
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | MONIKA STANISZEWSKA | -- | 2667 | 2023-04-28 16:07:00 | | | |
| 2 | Jessie Wu | + 3 word(s) | 2670 | 2023-05-04 05:50:18 | | |
Video Upload Options
Anaphylaxis is one of the most life-threatening and intensive allergic reactions. Unlike anaphylactoid reaction, it is an immunoglobulin E-mediated response. Its symptoms can occur in multiple organ systems, such as cutaneous, respiratory, cardiovascular, and others. Mast cells together with basophils are the first cells that are responding to IgE-mediated anaphylaxis. Mast cells (MCs) are the immune cells distributed throughout nearly all tissues, mainly in the skin, near blood vessels and lymph vessels, nerves, lungs, and the intestines. Although MCs are essential to the healthy immune response, their overactivity and pathological states can lead to numerous health hazards. The side effect of mast cell activity is usually caused by degranulation. It can be triggered by immunological factors, such as immunoglobulins, lymphocytes, or antigen–antibody complexes, and non-immune factors, such as radiation and pathogens. An intensive reaction of mast cells can even lead to anaphylaxis, one of the most life-threatening allergic reactions. What is more, mast cells play a role in the tumor microenvironment by modulating various events of tumor biology, such as cell proliferation and survival, angiogenesis, invasiveness, and metastasis.
1. Mast Cells as a Therapeutic Target in Allergic Inflammation
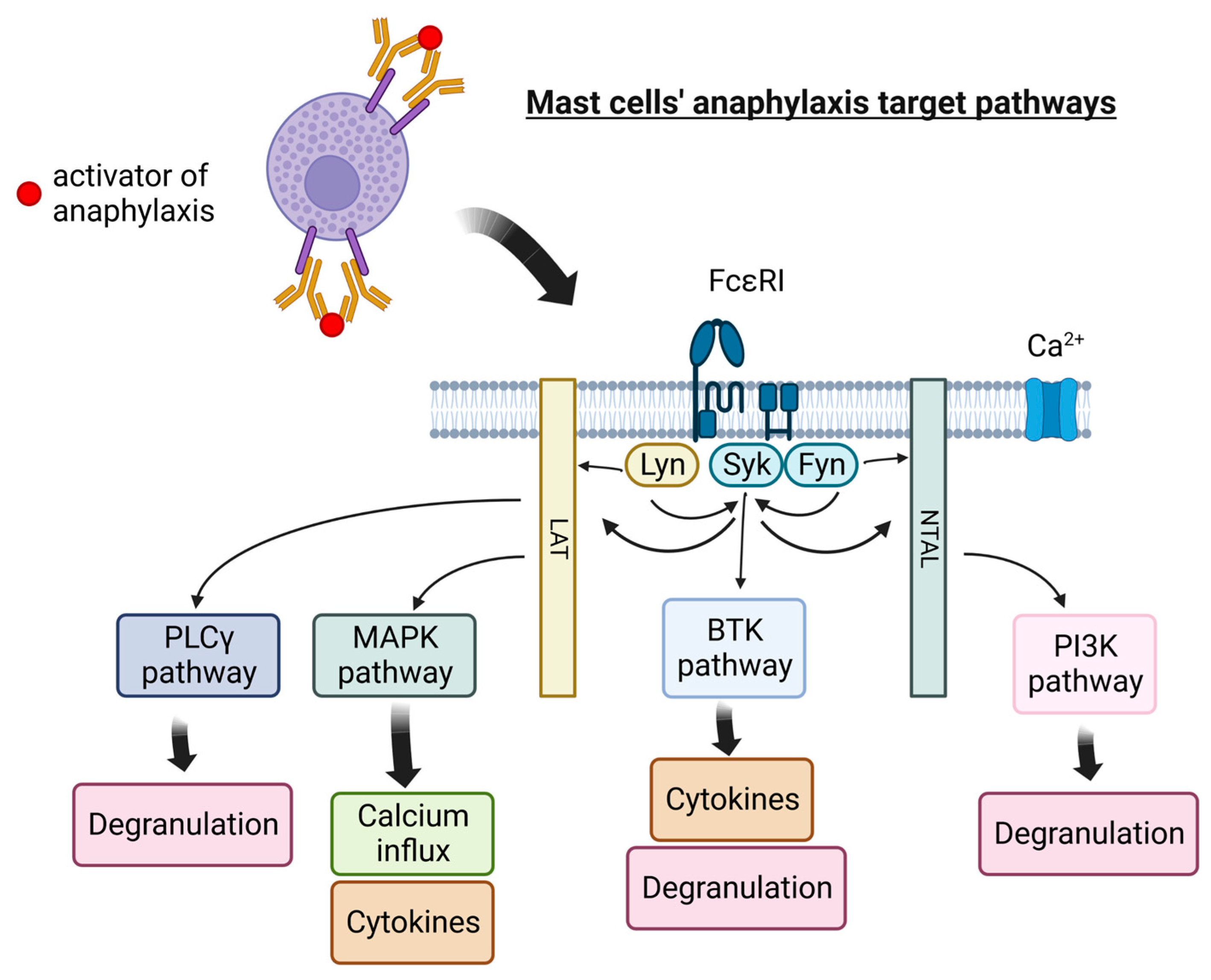
2. Mast Cell-Targeted Strategies in Cancer Therapy
References
- Kolkhir, P.; Elieh-Ali-Komi, D.; Metz, M.; Siebenhaar, F.; Maurer, M. Understanding human mast cells: Lesson from therapies for allergic and non-allergic diseases. Nat. Rev. Immunol. 2022, 22, 294–308.
- Webb, L.M.; Lieberman, P. Anaphylaxis: A review of 601 cases. Ann. Allergy Asthma Immunol. 2006, 97, 39–43.
- Gasser, P.; Eggel, A. Targeting IgE in allergic disease. Curr. Opin. Immunol. 2018, 54, 86–92.
- Rigoni, A.; Colombo, M.P.; Pucillo, C. Mast cells, basophils and eosinophils: From allergy to cancer. Semin. Immunol. 2018, 35, 29–34.
- Brown, J.C.; Simons, E.; Rudders, S.A. Epinephrine in the Management of Anaphylaxis. J. Allergy Clin. Immunol. Pract. 2020, 8, 1186–1195.
- Kemp, S.F.; Lockey, R.F.; Simons, F.E.R. Epinephrine: The Drug of Choice for Anaphylaxis—A Statement of the World Allergy Organization. World Allergy Organ. J. 2008, 1, S18–S26.
- Sampson, H.A.; Muñoz-Furlong, A.; Campbell, R.L.; Adkinson, N.F.; Allan Bock, S.; Branum, A.; Brown, S.G.A.; Camargo, C.A.; Cydulka, R.; Galli, S.J.; et al. Second Symposium on the Definition and Management of Anaphylaxis: Summary Report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. Ann. Emerg. Med. 2006, 47, 373–380.
- Li, L.; Wang, Z.; Cui, L.; Xu, Y.; Guan, K.; Zhao, B. Anaphylactic risk related to omalizumab, benralizumab, reslizumab, mepolizumab, and dupilumab. Clin. Transl. Allergy 2021, 11, e12038.
- Otani, T.; Iwamoto, H.; Horimasu, Y.; Yamaguchi, K.; Sakamoto, S.; Masuda, T.; Miyamoto, S.; Nakashima, T.; Fujitaka, K.; Hamada, H.; et al. Effect of dupilumab in a patient with severe asthma complicated with recurrent anaphylaxis: A case report. J. Investig. Allergol. Clin. Immunol. 2022, 33, 3.
- Jang, H.-Y.; Ha, D.H.; Rah, S.-Y.; Lee, D.-H.; Lee, S.-M.; Park, B.-H. Sirtuin 6 is a negative regulator of FcεRI signaling and anaphylactic responses. J. Allergy Clin. Immunol. 2022, 149, 156–167.e7.
- Dispenza, M.C.; Krier-Burris, R.A.; Chhiba, K.D.; Undem, B.J.; Robida, P.A.; Bochner, B.S. Bruton’s tyrosine kinase inhibition effectively protects against human IgE-mediated anaphylaxis. J. Clin. Investig. 2020, 130, 4759–4770.
- Morris, S.C.; Perkins, C.; Potter, C.; Parsons, D.; Schuman, R.; Khodoun, M.V.; Samavedam, U.; Strait, R.; Finkelman, F.D. Optimizing drug inhibition of IgE-mediated anaphylaxis in mice. J. Allergy Clin. Immunol. 2022, 149, 671–684.e9.
- Chang, Y.; Fan, T.; Huang, J. Anemoside B4 protects against IgE-dependent allergic responses by suppressing the PLC/IP3 and JAK/STAT3 pathways. Chem. Biol. Interact. 2022, 366, 110153.
- Ye, Y.; Sun, J.; Wang, L.; Zhu, J.; Cui, W.; Hou, H.; Zhang, J.; Zhou, C.; Yan, X. Isolation and Purification of Fucoxanthin from Brown Seaweed Sargassum horneri Using Open ODS Column Chromatography and Ethanol Precipitation. Molecules 2021, 26, 3777.
- Li, S.; Zhang, Y.; Veeraraghavan, V.P.; Mohan, S.K.; Ma, Y. Restorative Effect of Fucoxanthin in an Ovalbumin-Induced Allergic Rhinitis Animal Model through NF-κB p65 and STAT3 Signaling. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 365–375.
- Zhong, W.-C.; Li, E.-C.; Hao, R.-R.; Zhang, J.-F.; Jin, H.-T.; Lin, S. Anti-anaphylactic potential of benzoylpaeoniflorin through inhibiting HDC and MAPKs from Paeonia lactiflora. Chin. J. Nat. Med. 2021, 19, 825–835.
- Xu, C.; Li, L.; Wang, C.; Jiang, J.; Li, L.; Zhu, L.; Jin, S.; Jin, Z.; Lee, J.J.; Li, G.; et al. Effects of G-Rh2 on mast cell-mediated anaphylaxis via AKT-Nrf2/NF-κB and MAPK-Nrf2/NF-κB pathways. J. Ginseng Res. 2022, 46, 550–560.
- Kee, J.-Y.; Hong, S.-H. Ginsenoside Rg3 suppresses mast cell–mediated allergic inflammation via mitogen-activated protein kinase signaling pathway. J. Ginseng Res. 2019, 43, 282–290.
- Park, E.-K.; Choo, M.-K.; Kim, E.-J.; Han, M.J.; Kim, D.-H. Antiallergic Activity of Ginsenoside Rh2. Biol. Pharm. Bull. 2003, 26, 1581–1584.
- Park, E.-K.; Choo, M.-K.; Han, M.J.; Kim, D.-H. Ginsenoside Rh1 Possesses Antiallergic and Anti-Inflammatory Activities. Int. Arch. Allergy Immunol. 2004, 133, 113–120.
- Ding, Y.; Wang, Y.; Li, C.; Zhang, Y.; Hu, S.; Gao, J.; Liu, R.; An, H. α-Linolenic acid attenuates pseudo-allergic reactions by inhibiting Lyn kinase activity. Phytomedicine 2021, 80, 153391.
- Rivera, J.; Olivera, A. Src family kinases and lipid mediators in control of allergic inflammation. Immunol. Rev. 2007, 217, 255–268.
- Wang, Y.; Ding, Y.; Li, C.; Gao, J.; Wang, X.; An, H. Alpha-linolenic acid inhibits IgE-mediated anaphylaxis by inhibiting Lyn kinase and suppressing mast cell activation. Int. Immunopharmacol. 2022, 103, 108449.
- Ashikari, T.; Hachisu, M.; Nagata, K.; Ando, D.; Iizuka, Y.; Ito, N.; Ito, K.; Ikeda, Y.; Matsubara, H.; Yashiro, T.; et al. Salicylaldehyde Suppresses IgE-Mediated Activation of Mast Cells and Ameliorates Anaphylaxis in Mice. Int. J. Mol. Sci. 2022, 23, 8826.
- Gilfillan, A.M.; Beaven, M.A. Regulation of Mast Cell Responses in Health and Disease. Crit. Rev. Immunol. 2011, 31, 475–530.
- Weller, C.L.; Collington, S.J.; Williams, T.; Lamb, J.R. Mast cells in health and disease. Clin. Sci. 2011, 120, 473–484.
- Dawicki, W.; Marshall, J.S. New and emerging roles for mast cells in host defence. Curr. Opin. Immunol. 2007, 19, 31–38.
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454.
- Oldford, S.A.; Marshall, J.S. Mast cells as targets for immunotherapy of solid tumors. Mol. Immunol. 2015, 63, 113–124.
- Theoharides, T.C.; Alysandratos, K.-D.; Angelidou, A.; Delivanis, D.-A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2012, 1822, 21–33.
- Sobiepanek, A.; Kuryk, Ł.; Garofalo, M.; Kumar, S.; Baran, J.; Musolf, P.; Siebenhaar, F.; Fluhr, J.W.; Kobiela, T.; Plasenzotti, R.; et al. The Multifaceted Roles of Mast Cells in Immune Homeostasis, Infections and Cancers. Int. J. Mol. Sci. 2022, 23, 2249.
- Theoharides, T.C.; Conti, P. Mast cells: The JEKYLL and HYDE of tumor growth. Trends Immunol. 2004, 25, 235–241.
- Aponte-López, A.; Fuentes-Pananá, E.M.; Cortes-Muñoz, D.; Muñoz-Cruz, S. Mast Cell, the Neglected Member of the Tumor Microenvironment: Role in Breast Cancer. J. Immunol. Res. 2018, 2018, 2584243.
- Ammendola, M.; Sacco, R.; Sammarco, G.; Donato, G.; Montemurro, S.; Ruggieri, E.; Patruno, R.; Marech, I.; Cariello, M.; Vacca, A.; et al. Correlation between Serum Tryptase, Mast Cells Positive to Tryptase and Microvascular Density in Colo-Rectal Cancer Patients: Possible Biological-Clinical Significance. PLoS ONE 2014, 9, e99512.
- Elezoglu, B.; Tolunay, S. The relationship between the stromal mast cell number, microvessel density, c-erbb-2 staining and survival and prognostic factors in colorectal carcinoma. Turk. J. Pathol. 2012, 28, 110.
- Gulubova, M.; Vlaykova, T. Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer. J. Gastroenterol. Hepatol. 2009, 24, 1265–1275.
- Wu, X.; Zou, Y.; He, X.; Yuan, R.; Chen, Y.; Lan, N.; Lian, L.; Wang, F.; Fan, X.; Zeng, Y.; et al. Tumor-Infiltrating Mast Cells in Colorectal Cancer as a Poor Prognostic Factor. Int. J. Surg. Pathol. 2013, 21, 111–120.
- Ammendola, M.; Marech, I.; Sammarco, G.; Zuccalà, V.; Luposella, M.; Zizzo, N.; Patruno, R.; Crovace, A.; Ruggieri, E.; Zito, A.; et al. Infiltrating Mast Cells Correlate with Angiogenesis in Bone Metastases from Gastric Cancer Patients. Int. J. Mol. Sci. 2015, 16, 3237–3250.
- Ammendola, M.; Sacco, R.; Zuccalà, V.; Luposella, M.; Patruno, R.; Gadaleta, P.; Zizzo, N.; Gadaleta, C.; De Sarro, G.; Sammarco, G.; et al. Mast Cells Density Positive to Tryptase Correlate with Microvascular Density in both Primary Gastric Cancer Tissue and Loco-Regional Lymph Node Metastases from Patients That Have Undergone Radical Surgery. Int. J. Mol. Sci. 2016, 17, 1905.
- Micu, G.V.; Stăniceanu, F.; Sticlaru, L.C.; Popp, C.G.; Bastian, A.E.; Gramada, E.; Pop, G.; Mateescu, R.B.; Rimbaş, M.; Archip, B.; et al. Correlations Between the Density of Tryptase Positive Mast Cells (DMCT) and that of New Blood Vessels (CD105+) in Patients with Gastric Cancer. Rom. J. Intern. Med. 2016, 54, 113–120.
- Ribatti, D.; Guidolin, D.; Marzullo, A.; Nico, B.; Annese, T.; Benagiano, V.; Crivellato, E. Mast cells and angiogenesis in gastric carcinoma: Mast cells and angiogenesis in gastric carcinoma. Int. J. Exp. Pathol. 2010, 91, 350–356.
- Cai, S.-W.; Yang, S.-Z.; Gao, J.; Pan, K.; Chen, J.-Y.; Wang, Y.-L.; Wei, L.-X.; Dong, J.-H. Prognostic significance of mast cell count following curative resection for pancreatic ductal adenocarcinoma. Surgery 2011, 149, 576–584.
- Chang, D.Z.; Ma, Y.; Ji, B.; Wang, H.; Deng, D.; Liu, Y.; Abbruzzese, J.L.; Liu, Y.; Logsdon, C.D.; Hwu, P. Mast Cells in Tumor Microenvironment Promotes the In Vivo Growth of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2011, 17, 7015–7023.
- Porcelli, L.; Iacobazzi, R.; Di Fonte, R.; Serratì, S.; Intini, A.; Solimando, A.; Brunetti, O.; Calabrese, A.; Leonetti, F.; Azzariti, A.; et al. CAFs and TGF-β Signaling Activation by Mast Cells Contribute to Resistance to Gemcitabine/Nabpaclitaxel in Pancreatic Cancer. Cancers 2019, 11, 330.
- Strouch, M.J.; Cheon, E.C.; Salabat, M.R.; Krantz, S.B.; Gounaris, E.; Melstrom, L.G.; Dangi-Garimella, S.; Wang, E.; Munshi, H.G.; Khazaie, K.; et al. Crosstalk between Mast Cells and Pancreatic Cancer Cells Contributes to Pancreatic Tumor Progression. Clin. Cancer Res. 2010, 16, 2257–2265.
- Carpenco, E.; Ceauşu, R.A.; Cimpean, A.M.; Gaje, P.N.; Șaptefraţi, L.; Fulga, V.; David, V.; Raica, M. Mast Cells as an Indicator and Prognostic Marker in Molecular Subtypes of Breast Cancer. In Vivo 2019, 33, 743–748.
- Fakhrjou, A.; Naghavi-Behzad, M.; Montazeri, V.; Karkon-Shayan, F.; Norouzi-Panahi, L.; Piri, R. The relationship between histologic grades of invasive carcinoma of breast ducts and mast cell infiltration. South Asian J. Cancer 2016, 05, 005–007.
- Keser, S.H.; Kandemir, N.O.; Ece, D.; Gecmen, G.G.; Gul, A.E.; Barisik, N.O.; Sensu, S.; Buyukuysal, C.; Barut, F. Relationship of mast cell density with lymphangiogenesis and prognostic parameters in breast carcinoma. Kaohsiung J. Med. Sci. 2017, 33, 171–180.
- Marech, I.; Ammendola, M.; Sacco, R.; Capriuolo, G.S.; Patruno, R.; Rubini, R.; Luposella, M.; Zuccalà, V.; Savino, E.; Gadaleta, C.D.; et al. Serum tryptase, mast cells positive to tryptase and microvascular density evaluation in early breast cancer patients: Possible translational significance. BMC Cancer 2014, 14, 534.
- Reddy, S.M.; Reuben, A.; Barua, S.; Jiang, H.; Zhang, S.; Wang, L.; Gopalakrishnan, V.; Hudgens, C.W.; Tetzlaff, M.T.; Reuben, J.M.; et al. Poor Response to Neoadjuvant Chemotherapy Correlates with Mast Cell Infiltration in Inflammatory Breast Cancer. Cancer Immunol. Res. 2019, 7, 1025–1035.
- Carlini, M.J.; Dalurzo, M.C.L.; Lastiri, J.M.; Smith, D.E.; Vasallo, B.C.; Puricelli, L.I.; de Cidre, L.S.L. Mast cell phenotypes and microvessels in non–small cell lung cancer and its prognostic significance. Hum. Pathol. 2010, 41, 697–705.
- Imada, A.; Shijubo, N.; Kojima, H.; Abe, S. Mast cells correlate with angiogenesis and poor outcome in stage I lung adenocarcinoma. Eur. Respir. J. 2000, 15, 1087–1093.
- Takanami, I.; Takeuchi, K.; Naruke, M. Mast cell density is associated with angiogenesis and poor prognosis in pulmonary adenocarcinoma. Cancer 2000, 88, 2686–2692.
- Nonomura, N.; Takayama, H.; Nishimura, K.; Oka, D.; Nakai, Y.; Shiba, M.; Tsujimura, A.; Nakayama, M.; Aozasa, K.; Okuyama, A. Decreased number of mast cells infiltrating into needle biopsy specimens leads to a better prognosis of prostate cancer. Br. J. Cancer 2007, 97, 952–956.
- Pittoni, P.; Tripodo, C.; Piconese, S.; Mauri, G.; Parenza, M.; Rigoni, A.; Sangaletti, S.; Colombo, M.P. Mast Cell Targeting Hampers Prostate Adenocarcinoma Development but Promotes the Occurrence of Highly Malignant Neuroendocrine Cancers. Cancer Res. 2011, 71, 5987–5997.
- Bahri, R.; Kiss, O.; Prise, I.; Garcia-Rodriguez, K.M.; Atmoko, H.; Martínez-Gómez, J.M.; Levesque, M.P.; Dummer, R.; Smith, M.P.; Wellbrock, C.; et al. Human Melanoma-Associated Mast Cells Display a Distinct Transcriptional Signature Characterized by an Upregulation of the Complement Component 3 That Correlates With Poor Prognosis. Front. Immunol. 2022, 13, 861545.
- Ammendola, M.; Sacco, R.; Sammarco, G.; Luposella, M.; Patruno, R.; Gadaleta, C.D.; De Sarro, G.; Ranieri, G. Mast Cell-Targeted Strategies in Cancer Therapy. Transfus. Med. Hemotherapy 2016, 43, 109–113.
- Faustino-Rocha, A.I.; Gama, A.; Oliveira, P.A.; Vanderperren, K.; Saunders, J.H.; Pires, M.J.; Ferreira, R.; Ginja, M. Modulation of mammary tumor vascularization by mast cells: Ultrasonographic and histopathological approaches. Life Sci. 2017, 176, 35–41.
- Iqbal, N.; Iqbal, N. Imatinib: A Breakthrough of Targeted Therapy in Cancer. Chemother. Res. Pract. 2014, 2014, 357027.
- Cimpean, A.M.; Raica, M. The Hidden Side of Disodium Cromolyn: From Mast Cell Stabilizer to an Angiogenic Factor and Antitumor Agent. Arch. Immunol. Ther. Exp. 2016, 64, 515–522.
- Marech, I.; Ammendola, M.; Gadaleta, C.; Zizzo, N.; Oakley, C.; Gadaleta, C.D.; Ranieri, G. Possible biological and translational significance of mast cells density in colorectal cancer. World J. Gastroenterol. 2014, 20, 8910–8920. Available online: https://www.wjgnet.com/1007-9327/full/v20/i27/8910.htm (accessed on 9 March 2023).
- Alzforum. Mastinib. Available online: www.alzforum.org/therapeutics/mastinib (accessed on 20 January 2023).
- Dubreuil, P.; Letard, S.; Ciufolini, M.; Gros, L.; Humbert, M.; Castéran, N.; Borge, L.; Hajem, B.; Lermet, A.; Sippl, W.; et al. Masitinib (AB1010), a Potent and Selective Tyrosine Kinase Inhibitor Targeting KIT. PLoS ONE 2009, 4, e7258.
- Ribatti, D. Mast cells as therapeutic target in cancer. Eur. J. Pharmacol. 2016, 778, 152–157.
- Demetri, G.D.; van Oosterom, A.T.; Garrett, C.R.; Blackstein, M.E.; Shah, M.H.; Verweij, J.; McArthur, G.; Judson, I.R.; Heinrich, M.C.; Morgan, J.A.; et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006, 368, 1329–1338.
- Ohno, H.; Kosaki, G.; Kambayashi, J.; Imaoka, S.; Hirata, F. FOY: methanesulfonate as a serine proteinase inhibitor. I. Inhibition of thrombin and factor Xa in vitro. Thromb. Res. 1980, 19, 579–588.
- Tamura, Y.; Hirado, M.; Okamura, K.; Minato, Y.; Fujii, S. Synthetic inhibitors of trypsin, plasmin, kallikrein, thrombin, and C1 esterase. Biochim. Biophys. Acta BBA Enzymol. 1977, 484, 417–422.
- Yoon, W.-H.; Jung, Y.-J.; Kim, T.-D.; Li, G.; Park, B.-J.; Kim, J.-Y.; Lee, Y.-C.; Kim, J.-M.; Park, J.-I.; Park, H.-D.; et al. Gabexate Mesilate Inhibits Colon Cancer Growth, Invasion, and Metastasis by Reducing Matrix Metalloproteinases and Angiogenesis. Clin. Cancer Res. 2004, 10, 4517–4526.
- PubChem. Nafamostat Mesylate. Available online: Pubchem.ncbi.nlm.nih.gov/compound/Nafamostat-mesylate (accessed on 20 January 2023).




