavonoid polyphenols include phenolic acids, tannins, and stilbenes [2].
2. Flavonoids
Flavonoids, which are low-molecular-mass phenolic moieties, are plant-derived molecules that can be found in various regions of plants existing in nature. Vegetables utilize flavonoids to enable them to survive and protect themselves against plaque. They are one of the major distinctive groups of compounds found in higher plants. In most angiosperm families, several flavonoids are easily recognized as floral pigments [3]. Flavonoids can be put into different subgroups based on where the B ring is linked to the C ring and how much the C ring is unsaturated and oxidized. Isoflavones are flavonoids in which the B ring is connected to the C ring at position 3. The compounds in which the B ring is attached at position 4 are referred to as neoflavonoids. In addition, when the B ring is connected to position 2, they can be further divided into several subclasses based on the structure of the C ring. Flavanols, flavones, flavanonols, flavanones, and flavanols, also called anthocyanins, catechins, and chalcones, are all parts of these subclasses [4] (Figure 1).
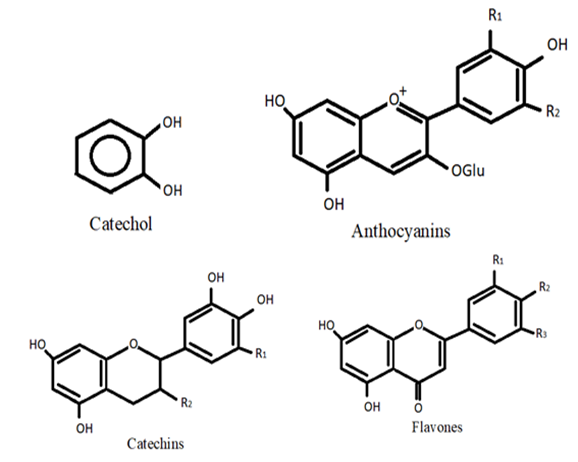
Figure 1. Flavonoids.
Choi et al. [5] have demonstrated the use of catechol to treat breast cancer, and Stat3 expression as a breast cancer stem cell (CSC) marker. According to them, catechol reduced the Stat3 expression and released IL-6, a CSC survival factor, inhibiting Stat3 signaling. Moon et al. [6] have reviewed the role of catechol in the enhancement of chemo and radio sensitivity by targeting AMPK/Hippo signaling in pancreatic cancer cells. Anthocyanins are usually found in fruits such as blueberries, raspberries, and black soybean, and also are found in vegetables, roots, legumes, cereals, and grains. These pigments dissolve in water. The blue and red colors of red grape and wine are because of the presence of phenolic moieties found in the skin of the berry and in the wine solution. Because they are easy to oxidize when under stress and because reactive oxygen species are present, these compounds are excellent antioxidants. They make fruits and vegetables better for human health, especially when it comes to degenerative and chronic diseases [7]. After 12 weeks of daily consumption of blueberry or Concord grape juice, the cognitive function in elderly persons improved [8][9]. Aged rats’ cognitive and motor abilities were enhanced by blueberries, bringing them up to par with young animals [10]. Catechins are naturally occurring polyphenolic flavanol compounds that can be present in a variety of culinary and medicinal plants, including tea, legumes, cocoa, berries, Fabaceae and Rubiaceae. Growing bodies of evidence have associated the consumption of foods high in catechins to the management of chronic human disorders such as inflammatory bowel disease (IBD) [11]. Baba et al. [12] have reported a positive impact on the working memory in Japanese adults between the ages of 50 to 60 years after 12 weeks of daily green tea catechins intake. Bernatoniene and Kopustinskiene [13] have reviewed the molecular characteristics of catechins, their antioxidant activity, and the mechanisms implicated in the prevention of oxidative-stress-related disorder. Flavones are a widely distributed flavonoid subgroup in plants that are produced by diverse pathways, depending on whether they possess a hydroxylated B-ring and C- or O-glycosylation [14]. Flavones have been substantially reported to be cancer-preventive. Diets rich in flavones reduce the risk of breast, skin, digestive, hematological, and prostate cancer. Flavones improve cardiovascular and neurological diseases besides cancer [15]. Reduced energy efficiency, decreased food intake, and weight loss have all been associated with a higher flavonoid intake. In order to prevent and treat obesity, flavonoids may provide a beneficial economic strategy with few, if any, adverse effects [16].
3. Phenolic Acids—Hydroxycinnamic and Hydroxybenzoic Acids
The two types of phenolic acids are hydroxycinnamic and hydroxybenzoic acids (Figure 2A,B). Hydroxybenzoic acids in the human diet are uncommon, which is why they are not thought to play a function in human health. Cinnamic acid and benzoic acid derivatives have C1-C6 and C3-C6 backbones, respectively [17]. Phenolic acids can have neuroprotective and pro-cognitive effects through a variety of processes that vary depending on the natural substances that make up this diverse category [18]. By modulating intracellular calcium concentrations, chlorogenic acid has been shown to protect primary neurons against glutamate neurotoxicity [19] and has shown to have both neuroprotective and inflammatory effects in microglial cells infected with the herpes simplex virus [20].
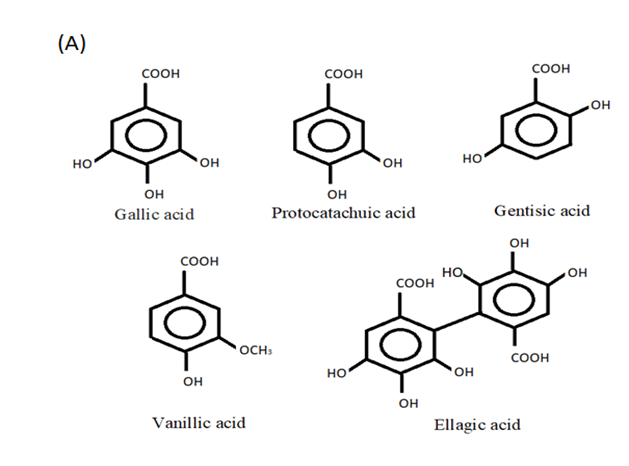
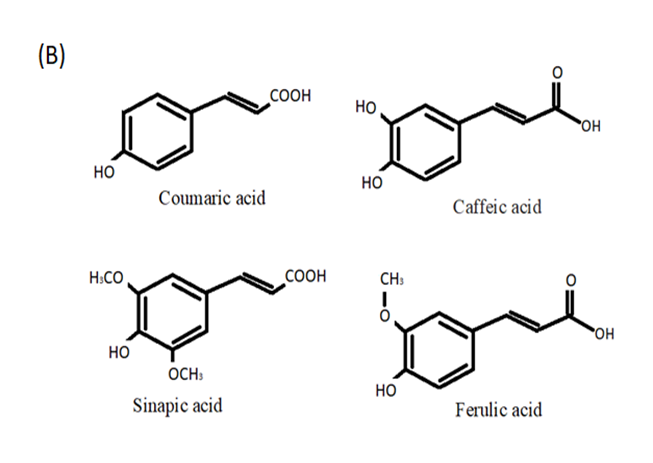
Figure 2. Phenolic acids: (A) hydroxycinnamic acid and (B) hydroxybenzoic acid.
4. Hydroxycinnamic Acids
Gallic acid is an effective antioxidant with neuroprotective and anti-inflammatory properties that has been shown to reduce cytokine levels in microglia cells and protect neurons from induced neurotoxicity by inhibiting NF-κB acetyltransferase [21]. Protocatechuic acid (PCA) is a form of naturally occurring phenolic acid that is extensively distributed in plants. PCA is a potent antioxidant with antibacterial, anticancer, antiasthma, antiulcer, anti-aging, antiathrogenic, antispasmodic, antitumoral, and neurological properties [22][23]. In experimental piglets, Hu et al. [24] found that dietary supplementation with PCA enhanced the expression of tight junction proteins such as claudin-1 and ZO-1 in the intestinal mucosa and reduced the serum concentration of thiobarbituric acid reactive substances (TBARS) when compared to control animals. Further, PCA supplementation has also been documented to reduce the levels of TNF and IL-2 in the blood.
Gentisic acid has numerous pharmacological activities, such as antioxidant, hepatoprotective, antimicrobial, anticarcinogenesis, analgesic, neuroprotective, and cardioprotective [25]. Gentisic acid increased the proliferation of keratinocytes. A Western blot analysis of proteins in the mitogen-activated protein (MAP) kinase signaling pathway revealed that ERK1/2 phosphorylation was augmented by gentisic acid supplementation. Thus, gentisic acid increases keratinocyte proliferation by phosphorylating EEK1/2 [26]. Vanillic acid has been shown to have antioxidant, anti-inflammatory, immunostimulatory, neuroprotective, hepatoprotective, cardioprotective, and antiapoptotic effects. According to reports, vanillic acid has the ability to reduce A1-42-induced cognitive impairment and oxidative stress, and hence aids in the treatment of Alzheimer’s disease [27]. In mice, vanillic acid reduces the severity of dextran sulfate sodium-induced ulcerative colitis, demonstrating its potential for controlling chronic intestinal inflammation [28]. Cikman et al. [29] have demonstrated that syringic acid lowered oxidative stress indicators and increased the antioxidant capacity in rats with L-arginine-induced acute pancreatic damage. Ellagic acid, an antioxidant active organic heterotetracyclic phenolic molecule found in grains and fruits, has been shown to have better accessibility to the brain and to increase the levels of antioxidant enzymes in aged rats [30].
5. Hydroxybenzoic Acids
Coumaric acid is a polyphenolic compound present in a wide range of plant-based diets, and studies have indicated that this phenolic molecule possesses neuroprotective properties [31]. An in vivo study has shown that coumaric acid supplementation reduces axonal degeneration in rat sciatic nerves and oxidative stress following ischemia/reperfusion [32]. Caffeic acid appears to have neuroprotective properties via reducing neuroinflammation and oxidative stress and, in particular, it was found to reduce sickness behavior and neuroinflammation in mice generated by lipopolysaccharides [33]. The phenethyl ester of caffeic acid has been demonstrated to be neuroprotective in rats exposed to ionizing radiation, reducing radiation-induced oxidative damage by improving SOD activity and lowering MDA levels in the brain [34]. Sinapic acid found in berries and cereals has been demonstrated to have neuroprotective properties in animal models of neurodegenerative diseases, including Alzheimer’s and Parkinson’s diseases [35][36]. Ferulic acid has shown its efficacy as a neuroprotective phenolic acid by increasing the levels of serotonin and norepinephrine in the mouse frontal cortex and hippocampus, but not dopamine levels, which are important in the pathophysiology associated with mood disorders [37].
6. Tannins
Tannins are polyphenols with 500 to 30,000 Da MW and are divided into pyrogallol tannins or hydrolysable tannins and proanthocyanidins or condensed tannins. Hydrolysable tannins are constituted of phenolic acid esters and a polyol, generally glucose, which are gallotannins and ellagitannins [38]. A tannin, a water-soluble polyphenol, is double-edged. According to studies, the amount of tannins that causes a disorder in one animal does not affect another of the same species [39]. Tannins prevent lipid peroxidation and scavenge pro-oxidant free radicals, and most tannin activities, including free radical-scavenging, depend on structure and polymerization [40][41]. Tannin-enriched fractions isolated from Myracrodruou urundeuva on rat primary mesencephalic cells decreased 6-hydroxydopamine-induced neuronal cell toxicity. It reversed 6-hydroxydopamine-induced lipid peroxidation, indicated by the down-regulation of thiobarbituric acid reactive substances [42]. Senobari et al. [43] have reviewed the anticancer, chemoprotective, and anti-inflammatory properties of ellagitannins and their derivatives. Various factors could modulate pro-inflammatory mediators and growth factors, such as IL-6, TNF, TGF, IL-1, IFN, and ellagitannins, that target cancer stem cells and disrupt stem cell signaling. Figure 3 represents the structures of gallotannins and ellagitannins.
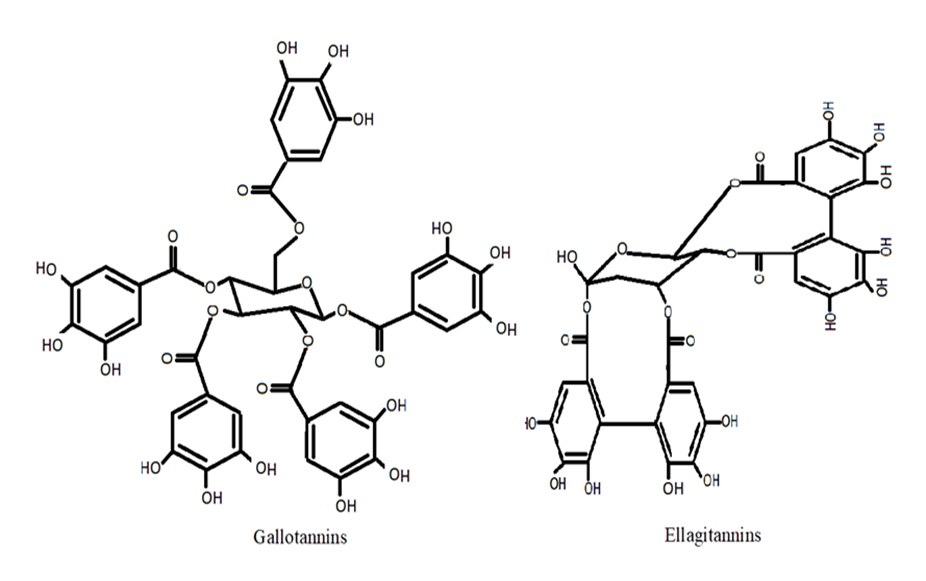
Figure 3. Structures of tannins.
7. Stilbenes
Although stilbenes are not considered to be flavonoids, their chemical makeup and biological activities are quite similar to those of flavonoids. Different plants naturally produce stilbenes, which are produced in reaction to pathogen infection (phytoalexins), exposure to ultraviolet radiation, and involvement in bacterial root nodulation and coloring [44]. A methylene bridge connects the two aromatic rings (rings A and B) that make up the chemical structure of stilbenes. Trans-resveratrol, a member of the stilbene family that has been hydroxylated at the 3, 5, and 4 positions, is one of the most well-known molecules [45] (Figure 4). Recent studies of Ma et al. [46] have revealed the neuroprotective activities of resveratrol in rat models of AD and diabetes. Resveratrol significantly increased the expression of Sirt1 and stopped memory loss, high levels of malondialdehyde, acetylcholinesterase, interleukin-6, and interleukin-1b, and lowered levels of superoxide dismutase (SOD), choline acetyltransferase (ChAT), and glutathione. In another study, resveratrol reduced the inflammation and oxidative stress in the kidneys of diabetic rats, and the treatment upregulated the gene and protein expression profile of NF-κB in them.
Figure 4. Stilbenes.
Exploring the therapeutic effects of natural polyphenols in the prevention and treatment of cardiovascular and neurological illnesses, particularly cancer, has received substantial attention in scientific research in recent years [47]. Plant sources have reported varied concentrations of these polyphenolic compounds (Table 1).
Table 1. Concentrations of polyphenolic compounds in some plant sources.
| Polyphenols | Examples | Source | Concentration (g/Kg) | References |
|---|---|---|---|---|
| Phenolic acid—Hydroxycinnamic acid | Gallic acid | Blackcurrant Primrose |
30–62 mg/kg dry weight 15,000 mg/kg |
[48] [49] |
| Protocatechuic acid | Acai fruit | 630 mg/kg | [50] | |
| Gentisic acid | Citrus fruit (Citrus paradisi) | 30,000 mg/kg | [51] | |
| Vanillic acid | Acai fruit | 1616 mg/kg | [50] | |
| Ellagic acid | Pomegranate Raspberry |
700 mg/kg dry weight (arils) 38,700 mg/kg dry weight (mesocarp) 1500 mg/kg dry weight 2637–3309 mg/kg fresh weight |
[52] [53] [54] |
|
| Phenolic acid—Hydroxybenzoic acid | Coumaric acid | Corn Barley |
242 mg/kg 75 mg/kg |
[55] |
| Caffeic acid | Cabbage (SA) | 42.5 mg/kg | [56] | |
| Sinapic acid | Lemon Strawberry Cranberries |
72.1 mg/kg 450.1 mg/kg 210 mg/kg |
[57] | |
| Ferulic acid | Acai fruit | 101 mg/kg | [50] | |
| Flavonoids | Anthocyanins | Plum peel Blueberry |
604.5 mg/kg 223.8 mg/kg |
[58] |
| Catechin | Acai fruit | 66.7 mg/kg | [50] | |
| Flavones | Fenugreek seed (Apigenin) (Luteolin) |
7310 mg/kg 5120 mg/kg |
[59] | |
| Tannins | Ellagitannins | Tea | 0.15 to 4.46 mg ellagic acid equivalent/g tea | [60] |
| Stilbenes | Resveratrol | Morus alba (fruit) Rumex japonicas (root) |
7.95 × 10−3 8.4 × 10−3 |
[61] |
Preclinical and clinical research suggest that an increased intake of polyphenol-rich diets protect against neurodegenerative disorders, cardiovascular diseases, cancer, diabetes, inflammatory disorders, and infectious illnesses. It has been suggested that the antioxidant activity of dietary polyphenols plays a crucial role in preventing oxidative-stress-induced diseases in humans [62]. The possible molecular targets of polyphenols orchestrating human health benefits as regulators, modulators, and anticancerous agents are represented in Figure 5.
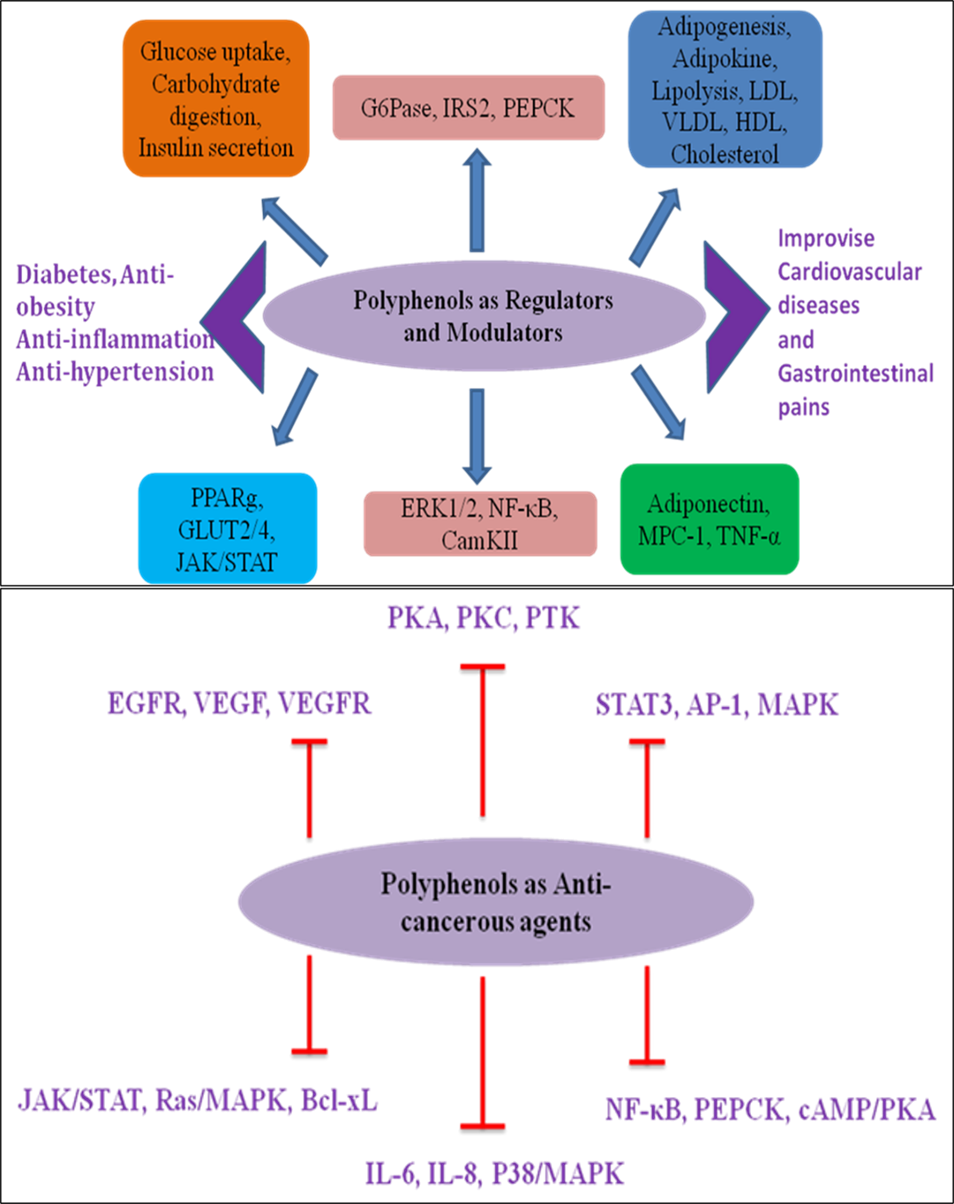
Figure 5. Possible molecular targets of polyphenols orchestrating human health benefits as regulators, modulators, and anticancerous agents.
References
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243.
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370.
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Choi, H.S.; Kim, J.H.; Kim, S.L.; Deng, H.Y.; Lee, D.; Kim, C.S.; Yun, B.S.; Lee, D.S. Catechol derived from aronia juice through lactic acid bacteria fermentation inhibits breast cancer stem cell formation via modulation Stat3/IL-6 signaling pathway. Mol. Carcinog. 2018, 57, 1467–1479.
- Moon, J.Y.; Ediriweera, M.K.; Ryu, J.Y.; Kim, H.Y.; Cho, S.K. Catechol enhances chemo-and radio-sensitivity by targeting AMPK/Hippo signaling in pancreatic cancer cells. Oncol. Rep. 2021, 45, 1133–1141.
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236.
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000.
- Miller, M.G.; Hamilton, D.A.; Joseph, J.A.; Shukitt-Hale, B. Dietary blueberry improves cognition among older adults in a randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2018, 57, 1169–1180.
- Shukitt-Hale, B.; Bielinski, D.F.; Lau, F.C.; Willis, L.M.; Carey, A.N.; Joseph, J.A. The beneficial effects of berries on cognition, motor behaviour and neuronal function in ageing. Br. J. Nutr. 2015, 114, 1542–1549.
- Fan, F.Y.; Sang, L.X.; Jiang, M. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules 2017, 22, 484.
- Baba, Y.; Inagaki, S.; Nakagawa, S.; Kaneko, T.; Kobayashi, M.; Takihara, T. Effect of daily intake of green tea catechins on cognitive function in middle-aged and older subjects: A randomized, placebo-controlled study. Molecules 2020, 25, 4265.
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965.
- Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From biosynthesis to health benefits. Plants 2016, 5, 27.
- Khan, A.U.; Dagur, H.S.; Khan, M.; Malik, N.; Alam, M.; Mushtaque, M. Therapeutic role of flavonoids and flavones in cancer prevention: Current trends and future perspectives. Eur. J. Med. Chem. Rep. 2021, 3, 100010.
- Bertoia, M.L.; Rimm, E.B.; Mukamal, K.J.; Hu, F.B.; Willett, W.C.; Cassidy, A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124 086 US men and women followed for up to 24 years. BMJ 2016, 352, i17.
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42.
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic acids and prevention of cognitive decline: Polyphenols with a neuroprotective role in cognitive disorders and Alzheimer’s disease. Nutrients 2022, 14, 819.
- Mikami, Y.; Yamazawa, T. Chlorogenic acid, a polyphenol in coffee, protects neurons against glutamate neurotoxicity. Life Sci. 2015, 139, 69–74.
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687.
- Kim, M.J.; Seong, A.R.; Yoo, J.Y.; Jin, C.H.; Lee, Y.H.; Kim, Y.J.; Lee, J.; Jun, W.J.; Yoon, H.G. Gallic acid, a histone acetyltransferase inhibitor, suppresses β-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation. Mol. Nutr. Food Res. 2011, 55, 1798–1808.
- Khan, A.K.; Rashid, R.; Fatima, N.; Mahmood, S.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological activities of protocatechuic acid. Acta Pol. Pharm. 2015, 72, 643–650.
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 952943.
- Hu, R.; He, Z.; Liu, M.; Tan, J.; Zhang, H.; Hou, D.X.; He, J.; Wu, S. Dietary protocatechuic acid ameliorates inflammation and up-regulates intestinal tight junction proteins by modulating gut microbiota in LPS-challenged piglets. J. Anim. Sci. Biotechnol. 2020, 11, 92.
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phytother. Res. 2020, 34, 729–741.
- Kim, M.; Kim, J.; Shin, Y.K.; Kim, K.Y. Gentisic acid stimulates keratinocyte proliferation through ERK1/2 phosphorylation. Int. J. Med. Sci. 2020, 17, 626.
- Sharma, N.; Tiwari, N.; Vyas, M.; Khurana, N.; Muthuraman, A.; Utreja, P. An overview of therapeutic effects of vanillic acid. Plant Arch. 2020, 20, 3053–3059.
- Kim, S.J.; Kim, M.C.; Um, J.Y.; Hong, S.H. The beneficial effect of vanillic acid on ulcerative colitis. Molecules 2010, 15, 7208–7217.
- Cikman, O.; Soylemez, O.; Ozkan, O.F.; Kiraz, H.A.; Sayar, I.; Ademoglu, S.; Taysi, S.; Karaayvaz, M. Antioxidant activity of syringic acid prevents oxidative stress in L-arginine–induced acute pancreatitis: An experimental study on rats. Int. Surg. 2015, 100, 891–896.
- Sowbhagya, R.; Anupama, S.K.; Bhagyalakshmi, D.; Anand, S.; Ravikiran, T. Modulatory effects of Decalepishamiltonii extract and its compounds on the antioxidant status of the aging rat brain. J. Pharm. Bioallied Sci. 2017, 9, 8–15.
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S.
- Guven, M.; Aras, A.B.; Akman, T.; Sen, H.M.; Ozkan, A.; Salis, O.; Sehitoglu, I.; Kalkan, Y.; Silan, C.; Deniz, M.; et al. Neuroprotective effect of p-coumaric acid in rat model of embolic cerebral ischemia. Iran. J. Basic Med. Sci. 2015, 18, 356–363.
- Mallik, S.B.; Mudgal, J.; Nampoothiri, M.; Hall, S.; Anoopkumar-Dukie, S.; Grant, G.; Rao, C.M.; Arora, D. Caffeic acid attenuates lipopolysaccharide-induced sickness behaviour and neuroinflammation in mice. Neurosci. Lett. 2016, 632, 218–223.
- Alkis, H.E.; Kuzhan, A.; Dirier, A.; Tarakcioglu, M.; Demir, E.; Saricicek, E.; Demir, T.; Ahlatci, A.; Demirci, A.; Cinar, K.; et al. Neuroprotective effects of propolis and caffeic acid phenethyl ester (CAPE) on the radiation-injured brain tissue (Neuroprotective effects of propolis and CAPE). Int. J. Radiat. Res. 2015, 13, 297–303.
- Shin, D.S.; Kim, K.W.; Chung, H.Y.; Yoon, S.; Moon, J.O. Effect of sinapic acid against carbon tetrachloride-induced acute hepatic injury in rats. Arch. Pharm. Res. 2013, 36, 626–633.
- Lee, H.E.; Kim, D.H.; Park, S.J.; Kim, J.M.; Lee, Y.W.; Jung, J.M.; Lee, C.H.; Hong, J.G.; Liu, X.; Cai, M.; et al. Neuroprotective effect of sinapic acid in a mouse model of amyloid β1–42 protein-induced Alzheimer’s disease. Pharmacol. Biochem. Behav. 2012, 103, 260–266.
- Chen, J.; Lin, D.; Zhang, C.; Li, G.; Zhang, N.; Ruan, L.; Yan, Q.; Li, J.; Yu, X.; Xie, X.; et al. Antidepressant-like effects of ferulic acid: Involvement of serotonergic andnorepinergic systems. Metab. Brain Dis. 2015, 30, 129–136.
- Farha, A.K.; Yang, Q.Q.; Kim, G.; Li, H.B.; Zhu, F.; Liu, H.Y.; Gan, R.Y.; Corke, H. Tannins as an alternative to antibiotics. Food Biosci. 2020, 38, 100751.
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2021, 40, 432–444.
- Okuda, T. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry 2005, 66, 2012–2031.
- Jerez, M.; Tourio, S.; Sineiro, J.; Torres, J.L.; Nuez, M.J. Procyanidins from pine bark: Relationships between structure, composition and antiradical activity. Food Chem. 2007, 104, 518–527.
- Nobre-Junior, H.V.; Maia, F.D.; de Oliveira, R.A.; Bandeira, M.A.; do Ó Pessoa, C.; Moraes, M.O.; Cunha, G.M.; Viana, G.S. Neuroprotective actions of Tannins from Myracrodruonurundeuva on 6-hydroxydopamine-induced neuronal cell death. J. Herbs Spices Med. Plants 2008, 13, 41–57.
- Senobari, Z.; Karimi, G.; Jamialahmadi, K. Ellagitannins, promising pharmacological agents for the treatment of cancer stem cells. Phytother. Res. 2022, 36, 231–242.
- Lim, C.G.; AG Koffas, M. Bioavailability and recent advances in the bioactivity of flavonoid and stilbene compounds. Curr. Org. Chem. 2010, 14, 1727–1751.
- King, R.E.; Bomser, J.A.; Min, D.B. Bioactivity of Resveratrol. Compr. Rev. Food Sci. Food Saf. 2006, 5, 65–70.
- Ma, X.; Sun, Z.; Han, X.; Li, S.; Jiang, X.; Chen, S.; Zhang, J.; Lu, H. Neuroprotective effect of resveratrol via activation of Sirt1 signaling in a rat model of combined diabetes and Alzheimer’s disease. Front. Neurosci. 2020, 13, 1400.
- Pechanova, O.; Dayar, E.; Cebova, M. Therapeutic potential of polyphenols-loaded polymeric nanoparticles in cardiovascular system. Molecules 2020, 25, 3322.
- Stohr, H.; Herrmann, K. The phenolics of fruits. VI. The phenolics of currants, gooseberries and blueberries. Changes in phenolic acids and catechins during development of black currants (author’s transl). Z. Lebensm. Unters. Forsch. 1975, 159, 31–37.
- Karamac, M.; Kosinska, A.; Pegg, R.B. Content of gallic acid in selected plant extracts. Polish J. Food Nutr. Sci. 2006, 15, 55.
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.). J. Agric.Food Chem. 2008, 56, 4631–4636.
- Van Lelyveld, L.J.; Van Vuuren, S.P.; Visser, G. Gentisic acid concentration in healthy and greening infected fruit albedo and leaves of citrus species and cultivars. S. Afr. J. Plant Soil 1988, 5, 209–211.
- García-Villalba, R.; Espín, J.C.; Aaby, K.; Alasalvar, C.; Heinonen, M.; Jacobs, G.; Voorspoels, S.; Koivumäki, T.; Kroon, P.A.; Pelvan, E.; et al. Validated Method for the Characterization and Quantification of Extractable and Nonextractable Ellagitannins after Acid Hydrolysis in Pomegranate Fruits, Juices, and Extracts. J. Agric. Food Chem. 2015, 63, 6555–6566.
- Daniel, E.M.; Krupnick, A.S.; Heur, Y.-H.; Blinzler, J.A.; Nims, R.W.; Stoner, G.D. Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J. Food Compos. Anal. 1989, 2, 338–349.
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619.
- Ndolo, V.U.; Beta, T. Comparative studies on composition and distribution of phenolic acids in cereal grain botanical fractions. Cereal Chem. 2014, 91, 522–530.
- Li, P.; Wang, X.Q.; Wang, H.Z.; Wu, Y.N. High performance liquid chromatographic determination of phenolic acids in fruits and vegetables. Biomed. Environ. Sci. 1993, 6, 389–398.
- Xuan, T.D.; Bach, D.T.; Dat, T.D. Involvement of phenolics, flavonoids and phenolic acids in high yield characteristics of rice (Oryza sativa L.). Int. Lett. Nat. Sci. 2018, 68, 19–26.
- Shehata, W.A.; Akhtar, S.; Alam, T. Extraction and estimation of anthocyanin content and antioxidant activity of some common fruits. Trends Appl. Sci. Res. 2020, 15, 179–186.
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949.
- Yang, X.; Tomás-Barberán, F.A. Tea Is a Significant Dietary Source of Ellagitannins and Ellagic Acid. J. Agric. Food Chem. 2019, 67, 5394–5404.
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404.
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.8K
Revisions:
2 times
(View History)
Update Date:
04 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





