
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | V Mahalakshmi | -- | 1496 | 2023-04-28 14:14:45 | | | |
| 2 | Dean Liu | Meta information modification | 1496 | 2023-05-04 03:17:07 | | | | |
| 3 | Dean Liu | -2 word(s) | 1494 | 2023-05-05 03:17:56 | | | | |
| 4 | V Mahalakshmi | + 375 word(s) | 1869 | 2023-05-17 15:31:01 | | | | |
| 5 | Vimalanathan ArunPrasanna | Meta information modification | 1869 | 2023-05-25 14:15:06 | | |
Video Upload Options
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which caused coronavirus diseases (COVID-19) in late 2019 in China created a devastating economical loss and loss of human lives.11 variants have been identified with minimum to maximum severity of infection and surges in cases. Bacterial co-infection/secondary infection is identified during viral respiratory infection, which is a vital reason for morbidity and mortality. The occurrence of secondary infections is an additional burden to the healthcare system; therefore, the quick diagnosis of both COVID-19 and secondary infections will reduce work pressure on healthcare workers. Therefore, well-established support from Artificial Intelligence (AI) could reduce the stress in healthcare and even help in creating novel products to defend against the coronavirus. AI is one of the rapidly growing fields with numerous applications for the healthcare sector.
1. AI in Utility Services
2. AI for Researchers
3. ML in Oil and Gas
4. AI-Based Decision-Making in the Hospital
5. Telemonitoring during COVID-19
6. Electrospun nanofiber mask by nanotechnology
Another major breakthrough in controlling the airborne transmission of SARS-CoV-2 is through the advanced electrospun nanofibrous air filters adopted masks. Electrospun nanofibers are not a new material to the medical field, these fibers are used in various medical applications for the past two decades, Abutaleb et al., (2021) have described various biodegradable nanofiber materials from natural resources for medical applications [134]. Nanotechnology is highly reliable in developing effective, scalable and cheaper air filters for mask and respirators. Electrospun technique have the capacity to produce a lesser pore size to several micrometers, when compared to commercial filters ES fibers capture smaller airborne particles [134,135]. In this study, a coronavirus aerosol test against ES mask was carried out. A polypropylene fabric was subjected to electrospun with polyvinylidene fluoride PVDF20 and PVDF30. This layer was soaked in to a polyelectrolyte poly(ethylenimine) (PEI) and poly(vinylphosphonic acid) (PVPA) which is positively or negatively charged to enhance the electrostatic attraction for virus removal. The diameter of this PVDF nanofiber is found to be 0.2 – 1.3µm, but other commercial face mask and neck gaiter in this study showed fiber diameter of 5.7 ± 2.8 and 12.0 ± 1.0µm. Usually fibers with larger diameter and pore size are less effective in airborne particle filter, but lesser in diameter and pore size are highly effective in filtration of aerosol. This is achieved by increasing the ES spinning time, which exhibit highest removal of corona virus aerosol up to 99.9% for PVDF30 and 99.1% for PVDF20 Figure 6 [136]. The reuse of facemask is an important question in this pandemic, a study carried out to identify melt-blown filter used in N95 face mask and nanofiber filter face mask reusability test with 75% ethanol treatment. It shows, there is a loss of filtering efficiency in N95 mask after ethanol treatment, but nanofiber filter mask shows promising results of filtering capacity up to 97 -99% irrespective of cleaning method [137]. The economically cheaper materials like ES fabrics are highly recommended for mask production in this pandemic time. This ES air filters are more competitive against other commercial filters in market and portable ES apparatus can be much helpful in rapid preparation of ES masks at home and for minor populations [134].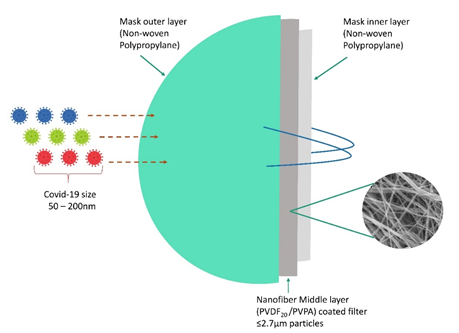
Figure 1. Illustration of a nanofiber mask with 3 layers of protection against micro-aerosol droplets with corona virus.
6. AI-Based Law in Various Countries
| Country | Law/Regulation | Purpose of This Law | Date Effective |
|---|---|---|---|
| USA | No Vaccine Passports Act [12] | Relaxing the restrictions of forcing vaccine certificate | 4 August 2021 |
| The Netherlands (Red Cross) | 510 Data Responsibility Policy [13] | Data protection | 12 November 2018 |
| United Kingdom | Contact-tracing app (General Data Protection Regulation (UK GDPR) and Data Protection Act (DPA) 2018) [14] | Data protection and digital COVID tracking app | May 2020 |
| European Union | General Data Protection Regulation [15] | Data protection of public and health records | 27 April 2016 |
| European Union | Medical Devices Regulations 2017/745 (MDR) [16] | Protection of patients from medical device, protection of produced data using this device | 5 April 2017 |
| European Union | The 2017/746 In Vitro Diagnostic Medical Devices Regulation (IVDR) [17] | Protection of patient health and users, quality and safety of in vitro medical devices | 25 January 2022 |
| European Union | Regulation of the European Parliament and of the council laying down harmonized rules on AI (Artificial Intelligence ACT) and amending certain union legislative acts [18] | Facilitate and creating innovation in AI, creating trusted AI applications | 21 April 2021 |
| European Union | Civil Law Rules on Robotics [19] | Implementation of AI robotics | 16 February 2017 |
| Singapore | Personal Data Protection Act 2012 [20] | Data protection | 31 December 2021 |
| Australia | Therapeutic Goods (Medical Devices) Regulations 2002 [21] | Clinical decision support software | 25 February 2021 |
| China | Notice of the State Council Issuing the New Generation of Artificial Intelligence Development Plan. State Council Document. No. 35. 2017 [22] | Healthcare and management | 8 July 2017 |
| Kingdom of Saudi Arabia | Guidance on Software as a Medical Device/SFDA MDS-G23 [23] | AI- and BigData-based medical software to diagnose and predict patient conditions | 27 April 2021 |
| Russia | Development of AI in healthcare up to 2030, approved on 10 October 2019, No. 490 [24] | Software as medical device in healthcare | 10 October 2019 |
| South Korea | Medical Devices Act No. 15945, 11 December 2018 [25] | Software as medical device in healthcare | 11 December 2008 |
| Singapore | Standalone Medical Mobile Applications (SaMD) and Qualification of Clinical Decision Support Software (CDSS) [26] | Clinical decision support software | 19 July 2021 |
| China | Cybersecurity Law of the People’s Republic of China [27] | To preserve cyberspace sovereignty and national security | 7 November 2016 |
| Malaysia | Medical Device Act 737-2012 [28] | Medical device, software regulation in healthcare | 30 January 2012 |
| Emirate of Abu Dhabi | Artificial Intelligence (AI) in the Healthcare Sector of the Emirate of Abu Dhabi, Policy/AI/0.9, Version 0.9 [29] | Health system monitors, analysis, and public health observation | 30 April 2018 |
| Canada | Digital Charter Implementation Act, 2022 (Bill C-27) [30] | Protection of personal information, data, and health records, along with any serious direct cause to patients by AI | 16 June 2022 |
| Brazil | LGPD–General Personal Data Protection Law (Federal Law no. 13,709/2018) [31] | AI regulation in health sector of Brazil | 14 August 2018 |
| Brazil | Brazilian Artificial Intelligence Bill (Bill No. 21/2020) [32] | Development and applying of AI in various sectors of Brazil | 29 September 2021 |
References
- Suvarna, M.; Katragadda, A.; Sun, Z.; Choh, Y.B.; Chen, Q.; PS, P.; Wang, X. A machine learning framework to quantify and assess the impact of COVID-19 on the power sector: An Indian context. Adv. Appl. Energy 2022, 5, 100078.
- Norouzi, N.; Zarazua de Rubens, G.; Choubanpishehzafar, S.; Enevoldsen, P. When pandemics impact economies and climate change: Exploring the impacts of COVID-19 on oil and electricity demand in China. Energy Res. Soc. Sci. 2020, 68, 101654.
- Soni, S.; Roberts, K. An evaluation of two commercial deep learning-based information retrieval systems for COVID-19 literature. J. Am. Med. Informatics Assoc. 2021, 28, 132–137.
- Ou, S.; He, X.; Ji, W.; Chen, W.; Sui, L.; Gan, Y.; Lu, Z.; Lin, Z.; Deng, S.; Przesmitzki, S.; et al. Machine learning model to project the impact of COVID-19 on US motor gasoline demand. Nat. Energy 2020, 5, 666–673.
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Applications of Artificial Intelligence (AI) for cardiology during COVID-19 pandemic. Sustain. Oper. Comput. 2021, 2, 71–78.
- Gleichgerrcht, E.; Munsell, B.; Bhatia, S.; Vandergrift, W.A.; Rorden, C.; McDonald, C.; Edwards, J.; Kuzniecky, R.; Bonilha, L. Deep learning applied to whole-brain connectome to determine seizure control after epilepsy surgery. Epilepsia 2018, 59, 1643–1654.
- Dias, R.D.; Shah, J.A.; Zenati, M.A. Artificial intelligence in cardiothoracic surgery. Minerva Cardioangiol. 2020, 68, 532–538.
- Shabbir, A.; Shabbir, M.; Javed, A.R.; Rizwan, M.; Iwendi, C.; Chakraborty, C. Exploratory data analysis, classification, comparative analysis, case severity detection, and internet of things in COVID-19 telemonitoring for smart hospitals. J. Exp. Theor. Artif. Intell. 2022, 1–28.
- Cingolani, M.; Scendoni, R.; Fedeli, P.; Cembrani, F. Artificial intelligence and digital medicine for integrated home care services in Italy: Opportunities and limits. Front. Public Health 2023, 10, 1095001.
- Panicacci, S.; Donati, M.; Lubrano, A.; Vianello, A.; Ruiu, A.; Melani, L.; Tomei, A.; Fanucci, L. Telemonitoring in the Covid-19 Era: The Tuscany Region Experience. Healthcare 2021, 9, 516.
- Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices. Available online: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices (accessed on 14 November 2022).
- USA, Congress.Gov. No Vaccine Passports Act. Available online: https://www.congress.gov/bill/117th-congress/house-bill/2384?s=1&r=89 (accessed on 14 November 2022).
- Van Der Maarten, V. Data Responsibility V2.2–510 Global. Available online: https://centre.humdata.org/data-responsibility/ (accessed on 28 October 2022).
- UK General Data Protection Regulation (UK GDPR) and Data Protection Act (DPA). 2018. Available online: https://www.gov.uk/government/publications/nhs-covid-19-app-privacy-information/nhs-covid-19-app-privacy-notice#lawful-basis (accessed on 28 October 2022).
- General Data Protection Regulation. Available online: http://data.europa.eu/eli/reg/2016/679/oj (accessed on 28 October 2022).
- EU, Medical AI Tools. 2017/745 Medical Devices Regulations (MDR). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0745 (accessed on 28 October 2022).
- EU, Medical AI Tools. The 2017/746 In Vitro Diagnostic Medical Devices Regulation (IVDR). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32022R0112 (accessed on 28 October 2022).
- European Commission Proposal for a Regulation of the European Parliament and of the Council Laying Down Harmonised Rules on Artificial Intelligence (Artificial Intelligence Act) and Amending Certain Union Legislative Acts. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1623335154975&uri=CELEX%3A52021PC0206 (accessed on 30 October 2022).
- European Parliament Resolution of 16 February 2017 with Recommendations to the Commission on Civil Law Rules on Robotics (2015/2103(INL)). Available online: https://www.europarl.europa.eu/doceo/document/TA-8-2017-0051_EN.pdf (accessed on 30 October 2022).
- Government of Singapore Personal Data Protection Act 2012—Singapore Statutes Online. Available online: https://sso.agc.gov.sg/Act/PDPA2012?ProvIds=P1I-#pr4- (accessed on 30 October 2022).
- Therapeutic Goods Administration, Therapeutic Goods (Medical Devices) Regulations 2002. Available online: https://www.legislation.gov.au/Details/F2021C00217/Download (accessed on 30 October 2022).
- The State Council Notice of the State Council on Issuing the Development Plan for the New Generation of Artificial Intelligence. Available online: https://flia.org/wp-content/uploads/2017/07/A-New-Generation-of-Artificial-Intelligence-Development-Plan-1.pdf (accessed on 30 October 2022).
- Saudi Food and Drug Authority—Guidance on Software as a Medical Device, SFDA MDS-G23. Available online: https://www.sfda.gov.sa/sites/default/files/2021-04/SFDAArtificialIntelligenceEn.pdf (accessed on 30 October 2022).
- Gusev, A.V.; Morozov, S.P.; Kutichev, V.A.; Novitsky, R.E. Legal regulation of artificial intelligence software in healthcare in the Russian Federation. Med. Technol. Assess. Choice 2021, 1, 36.
- Medical Devices Act—South Korea. Available online: https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=50798&type=sogan&key=31 (accessed on 14 November 2022).
- Singapore, Health Services Authority. Guidelines on Risk Classification of Standalone Medical Mobile Applications (SaMD) and Qualification of Clinical Decision Support Software (CDSS). Available online: https://www.hsa.gov.sg/announcements/regulatory-updates/consultation-on-regulatory-guidelines-for-classification-of-standalone-medical-mobile-applications-(samd)-and-qualification-of-clinical-decision-support-software-(cdss) (accessed on 14 November 2022).
- Standing Committee of the National People’s Congress Cybersecurity Law of the People’s Republic of China. Available online: http://www.xinhuanet.com//politics/2016-11/07/c_1119867015_2.htm (accessed on 30 October 2022).
- Gazette, G. Medical Device Act 2012 (ACT 737). Available online: www.federalgazette.agc.gov.my/outputaktap/20120209 (accessed on 14 November 2022).
- Abu Dhabi Department of Health Policy on Use of Artificial Intelligence (AI) in the Healthcare Sector of the Emirate of Abu Dhabi. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwi6iNbo0K77AhWjgv0HHdg5D-wQFnoECBAQAQ&url=https%3A%2F%2Fwww.doh.gov.ae%2F-%2Fmedia%2FE9C1470A575146B18015DEBE57E47F8D.ashx&usg=AOvVaw0TgyUjO4zetznNvkZiRKkt (accessed on 14 November 2022).
- House of Commons Digital Charter Implementation Act. Available online: https://www.parl.ca/DocumentViewer/en/43-2/bill/C-11/first-reading (accessed on 14 November 2022).
- Brazil. Law No 13, 709, of 14 August 2018 General Personal Data Protection Law (LGPD). Available online: http://www.planalto.gov.br/ccivil_03/_ato2015-2018/2018/lei/l13709.htm (accessed on 14 November 2022).
- Brazilian Artificial Intelligence Bill (Bill No. 21/2020). Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiY57269K_7AhVIh_0HHfGcDTQQFnoECAoQAQ&url=https%3A%2F%2Flegis.senado.leg.br%2Fsdleg-getter%2Fdocumento%2Fdownload%2Fa08e2a4b-da0c-4e58-8556-4e9f360e4c42&usg=AOvVaw15XW (accessed on 14 November 2022).





