
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vaughan S Langford | -- | 3243 | 2023-04-28 06:01:48 | | | |
| 2 | Camila Xu | + 22 word(s) | 3265 | 2023-04-28 07:21:53 | | |
Video Upload Options
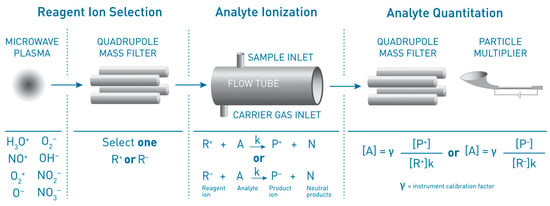
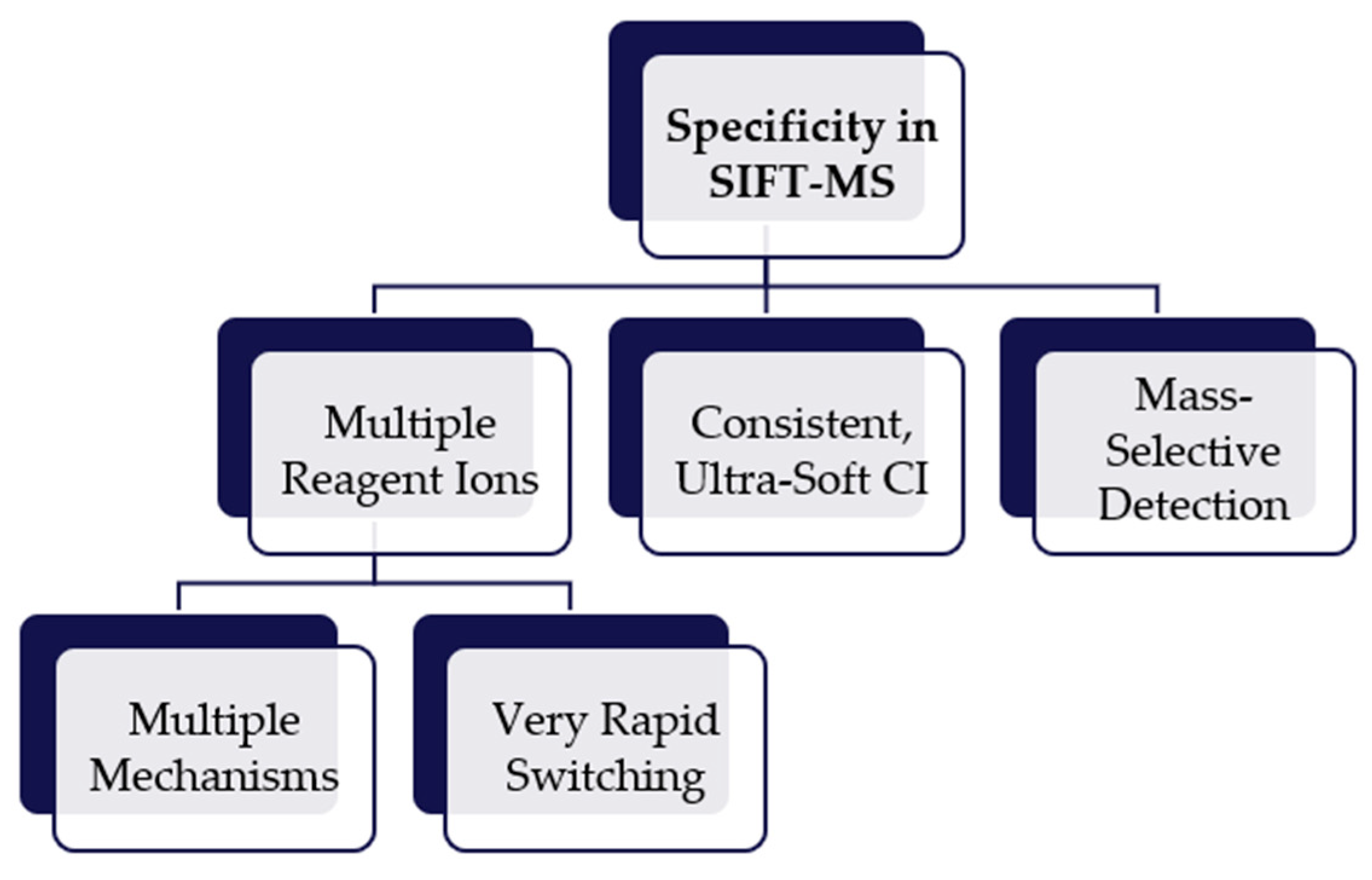
Selected ion flow tube mass spectrometry (SIFT-MS) uses soft chemical ionization (CI) to generate mass-selected reagent ions that can rapidly react with and quantify VOCs down to part-per-trillion concentrations (by volume, pptV). Up to eight reagent ions (H3O+, NO+, O2+, O-, OH-, O2-, NO2- and NO3-) obtained from a microwave discharge in air are available on SIFT-MS instruments. These reagent ions react with VOCs and other trace analytes in well-controlled ion-molecule reactions, but they do not react with the major components of air (N2, O2, CO2 and Ar). This enables direct, real-time analysis of air samples to be achieved at trace and ultra-trace levels without pre-concentration. Rapid switching between reagent ions provides high selectivity because the multiple reaction mechanisms give independent measurements of each analyte.
1. Introduction
2. The SIFT-MS Technique—An Overview
2.1. Instrument Overview
2.2. Breadth of Analysis
| Mechanism Name | Reagent Ion(s) | General Equation | Shift Relative to Parent Ion |
|---|---|---|---|
| Proton transfer (PT) | H3O+ | AH+ + B → BH+ + A | +1 (simple PT) −17 (loss of OH moiety) |
| Electron transfer (ET) | NO+, O2+•, O2−• | A+/− + B → B+/− + A | 0 |
| Dissociative ET | NO+, O2+• | A+ + B → C+ + Products | Varies |
| Hydride abstraction | NO+, O2+• | A+ + B-H → B+ + HA | −1 |
| Association | NO+, O−•, O2−•, OH− | A+/− + B + M → {B.A}+/− + M | +Reagent m/z (e.g., +30 for NO+) |
| Proton abstraction | O−•, O2−•, OH−, NO2−, NO3− | A− + BH → B− + AH | −1 |
| Mechanism Name | Reagent Ion(s) | General Equation | Standard Product * m/z |
| Hydrogen atom transfer | O−• | A− + BH → AH− + B− | Reagent ion m/z − 1 ** |
| Displacement | O−•, OH− | A− + RB → RA + B− (R = alkyl) |
−35 and −37 for B = Cl −79 and −81 for B = Br |
| Elimination | O−•, OH− | A− + RB → R’ + R”A + B− (R’ = alkene; R” = alkyl) |
−35 and −37 for B = Cl −79 and −81 for B = Br |
| Associative detachment | O−•, O2−•, OH− | A− + B → AB + e− (e− = electron) |
No product detected (e−) |
| Compound | Reference(s) | H3O+ | NO+ | O2+• | O−• | O2−• | OH− | NO2− | NO3− |
|---|---|---|---|---|---|---|---|---|---|
| Ammonia | [7][8] | ✓ | ✓ | ✓ | |||||
| Benzene | [9] | ✓ | ✓ | ✓ | |||||
| Formaldehyde | [10] | ✓ | ✓ | ||||||
| Hydrogen sulfide | [11][12][13] | ✓ | ✓ | ✓ | ✓ | ||||
| Ethanol | [14] | ✓ | ✓ | ✓ | ✓ | ||||
| Pyridine | [7][15] | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Methyl bromide | [16][17] | ✓ | ✓ | ✓ | ✓ | ||||
| Carbon dioxide | [18][19][20][21] | ✓ | ✓ | ✓ | |||||
| Hydrogen chloride | [21] | ✓ | ✓ | ✓ | ✓ | ||||
| Sulfur dioxide | [19][21][22] | ✓ | ✓ | ✓ | ✓ |
2.3. Specificity of Analysis
| Reagent Ion | Acetone | Propanal | ||||
|---|---|---|---|---|---|---|
| Formula | m/z | br (%) | Formula | m/z | br (%) | |
| H3O+ | (CH3)2CO.H+ | 59 | 100 | CH3CH2CHO.H+ | 59 | 100 |
| NO+ | (CH3)2CO.NO+ | 88 | 100 | CH3CH2CO+ | 57 | 100 |
| O2+• | (CH3)2CO+• CH3CO+• |
58 43 |
60 40 |
CH3CH2CHO+• CH3CH2CO+ |
58 57 |
50 50 |

2.4. Quantitation in SIFT-MS
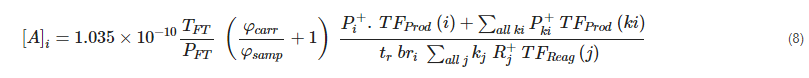
-
TFT is the temperature in the flow tube in Kelvin (K)
-
PFT is the pressure in the flow tube in torr
-
kj is the rate coefficient for reaction of reagent ion Rj+ with the analyte (in cm3 molecule−1 s−1)
-
tr is the reaction time (in seconds)
-
φcarr is the carrier gas flow in torr L s−1
-
φsamp is the sample flow in torr L s−1
-
Pi+ is the primary product ion signal (in counts per second, cps) for primary product ion i counted by the particle multiplier detector
-
TFProd(i) is the transmission factor for the primary product ion Pi+ (dimensionless)
-
Pki+ is the secondary product ion signal (in cps) for secondary product ion k derived from primary product ion i
-
TFProd(ki) is the transmission factor for the secondary product ion Pki+ (dimensionless)
-
Rj+ is the reagent ion signal (in cps) for the injected reagent ion (j = 0) and its water cluster ions (if appropriate; j = 1, 2, 3)
-
TFReag(j) is the transmission factor for the reagent ion Rj+ (dimensionless)
-
bri is the branching ratio for primary product ion i (0 < i ≤ 1 for calculation purposes, but ordinarily tabulated as a percentage; see, e.g., Table 3).
-
Instrument operating parameters: TFT, PFT, φcarr, and φsamp
-
The instrument’s automated performance check on a certified gas standard: tr, TFProd(i), TFProd(ki), and TFReag(j)
-
Software library: kj and bri (clearly, together with the m/z at which the relevant reagent and product ions will be located)
-
Measurement of sample: Pi+, Pki+, and Rj+.
References
- Dettmer-Wilde, K.; Engewald, W. Practical Gas Chromatography; Springer: Heidelberg, Germany, 2014; 902p.
- Fanali, S.; Haddad, P.R.; Poole, C.F.; Riekkola, M.-L. Liquid Chromatography: Fundamentals and Instrumentation, 2nd ed.; Elsevier: Oxford, UK, 2017; 784p.
- Taylor, A.J.; Beauchamp, J.D.; Langford, V.S. Non-destructive and high-throughput–APCI-MS, PTR-MS and SIFT-MS as methods of choice for exploring flavor release. In Dynamic Flavor: Capturing Aroma Release using Real-Time Mass Spectrometry; Beauchamp, J.D., Ed.; American Chemical Society: Washington, DC, USA, 2021; pp. 1–16.
- McEwan, M.J. Direct analysis mass spectrometry. In Ion Molecule Attachment Reactions: Mass Spectrometry; Fujii, T., Ed.; Springer: New York, NY, USA, 2015; pp. 263–317.
- Smith, D.; McEwan, M.J.; Španěl, P. Understanding gas phase ion chemistry is the key to reliable selected ion flow tube-mass spectrometry analyses. Anal. Chem. 2020, 92, 12750–12762.
- Smith, D.; Španěl, P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom. Rev. 2005, 24, 661–700.
- Španěl, P.; Smith, D. Selected ion flow tube studies of the reactions of H3O+, NO+, and O2+ with several amines and some other nitrogen-containing molecules. Int. J. Mass Spectrom. 1998, 176, 203–211.
- Smith, D.; Bloor, R.; George, C.; Pysanenko, A.; Španěl, P. Release of toxic ammonia and volatile organic compounds by heated cannabis and their relation to tetrahydrocannabinol content. Anal. Methods 2015, 7, 4104–4110.
- Španěl, P.; Smith, D. SIFT studies of the reactions of H3O+, NO+ and O2+ with several aromatic and aliphatic hydrocarbons. Int. J. Mass Spectrom. 1998, 181, 1–10.
- Španěl, P.; Ji, Y.; Smith, D. SIFT studies of the reactions of H3O+, NO+ and O2+ with a series of aldehydes and ketones. Int. J. Mass Spectrom. Ion Process. 1997, 165, 25–37.
- Williams, T.L.; Adams, N.G.; Babcock, L.M. Selected ion flow tube studies of H3O+(H2O)0.1 reactions with sulfides and thiols. Int. J. Mass Spectrom. 1998, 172, 149–159.
- Španěl, P.; Smith, D. Selected ion flow tube studies of the reactions of H3O+, NO+, and O2+ with some organosulphur molecules. Int. J. Mass Spectrom. 1998, 176, 167–176.
- LabSyft Software: Compound Library; Syft Technologies Limited: Christchurch, New Zealand, 2017.
- Španěl, P.; Smith, D. SIFT studies of the reactions of H3O+, NO+ and O2+ with a series of alcohols. Int. J. Mass Spectrom. Ion Process. 1997, 167, 375–388.
- Guo, Y.; Grabowski, J.J. Mechanistic insight into the reactions of O− with aromatic compounds and the synthesis of didehydroaromatic anions in the gas phase. Int. J. Mass Spectrom. Ion Process. 1992, 117, 299–326.
- Španěl, P.; Smith, D. Selected ion flow tube studies of the reactions of H3O+, NO+, and O2+ with several aromatic and aliphatic monosubstituted halocarbons. Int. J. Mass Spectrom. 1999, 189, 213–223.
- Thomas, R.; Liu, Y.; Mayhew, C.A.; Peverall, R. Selected ion flow tube studies of the gas phase reactions of O−, O2− and OH− with a variety of brominated compounds. Int. J. Mass Spectrom. Ion Process. 1996, 155, 163–183.
- Fehsenfeld, F.C.; Ferguson, E.E. Laboratory studies of negative ion reactions with atmospheric trace constituents. J. Chem. Phys. 1974, 61, 3181–3193.
- Fahey, D.W.; Böhringer, H.; Fehsenfeld, F.C.; Ferguson, E.E. Reaction rate constants for O2−(H2O)n ions n = 0–4 with O3, NO, SO2 and CO2. J. Chem. Phys. 1982, 76, 1799–1805.
- Smith, D.; Pysanenko, A.; Španěl, P. The quantification of carbon dioxide in humid air and exhaled breath by selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 1419–1425.
- Hera, D.; Langford, V.S.; McEwan, M.J.; McKellar, T.I.; Milligan, D.B. Negative reagent ions for real time detection using SIFT-MS. Environments 2017, 4, 16.
- Böhringer, H.; Fahey, D.W.; Fehsenfeld, F.C.; Ferguson, E.E. Temperature dependence of the three-body association of Cl−, NO2−, and NO3− with SO2. J. Chem. Phys. 1984, 81, 2696–2698.
- Španěl, P.; Smith, D. Progress in SIFT-MS: Breath analysis and other applications. Mass Spectrom. Rev. 2011, 30, 236–267.
- Smith, D.; Španěl, P.; Demarais, N.; Langford, V.S.; McEwan, M.J. Recent developments and applications of selected ion flow tube mass spectrometry, SIFT-MS. Mass Spec. Rev. 2023, e21835.
- United States Environmental Protection Agency. Understanding Global Warming Potentials. Available online: https://www.epa.gov/ghgemissions/understanding-global-warming-potentials (accessed on 17 January 2023).
- Wilson, P.F.; Freeman, C.G.; McEwan, M.J. Reactions of small hydrocarbons with H3O+, O2+ and NO+ ions. Int. J. Mass Spectrom. 2003, 229, 143–149.
- Dryahina, K.; Smith, D.; Španěl, P. Quantification of methane in humid air and exhaled breath using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 1296–1304.
- Ikezoe, Y.; Matsuoka, S.; Takebe, M.; Viggiano, A.A. Gas Phase Reaction Rate Constants through 1986; Maruzen: Tokyo, Japan, 1987.
- Kao, L.W.; Kristine, A.; Nañagas, K.A. Toxicity associated with carbon monoxide. Clin. Lab. Med. 2006, 26, 99–125.
- Reineccius, G. Flavor Chemistry and Technology; CRC Press: Boca Raton, FL, USA, 2006; p. 63.
- Langford, V.S.; Padayachee, D.; McEwan, M.J.; Barringer, S.A. Comprehensive odorant analysis for on-line applications using selected ion flow tube mass spectrometry (SIFT-MS). Flavour Fragr. J. 2019, 34, 393–410.
- Lehnert, A.-S.; Perreca, E.; Gershenzon, J.; Pohnert, G.; Trumbore, S.E. Simultaneous real-time measurement of isoprene and 2-methyl-3-buten-2-ol emissions from trees using SIFT-MS. Front. Plant Sci. 2020, 11, 578204.
- Allpress, C.; Crittenden, D.; Ma, J.; McEwan, M.; Robinson, S.; Wilson, P.; Wu, M. Real-time differentiation of ethylbenzene and the xylenes using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2019, 33, 1844–1849.
- Perkins, M.J.; Langford, V.S. Application of headspace-SIFT-MS to direct analysis of hazardous volatiles in drinking water. Environments 2022, 9, 124.
- Španěl, P.; Smith, D. Influence of water vapour on SIFT-MS analyses of trace gases in humid air and breath. Rapid Commun. Mass Spectrom. 2000, 14, 1898–1906.
- Španěl, P.; Dryahina, K.; Smith, D. A general method for the calculation of absolute trace gas concentrations in air and breath from selected ion flow tube mass spectrometry data. Int. J. Mass Spectrom. 2006, 249, 230–239.
- LabSyft Software, Version 1.0 and Above; Syft Technologies Limited: Christchurch, New Zealand. Available online: www.syft.com (accessed on 15 January 2023).
- Langford, V.S.; Graves, I.; McEwan, M.J. Rapid monitoring of volatile organic compounds: A comparison between gas chromatography/mass spectrometry and selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 10–18.
- Hastie, C.; Thompson, A.; Perkins, M.J.; Langford, V.S.; Eddleston, M.; Homer, N. Selected ion flow tube-mass spectrometry (SIFT-MS) as an alternative to gas chromatography/mass spectrometry (GC/MS) for the analysis of cyclohexanone and cyclohexanol in plasma. ACS Omega 2021, 6, 32818–32822.
- Kaus, C. Method Development for Continuous Monitoring of Selected VOC Test Gases by GC and SIFT-MS and Its Use for Verifying a New Dosing System for Test Gas Generation. Ph.D. Dissertation, University of Wuppertal, Wuppertal, Germany, 2021.
- Perkins, M.J.; Langford, V.S. Standard validation protocol for selected ion flow tube mass spectrometry methods applied to direct headspace analysis of aqueous volatile organic compounds. Anal. Chem. 2021, 93, 8386–8392.
- Biba, E.; Perkins, M.J.; Langford, V.S. Stimuli to the Revision Process: High-throughput residual solvent analysis using selected ion flow tube mass spectrometry (SIFT-MS). United States Pharmacopeia. Pharm. Forum 2021, 47, 1. Available online: https://online.usppf.com/usppf/document/GUID-2EE1BF6B-C82B-4F11-8E0B-C5520A4E8C3D_10101_en-US (accessed on 15 January 2023).
- Prince, B.J.; Milligan, D.B.; McEwan, M.J. Application of selected ion flow tube mass spectrometry to real-time atmospheric monitoring. Rapid Commun. Mass Spectrom. 2010, 24, 1763–1769.
- Perkins, M.J.; Langford, V.S. Application of routine analysis procedures to a direct mass spectrometry technique: Selected ion flow tube mass spectrometry (SIFT-MS). Rev. Sep. Sci. 2021, 3, e21003.




