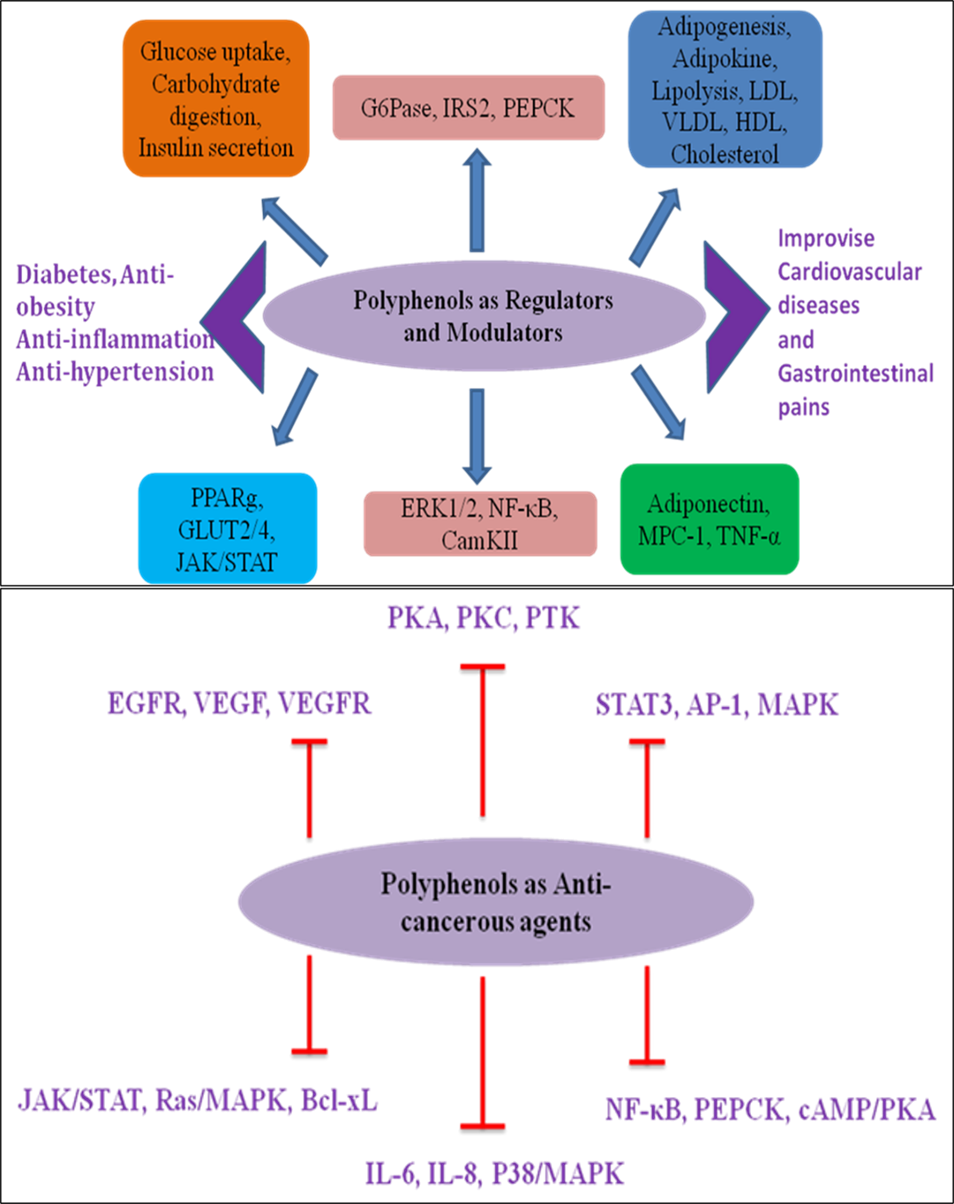
Polyphenols are the secondary metabolites synthesized by the plants as a part of defense machinery. Owing to their antioxidant, anti-inflammatory, anticancerous, antineoplastic, and immunomodulatory effects, natural polyphenols have been used for a long time to prevent and treat a variety of diseases. Polyphenols are plant secondary metabolites, with over 8000 polyphenolic compounds discovered to date, and have a variety of complicated structures. The most common and abundant polyphenols are phenolic acids, phenolic alcohols, flavonoids, lignans, and stiblins, which are available in the plant kingdom.
- polyphenols
- bioavailability
- nanoformulations
- human health
- nanonization
1. Classification of Polyphenols
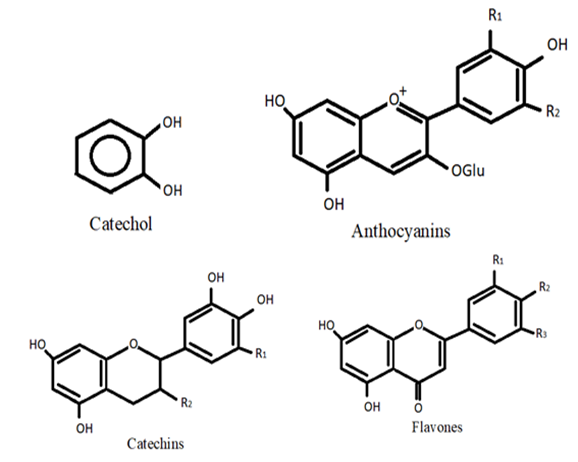
2. Flavonoids
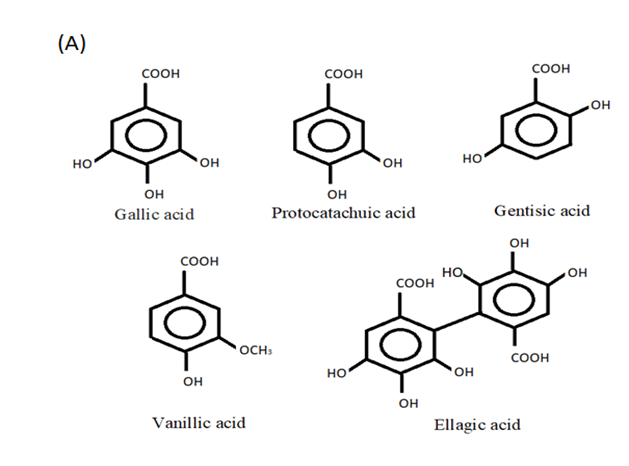
3. Phenolic Acids—Hydroxycinnamic and Hydroxybenzoic Acids
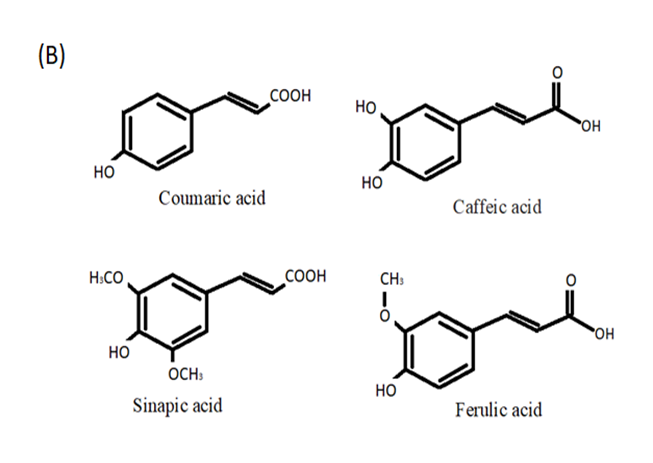
4. Hydroxycinnamic Acids
5. Hydroxybenzoic Acids
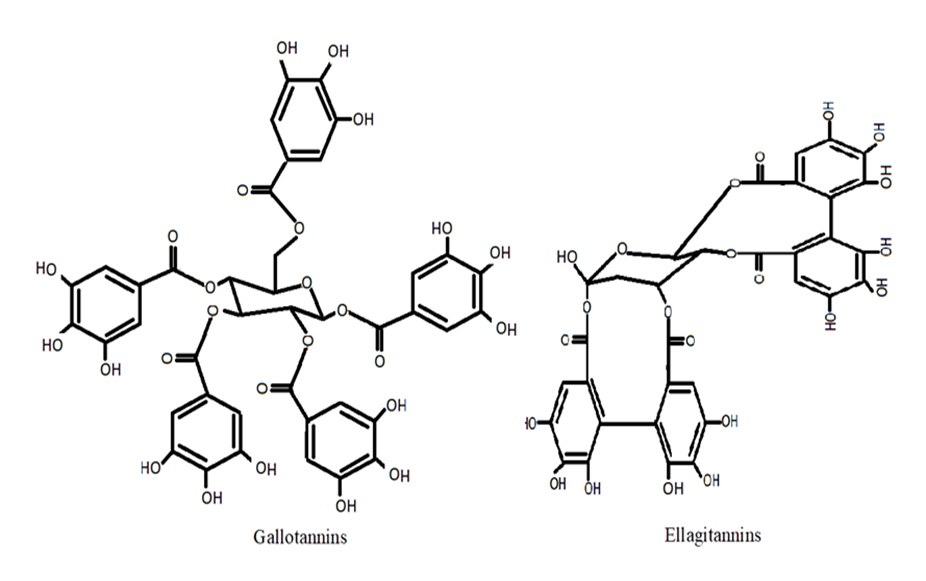
6. Tannins
7. Stilbenes
| Polyphenols | Examples | Source | Concentration (g/Kg) | References |
|---|---|---|---|---|
| Phenolic acid—Hydroxycinnamic acid | Gallic acid | Blackcurrant Primrose |
30–62 mg/kg dry weight 15,000 mg/kg |
[48] [49] |
| Protocatechuic acid | Acai fruit | 630 mg/kg | [50] | |
| Gentisic acid | Citrus fruit (Citrus paradisi) | 30,000 mg/kg | [51] | |
| Vanillic acid | Acai fruit | 1616 mg/kg | [50] | |
| Ellagic acid | Pomegranate Raspberry |
700 mg/kg dry weight (arils) 38,700 mg/kg dry weight (mesocarp) 1500 mg/kg dry weight 2637–3309 mg/kg fresh weight |
[52] [53] [54] |
|
| Phenolic acid—Hydroxybenzoic acid | Coumaric acid | Corn Barley |
242 mg/kg 75 mg/kg |
[55] |
| Caffeic acid | Cabbage (SA) | 42.5 mg/kg | [56] | |
| Sinapic acid | Lemon Strawberry Cranberries |
72.1 mg/kg 450.1 mg/kg 210 mg/kg |
[57] | |
| Ferulic acid | Acai fruit | 101 mg/kg | [50] | |
| Flavonoids | Anthocyanins | Plum peel Blueberry |
604.5 mg/kg 223.8 mg/kg |
[58] |
| Catechin | Acai fruit | 66.7 mg/kg | [50] | |
| Flavones | Fenugreek seed (Apigenin) (Luteolin) |
7310 mg/kg 5120 mg/kg |
[59] | |
| Tannins | Ellagitannins | Tea | 0.15 to 4.46 mg ellagic acid equivalent/g tea | [60] |
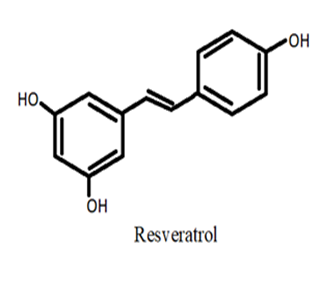
| Stilbenes | Resveratrol | Morus alba (fruit) Rumex japonicas (root) |
7.95 × 10−3 8.4 × 10−3 |
[61] |
This entry is adapted from the peer-reviewed paper 10.3390/life12101639
References
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243.
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370.
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Choi, H.S.; Kim, J.H.; Kim, S.L.; Deng, H.Y.; Lee, D.; Kim, C.S.; Yun, B.S.; Lee, D.S. Catechol derived from aronia juice through lactic acid bacteria fermentation inhibits breast cancer stem cell formation via modulation Stat3/IL-6 signaling pathway. Mol. Carcinog. 2018, 57, 1467–1479.
- Moon, J.Y.; Ediriweera, M.K.; Ryu, J.Y.; Kim, H.Y.; Cho, S.K. Catechol enhances chemo-and radio-sensitivity by targeting AMPK/Hippo signaling in pancreatic cancer cells. Oncol. Rep. 2021, 45, 1133–1141.
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236.
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000.
- Miller, M.G.; Hamilton, D.A.; Joseph, J.A.; Shukitt-Hale, B. Dietary blueberry improves cognition among older adults in a randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2018, 57, 1169–1180.
- Shukitt-Hale, B.; Bielinski, D.F.; Lau, F.C.; Willis, L.M.; Carey, A.N.; Joseph, J.A. The beneficial effects of berries on cognition, motor behaviour and neuronal function in ageing. Br. J. Nutr. 2015, 114, 1542–1549.
- Fan, F.Y.; Sang, L.X.; Jiang, M. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules 2017, 22, 484.
- Baba, Y.; Inagaki, S.; Nakagawa, S.; Kaneko, T.; Kobayashi, M.; Takihara, T. Effect of daily intake of green tea catechins on cognitive function in middle-aged and older subjects: A randomized, placebo-controlled study. Molecules 2020, 25, 4265.
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965.
- Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From biosynthesis to health benefits. Plants 2016, 5, 27.
- Khan, A.U.; Dagur, H.S.; Khan, M.; Malik, N.; Alam, M.; Mushtaque, M. Therapeutic role of flavonoids and flavones in cancer prevention: Current trends and future perspectives. Eur. J. Med. Chem. Rep. 2021, 3, 100010.
- Bertoia, M.L.; Rimm, E.B.; Mukamal, K.J.; Hu, F.B.; Willett, W.C.; Cassidy, A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124 086 US men and women followed for up to 24 years. BMJ 2016, 352, i17.
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42.
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic acids and prevention of cognitive decline: Polyphenols with a neuroprotective role in cognitive disorders and Alzheimer’s disease. Nutrients 2022, 14, 819.
- Mikami, Y.; Yamazawa, T. Chlorogenic acid, a polyphenol in coffee, protects neurons against glutamate neurotoxicity. Life Sci. 2015, 139, 69–74.
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687.
- Kim, M.J.; Seong, A.R.; Yoo, J.Y.; Jin, C.H.; Lee, Y.H.; Kim, Y.J.; Lee, J.; Jun, W.J.; Yoon, H.G. Gallic acid, a histone acetyltransferase inhibitor, suppresses β-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation. Mol. Nutr. Food Res. 2011, 55, 1798–1808.
- Khan, A.K.; Rashid, R.; Fatima, N.; Mahmood, S.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological activities of protocatechuic acid. Acta Pol. Pharm. 2015, 72, 643–650.
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 952943.
- Hu, R.; He, Z.; Liu, M.; Tan, J.; Zhang, H.; Hou, D.X.; He, J.; Wu, S. Dietary protocatechuic acid ameliorates inflammation and up-regulates intestinal tight junction proteins by modulating gut microbiota in LPS-challenged piglets. J. Anim. Sci. Biotechnol. 2020, 11, 92.
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phytother. Res. 2020, 34, 729–741.
- Kim, M.; Kim, J.; Shin, Y.K.; Kim, K.Y. Gentisic acid stimulates keratinocyte proliferation through ERK1/2 phosphorylation. Int. J. Med. Sci. 2020, 17, 626.
- Sharma, N.; Tiwari, N.; Vyas, M.; Khurana, N.; Muthuraman, A.; Utreja, P. An overview of therapeutic effects of vanillic acid. Plant Arch. 2020, 20, 3053–3059.
- Kim, S.J.; Kim, M.C.; Um, J.Y.; Hong, S.H. The beneficial effect of vanillic acid on ulcerative colitis. Molecules 2010, 15, 7208–7217.
- Cikman, O.; Soylemez, O.; Ozkan, O.F.; Kiraz, H.A.; Sayar, I.; Ademoglu, S.; Taysi, S.; Karaayvaz, M. Antioxidant activity of syringic acid prevents oxidative stress in L-arginine–induced acute pancreatitis: An experimental study on rats. Int. Surg. 2015, 100, 891–896.
- Sowbhagya, R.; Anupama, S.K.; Bhagyalakshmi, D.; Anand, S.; Ravikiran, T. Modulatory effects of Decalepishamiltonii extract and its compounds on the antioxidant status of the aging rat brain. J. Pharm. Bioallied Sci. 2017, 9, 8–15.
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S.
- Guven, M.; Aras, A.B.; Akman, T.; Sen, H.M.; Ozkan, A.; Salis, O.; Sehitoglu, I.; Kalkan, Y.; Silan, C.; Deniz, M.; et al. Neuroprotective effect of p-coumaric acid in rat model of embolic cerebral ischemia. Iran. J. Basic Med. Sci. 2015, 18, 356–363.
- Mallik, S.B.; Mudgal, J.; Nampoothiri, M.; Hall, S.; Anoopkumar-Dukie, S.; Grant, G.; Rao, C.M.; Arora, D. Caffeic acid attenuates lipopolysaccharide-induced sickness behaviour and neuroinflammation in mice. Neurosci. Lett. 2016, 632, 218–223.
- Alkis, H.E.; Kuzhan, A.; Dirier, A.; Tarakcioglu, M.; Demir, E.; Saricicek, E.; Demir, T.; Ahlatci, A.; Demirci, A.; Cinar, K.; et al. Neuroprotective effects of propolis and caffeic acid phenethyl ester (CAPE) on the radiation-injured brain tissue (Neuroprotective effects of propolis and CAPE). Int. J. Radiat. Res. 2015, 13, 297–303.
- Shin, D.S.; Kim, K.W.; Chung, H.Y.; Yoon, S.; Moon, J.O. Effect of sinapic acid against carbon tetrachloride-induced acute hepatic injury in rats. Arch. Pharm. Res. 2013, 36, 626–633.
- Lee, H.E.; Kim, D.H.; Park, S.J.; Kim, J.M.; Lee, Y.W.; Jung, J.M.; Lee, C.H.; Hong, J.G.; Liu, X.; Cai, M.; et al. Neuroprotective effect of sinapic acid in a mouse model of amyloid β1–42 protein-induced Alzheimer’s disease. Pharmacol. Biochem. Behav. 2012, 103, 260–266.
- Chen, J.; Lin, D.; Zhang, C.; Li, G.; Zhang, N.; Ruan, L.; Yan, Q.; Li, J.; Yu, X.; Xie, X.; et al. Antidepressant-like effects of ferulic acid: Involvement of serotonergic andnorepinergic systems. Metab. Brain Dis. 2015, 30, 129–136.
- Farha, A.K.; Yang, Q.Q.; Kim, G.; Li, H.B.; Zhu, F.; Liu, H.Y.; Gan, R.Y.; Corke, H. Tannins as an alternative to antibiotics. Food Biosci. 2020, 38, 100751.
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2021, 40, 432–444.
- Okuda, T. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry 2005, 66, 2012–2031.
- Jerez, M.; Tourio, S.; Sineiro, J.; Torres, J.L.; Nuez, M.J. Procyanidins from pine bark: Relationships between structure, composition and antiradical activity. Food Chem. 2007, 104, 518–527.
- Nobre-Junior, H.V.; Maia, F.D.; de Oliveira, R.A.; Bandeira, M.A.; do Ó Pessoa, C.; Moraes, M.O.; Cunha, G.M.; Viana, G.S. Neuroprotective actions of Tannins from Myracrodruonurundeuva on 6-hydroxydopamine-induced neuronal cell death. J. Herbs Spices Med. Plants 2008, 13, 41–57.
- Senobari, Z.; Karimi, G.; Jamialahmadi, K. Ellagitannins, promising pharmacological agents for the treatment of cancer stem cells. Phytother. Res. 2022, 36, 231–242.
- Lim, C.G.; AG Koffas, M. Bioavailability and recent advances in the bioactivity of flavonoid and stilbene compounds. Curr. Org. Chem. 2010, 14, 1727–1751.
- King, R.E.; Bomser, J.A.; Min, D.B. Bioactivity of Resveratrol. Compr. Rev. Food Sci. Food Saf. 2006, 5, 65–70.
- Ma, X.; Sun, Z.; Han, X.; Li, S.; Jiang, X.; Chen, S.; Zhang, J.; Lu, H. Neuroprotective effect of resveratrol via activation of Sirt1 signaling in a rat model of combined diabetes and Alzheimer’s disease. Front. Neurosci. 2020, 13, 1400.
- Pechanova, O.; Dayar, E.; Cebova, M. Therapeutic potential of polyphenols-loaded polymeric nanoparticles in cardiovascular system. Molecules 2020, 25, 3322.
- Stohr, H.; Herrmann, K. The phenolics of fruits. VI. The phenolics of currants, gooseberries and blueberries. Changes in phenolic acids and catechins during development of black currants (author’s transl). Z. Lebensm. Unters. Forsch. 1975, 159, 31–37.
- Karamac, M.; Kosinska, A.; Pegg, R.B. Content of gallic acid in selected plant extracts. Polish J. Food Nutr. Sci. 2006, 15, 55.
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.). J. Agric.Food Chem. 2008, 56, 4631–4636.
- Van Lelyveld, L.J.; Van Vuuren, S.P.; Visser, G. Gentisic acid concentration in healthy and greening infected fruit albedo and leaves of citrus species and cultivars. S. Afr. J. Plant Soil 1988, 5, 209–211.
- García-Villalba, R.; Espín, J.C.; Aaby, K.; Alasalvar, C.; Heinonen, M.; Jacobs, G.; Voorspoels, S.; Koivumäki, T.; Kroon, P.A.; Pelvan, E.; et al. Validated Method for the Characterization and Quantification of Extractable and Nonextractable Ellagitannins after Acid Hydrolysis in Pomegranate Fruits, Juices, and Extracts. J. Agric. Food Chem. 2015, 63, 6555–6566.
- Daniel, E.M.; Krupnick, A.S.; Heur, Y.-H.; Blinzler, J.A.; Nims, R.W.; Stoner, G.D. Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J. Food Compos. Anal. 1989, 2, 338–349.
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619.
- Ndolo, V.U.; Beta, T. Comparative studies on composition and distribution of phenolic acids in cereal grain botanical fractions. Cereal Chem. 2014, 91, 522–530.
- Li, P.; Wang, X.Q.; Wang, H.Z.; Wu, Y.N. High performance liquid chromatographic determination of phenolic acids in fruits and vegetables. Biomed. Environ. Sci. 1993, 6, 389–398.
- Xuan, T.D.; Bach, D.T.; Dat, T.D. Involvement of phenolics, flavonoids and phenolic acids in high yield characteristics of rice (Oryza sativa L.). Int. Lett. Nat. Sci. 2018, 68, 19–26.
- Shehata, W.A.; Akhtar, S.; Alam, T. Extraction and estimation of anthocyanin content and antioxidant activity of some common fruits. Trends Appl. Sci. Res. 2020, 15, 179–186.
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949.
- Yang, X.; Tomás-Barberán, F.A. Tea Is a Significant Dietary Source of Ellagitannins and Ellagic Acid. J. Agric. Food Chem. 2019, 67, 5394–5404.
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404.
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470.
