Selected ion flow tube mass spectrometry (SIFT-MS) uses soft chemical ionization (CI) to generate mass-selected reagent ions that can rapidly react with and quantify VOCs down to part-per-trillion concentrations (by volume, pptV). Up to eight reagent ions (H3O+, NO+, O2+, O-, OH-, O2-, NO2- and NO3-) obtained from a microwave discharge in air are available on SIFT-MS instruments. These reagent ions react with VOCs and other trace analytes in well-controlled ion-molecule reactions, but they do not react with the major components of air (N2, O2, CO2 and Ar). This enables direct, real-time analysis of air samples to be achieved at trace and ultra-trace levels without pre-concentration. Rapid switching between reagent ions provides high selectivity because the multiple reaction mechanisms give independent measurements of each analyte.
- SIFT-MS
- direct-injection mass spectrometry
- DIMS
1. Introduction
2. The SIFT-MS Technique—An Overview
2.1. Instrument Overview
All SIFT-MS instruments have three zones. In the first region, reagent ions are generated continuously in a plasma created at low pressure using a microwave discharge. For the positively charged reagent ions, H3O+, NO+, and O2+•, the ion source is operated at approximately 54 kPa (400 torr), while for the negatively charged reagent ions (O−•, O2−•, OH−, NO2−, and NO3−) it is operated at approximately twice this pressure to facilitate electron attachment. Multiple reagent ions provide two significant benefits that are elaborated below: (1) their different ionization properties enable a wide range of compounds to be detected, and (2) they are foundational to achieving specific analysis. The plasma containing a mixture of potential reagent ions is then passed into a quadrupole mass filter (QMF) that, using software control, selects the appropriate, single-reagent ion type (by its m/z; e.g., H3O+ with m/z = 19) for introduction as a pure stream of reagent ion into the second region, the reaction chamber (or flow tube). In the flow tube, reagent ions first encounter carrier gas molecules (either helium or nitrogen, depending on instrument application). Through collisions with the carrier gas, the energies of the reagent ions are reduced to approximately the temperature of the carrier gas itself (i.e., the reagent ions are “thermalized”) [7][5]. The sample is introduced after reagent ions have been in the flow tube for approximately 1 ms, then has a residence time of about 3 to 8 ms (configuration-dependent) for reaction with reagent ions. At the end of the flow tube, ions are sampled into the third region (detection), while the bulk of carrier and sample mixture is pumped away to exhaust. Note that the carrier gas and the bulk matrix must have ionization properties that render them essentially non-reactive with the reagent ions. Conveniently, this is the case for air, with which the reagent ions listed above either do not react or react only very slowly (nitrogen, oxygen, argon, carbon dioxide, and water). The detection region comprises, firstly, an ion guide that improves transmission of heavier product ions, second a QMF that transmits just the ions with a given m/z, and finally a particle multiplier detector that counts ions at each m/z. Typically operation in the linear range means that less than 10% of the reagent ion signal is consumed [6] and concentration is proportional to the ratio of product ion count divided by reagent ion count (Section 2.4). This is essentially an auto-normalization feature, correcting for any drift of ion signal, should it occur, and supports stable long-term operation. SIFT-MS instruments are completely computer controlled and can operate autonomously or via remote control. The stability of ionization means that—when required—re-calibration is an infrequent task (annual for many compounds due to drift less than 10%), and software supports the generation of laboratory-grade analytical results for non-technical operators for properly developed analytical methods.2.2. Breadth of Analysis
Although, as noted in the previous subsection, the SIFT-MS technique is ‘blind’ to the bulk components of air due to the low ionization energies of its reagent ions, it has remarkable breadth of analysis for VOCs and trace inorganic gases. This is due to the multiple reagent ions that ionize compounds via a wide range of ion–molecule reaction mechanisms (Table 1). These mechanisms have been reviewed in detail elsewhere [6,7][5][6]. Essentially, if the ionization properties of the target compound are a match for SIFT-MS, then it will be ionized and detected. Note, however, that unlike the electron ionization (EI) method used commonly with GC or photoionization detectors (PIDs), the different mechanisms yield a wider range of product ions, potentially increasing specificity for isobaric compounds.| Mechanism Name | Reagent Ion(s) | General Equation |
|---|
| Compound | Shift Relative to Parent Ion | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference(s) | H | 3O+ | NO+ | O2+• | O−• | O2−• | OH− | NO2− | NO3− | ||||
| Proton transfer (PT) | H3O+ | ||||||||||||
| Ammonia | [11,12][7][8] | AH+ + B → BH+ + A | +1 (simple PT) −17 (loss of OH moiety) |
||||||||||
| ✓ | ✓ | ✓ | Electron transfer (ET) | NO+, O2+•, O2−• | A+/− + B → B+/− + A | 0 | |||||||
| Benzene | [13][9] | ✓ | ✓ | ✓ | Dissociative ET | NO+, O2+• | A+ + B → C+ + Products | Varies | |||||
| Formaldehyde | [14][10] | ✓ | ✓ | Hydride abstraction | NO+, O2+• | A+ + B-H → B+ + HA | −1 | ||||||
| Association | NO+, O−•, O2−•, OH− | A+/− + B + M → {B.A}+/− + M | +Reagent m/z (e.g., +30 for NO+) |
||||||||||
| Proton abstraction | O−•, O2−•, OH−, NO | ||||||||||||
| Hydrogen sulfide | [15,16,17][11][12][13] | ✓ | 2−, NO3− | A− + BH → B− + AH | −1 | ||||||||
| Mechanism Name | Reagent Ion(s) | General Equation | Standard Product * m/z | ||||||||||
| [ | 16 | Hydrogen atom transfer | O−• | A− + BH → AH− + B− | Reagent ion m/z − 1 ** | ||||||||
| Displacement | O−•, OH− | A− + RB → RA + B− (R = alkyl) |
−35 and −37 for B = Cl −79 and −81 for B = Br |
||||||||||
| Elimination | O−•, OH− | A− + RB → R’ + R”A + B− (R’ = alkene; R” = alkyl) |
−35 and −37 for B = Cl −79 and −81 for B = Br |
||||||||||
| Associative detachment | O−•, O2−•, OH− | A− + B → AB + e− (e− = electron) |
No product detected (e−) |
2.3. Specificity of Analysis
Achieving specific analysis in moderately complex matrixes can be challenging for low-cost chemosensors. Eminent flavor chemist, Gary Reineccius wrote of the application of electronic noses (sensor arrays) in food flavor analysis that “one has no clear idea of what the instrument is responding to in making a judgement” [32][30]. In contrast, SIFT-MS achieves specific analysis in real-time by the combination of highly controlled, ultra-soft CI and MS, producing signals that are attributable to specific analytes. Table 1 illustrates the variety of common reaction mechanisms that occur for the standard SIFT-MS reagent ions and indicates the relative shifts from parent product ion m/z, or standard product ion m/z, for these mechanisms. Combining this behavior with the observation that different functional groups typically react in different ways [6[5][6],7], at least one unique ion for a given analyte is usually realized. It is important to reemphasize that when using the phrase “real-time specificity” in the context of SIFT-MS, a user is not limited to a single reagent ion in each analysis. By means of the first QMF (Figure 1), combined with software control, reagent ions generated in a given ion source setting (e.g., positive ions with H3O+, NO+, and O2+•) are switchable in tens of milliseconds. This means that target compounds can be analyzed specifically across different reagent ions, if necessary. This contrasts with the other DIMS techniques [3]. Note that where an ion source setting change is required—e.g., from positive ions to either of the negative ion settings—this is still near real time at approximately 10 s. Figure 2 summarizes the elements that contribute to specific analysis in SIFT-MS. Addition of a time-of-flight (TOF) mass spectrometer to SIFT-MS instruments (as has been done successfully for PTR-MS [3]) could further improve specificity, but at additional cost. However, regardless of the way that mass filtering is accomplished in SIFT-MS or PTR-MS, it is not reasonable to expect that the specificity of DIMS techniques will approach that of GC/MS with its temporal separation of analytes. GC/MS techniques—especially those utilizing high-resolution mass spectrometry—will remain the technique of choice for compound identification and analysis of very complex matrices.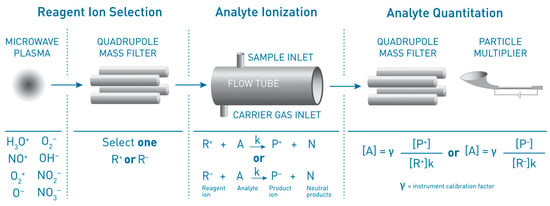 Figure 1. A schematic illustration of the SIFT-MS technique, showing the three zones in this continuous-analysis approach. Used with permission of Syft Technologies.
Figure 1. A schematic illustration of the SIFT-MS technique, showing the three zones in this continuous-analysis approach. Used with permission of Syft Technologies.
| Reagent Ion | Acetone | Propanal | ||||
|---|---|---|---|---|---|---|
| Formula | m/z | br (%) | Formula | m/z | br (%) | |
| H3O+ | (CH3)2CO.H+ | 59 | 100 | CH3CH2CHO.H+ | 59 | 100 |
| NO+ | (CH3)2CO.NO+ | 88 | 100 | CH3CH2CO+ | 57 | 100 |
| O2+• | (CH3)2CO+• CH3CO+• |
58 43 |
60 40 |
CH3CH2CHO+• CH3CH2CO+ |
58 57 |
50 50 |

2.4. Quantitation in SIFT-MS
The preceding subsections have described how SIFT-MS can analyze a wide range of compounds and generally do so with high specificity. It remains, then, to outline how quantitative results are obtained in SIFT-MS. Since the details of the gas-phase kinetic theory have been described elsewhere [6[6][35],37], and the detailed calculation of concentration presented [38][36], only a brief sketch will be given here. A future article will describe the calculation, as currently adapted in commercial SIFT-MS instruments, in more detail. In this aentrticley, the use of non-SI pressure units (torr) follows that of the SIFT-MS literature [38][36]. The master equation used in commercial instruments to determine the concentration of analyte A in air ([A]i in units of parts-per-billion by volume, ppbV) for each primary product ion (i) of A utilized in the analytical method is: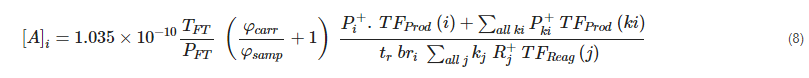
-
TFT is the temperature in the flow tube in Kelvin (K)
-
PFT is the pressure in the flow tube in torr
-
kj is the rate coefficient for reaction of reagent ion Rj+ with the analyte (in cm3 molecule−1 s−1)
-
tr is the reaction time (in seconds)
-
φcarr is the carrier gas flow in torr L s−1
-
φsamp is the sample flow in torr L s−1
-
Pi+ is the primary product ion signal (in counts per second, cps) for primary product ion i counted by the particle multiplier detector
- +
- (dimensionless)
- br
- i is the branching ratio for primary product ion i (0 < i ≤ 1 for calculation purposes, but ordinarily tabulated as a percentage; see, e.g., Table 3).
-
Instrument operating parameters: TFT, PFT, φcarr, and φsamp
-
The instrument’s automated performance check on a certified gas standard: tr, TFProd(i), TFProd(ki), and TFReag(j)
-
Software library: kj and bri (clearly, together with the m/z at which the relevant reagent and product ions will be located)
-
Measurement of sample: Pi+, Pki+, and Rj+.
- TF
- Prod
- (i) is the transmission factor for the primary product ion Pi+ (dimensionless)
-
Pki+ is the secondary product ion signal (in cps) for secondary product ion k derived from primary product ion i
-
TFProd(ki) is the transmission factor for the secondary product ion Pki+ (dimensionless)
-
Rj+ is the reagent ion signal (in cps) for the injected reagent ion (j = 0) and its water cluster ions (if appropriate; j = 1, 2, 3)
-
TFReag(j) is the transmission factor for the reagent ion Rj
References
- Dettmer-Wilde, K.; Engewald, W. Practical Gas Chromatography; Springer: Heidelberg, Germany, 2014; 902p.
- Fanali, S.; Haddad, P.R.; Poole, C.F.; Riekkola, M.-L. Liquid Chromatography: Fundamentals and Instrumentation, 2nd ed.; Elsevier: Oxford, UK, 2017; 784p.
- Taylor, A.J.; Beauchamp, J.D.; Langford, V.S. Non-destructive and high-throughput–APCI-MS, PTR-MS and SIFT-MS as methods of choice for exploring flavor release. In Dynamic Flavor: Capturing Aroma Release using Real-Time Mass Spectrometry; Beauchamp, J.D., Ed.; American Chemical Society: Washington, DC, USA, 2021; pp. 1–16.
- McEwan, M.J. Direct analysis mass spectrometry. In Ion Molecule Attachment Reactions: Mass Spectrometry; Fujii, T., Ed.; Springer: New York, NY, USA, 2015; pp. 263–317.
- Smith, D.; McEwan, M.J.; Španěl, P. Understanding gas phase ion chemistry is the key to reliable selected ion flow tube-mass spectrometry analyses. Anal. Chem. 2020, 92, 12750–12762.
- Smith, D.; Španěl, P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom. Rev. 2005, 24, 661–700.
- Španěl, P.; Smith, D. Selected ion flow tube studies of the reactions of H3O+, NO+, and O2+ with several amines and some other nitrogen-containing molecules. Int. J. Mass Spectrom. 1998, 176, 203–211.
- Smith, D.; Bloor, R.; George, C.; Pysanenko, A.; Španěl, P. Release of toxic ammonia and volatile organic compounds by heated cannabis and their relation to tetrahydrocannabinol content. Anal. Methods 2015, 7, 4104–4110.
- Španěl, P.; Smith, D. SIFT studies of the reactions of H3O+, NO+ and O2+ with several aromatic and aliphatic hydrocarbons. Int. J. Mass Spectrom. 1998, 181, 1–10.
- Španěl, P.; Ji, Y.; Smith, D. SIFT studies of the reactions of H3O+, NO+ and O2+ with a series of aldehydes and ketones. Int. J. Mass Spectrom. Ion Process. 1997, 165, 25–37.
- Williams, T.L.; Adams, N.G.; Babcock, L.M. Selected ion flow tube studies of H3O+(H2O)0.1 reactions with sulfides and thiols. Int. J. Mass Spectrom. 1998, 172, 149–159.
- Španěl, P.; Smith, D. Selected ion flow tube studies of the reactions of H3O+, NO+, and O2+ with some organosulphur molecules. Int. J. Mass Spectrom. 1998, 176, 167–176.
- LabSyft Software: Compound Library; Syft Technologies Limited: Christchurch, New Zealand, 2017.
- Španěl, P.; Smith, D. SIFT studies of the reactions of H3O+, NO+ and O2+ with a series of alcohols. Int. J. Mass Spectrom. Ion Process. 1997, 167, 375–388.
- Guo, Y.; Grabowski, J.J. Mechanistic insight into the reactions of O− with aromatic compounds and the synthesis of didehydroaromatic anions in the gas phase. Int. J. Mass Spectrom. Ion Process. 1992, 117, 299–326.
- Španěl, P.; Smith, D. Selected ion flow tube studies of the reactions of H3O+, NO+, and O2+ with several aromatic and aliphatic monosubstituted halocarbons. Int. J. Mass Spectrom. 1999, 189, 213–223.
- Thomas, R.; Liu, Y.; Mayhew, C.A.; Peverall, R. Selected ion flow tube studies of the gas phase reactions of O−, O2− and OH− with a variety of brominated compounds. Int. J. Mass Spectrom. Ion Process. 1996, 155, 163–183.
- Fehsenfeld, F.C.; Ferguson, E.E. Laboratory studies of negative ion reactions with atmospheric trace constituents. J. Chem. Phys. 1974, 61, 3181–3193.
- Fahey, D.W.; Böhringer, H.; Fehsenfeld, F.C.; Ferguson, E.E. Reaction rate constants for O2−(H2O)n ions n = 0–4 with O3, NO, SO2 and CO2. J. Chem. Phys. 1982, 76, 1799–1805.
- Smith, D.; Pysanenko, A.; Španěl, P. The quantification of carbon dioxide in humid air and exhaled breath by selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 1419–1425.
- Hera, D.; Langford, V.S.; McEwan, M.J.; McKellar, T.I.; Milligan, D.B. Negative reagent ions for real time detection using SIFT-MS. Environments 2017, 4, 16.
- Böhringer, H.; Fahey, D.W.; Fehsenfeld, F.C.; Ferguson, E.E. Temperature dependence of the three-body association of Cl−, NO2−, and NO3− with SO2. J. Chem. Phys. 1984, 81, 2696–2698.
- Španěl, P.; Smith, D. Progress in SIFT-MS: Breath analysis and other applications. Mass Spectrom. Rev. 2011, 30, 236–267.
- Smith, D.; Španěl, P.; Demarais, N.; Langford, V.S.; McEwan, M.J. Recent developments and applications of selected ion flow tube mass spectrometry, SIFT-MS. Mass Spec. Rev. 2023, e21835.
- United States Environmental Protection Agency. Understanding Global Warming Potentials. Available online: https://www.epa.gov/ghgemissions/understanding-global-warming-potentials (accessed on 17 January 2023).
- Wilson, P.F.; Freeman, C.G.; McEwan, M.J. Reactions of small hydrocarbons with H3O+, O2+ and NO+ ions. Int. J. Mass Spectrom. 2003, 229, 143–149.
- Dryahina, K.; Smith, D.; Španěl, P. Quantification of methane in humid air and exhaled breath using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 1296–1304.
- Ikezoe, Y.; Matsuoka, S.; Takebe, M.; Viggiano, A.A. Gas Phase Reaction Rate Constants through 1986; Maruzen: Tokyo, Japan, 1987.
- Kao, L.W.; Kristine, A.; Nañagas, K.A. Toxicity associated with carbon monoxide. Clin. Lab. Med. 2006, 26, 99–125.
- Reineccius, G. Flavor Chemistry and Technology; CRC Press: Boca Raton, FL, USA, 2006; p. 63.
- Langford, V.S.; Padayachee, D.; McEwan, M.J.; Barringer, S.A. Comprehensive odorant analysis for on-line applications using selected ion flow tube mass spectrometry (SIFT-MS). Flavour Fragr. J. 2019, 34, 393–410.
- Lehnert, A.-S.; Perreca, E.; Gershenzon, J.; Pohnert, G.; Trumbore, S.E. Simultaneous real-time measurement of isoprene and 2-methyl-3-buten-2-ol emissions from trees using SIFT-MS. Front. Plant Sci. 2020, 11, 578204.
- Allpress, C.; Crittenden, D.; Ma, J.; McEwan, M.; Robinson, S.; Wilson, P.; Wu, M. Real-time differentiation of ethylbenzene and the xylenes using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2019, 33, 1844–1849.
- Perkins, M.J.; Langford, V.S. Application of headspace-SIFT-MS to direct analysis of hazardous volatiles in drinking water. Environments 2022, 9, 124.
- Španěl, P.; Smith, D. Influence of water vapour on SIFT-MS analyses of trace gases in humid air and breath. Rapid Commun. Mass Spectrom. 2000, 14, 1898–1906.
- Španěl, P.; Dryahina, K.; Smith, D. A general method for the calculation of absolute trace gas concentrations in air and breath from selected ion flow tube mass spectrometry data. Int. J. Mass Spectrom. 2006, 249, 230–239.
- LabSyft Software, Version 1.0 and Above; Syft Technologies Limited: Christchurch, New Zealand. Available online: www.syft.com (accessed on 15 January 2023).
- Langford, V.S.; Graves, I.; McEwan, M.J. Rapid monitoring of volatile organic compounds: A comparison between gas chromatography/mass spectrometry and selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 10–18.
- Hastie, C.; Thompson, A.; Perkins, M.J.; Langford, V.S.; Eddleston, M.; Homer, N. Selected ion flow tube-mass spectrometry (SIFT-MS) as an alternative to gas chromatography/mass spectrometry (GC/MS) for the analysis of cyclohexanone and cyclohexanol in plasma. ACS Omega 2021, 6, 32818–32822.
- Kaus, C. Method Development for Continuous Monitoring of Selected VOC Test Gases by GC and SIFT-MS and Its Use for Verifying a New Dosing System for Test Gas Generation. Ph.D. Dissertation, University of Wuppertal, Wuppertal, Germany, 2021.
- Perkins, M.J.; Langford, V.S. Standard validation protocol for selected ion flow tube mass spectrometry methods applied to direct headspace analysis of aqueous volatile organic compounds. Anal. Chem. 2021, 93, 8386–8392.
- Biba, E.; Perkins, M.J.; Langford, V.S. Stimuli to the Revision Process: High-throughput residual solvent analysis using selected ion flow tube mass spectrometry (SIFT-MS). United States Pharmacopeia. Pharm. Forum 2021, 47, 1. Available online: https://online.usppf.com/usppf/document/GUID-2EE1BF6B-C82B-4F11-8E0B-C5520A4E8C3D_10101_en-US (accessed on 15 January 2023).
- Prince, B.J.; Milligan, D.B.; McEwan, M.J. Application of selected ion flow tube mass spectrometry to real-time atmospheric monitoring. Rapid Commun. Mass Spectrom. 2010, 24, 1763–1769.
- Perkins, M.J.; Langford, V.S. Application of routine analysis procedures to a direct mass spectrometry technique: Selected ion flow tube mass spectrometry (SIFT-MS). Rev. Sep. Sci. 2021, 3, e21003.
