
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nikolay Polyakov | -- | 3002 | 2023-04-27 09:53:06 | | | |
| 2 | Lindsay Dong | -2 word(s) | 3000 | 2023-04-28 02:43:12 | | |
Video Upload Options
Natural bioactive compounds have emerged as a strategy for Alzheimer’s disease treatment. Carotenoids, including astaxanthin, lycopene, lutein, fucoxanthin, crocin and others are natural pigments and antioxidants, and can be used to treat a variety of diseases, including Alzheimer’s disease. However, carotenoids, as oil-soluble substances with additional unsaturated groups, suffer from low solubility, poor stability and poor bioavailability. Therefore, the preparation of various nano-drug delivery systems from carotenoids is a current measure to achieve efficient application of carotenoids. Different carotenoid delivery systems can improve the solubility, stability, permeability and bioavailability of carotenoids to a certain extent to achieve Alzheimer’s disease efficacy.
1. Introduction
2. Carotenoids in Alzheimer’s Disease Treatment
2.1. Pathogenesis of Alzheimer’s Disease
2.2. Overview of Carotenoids
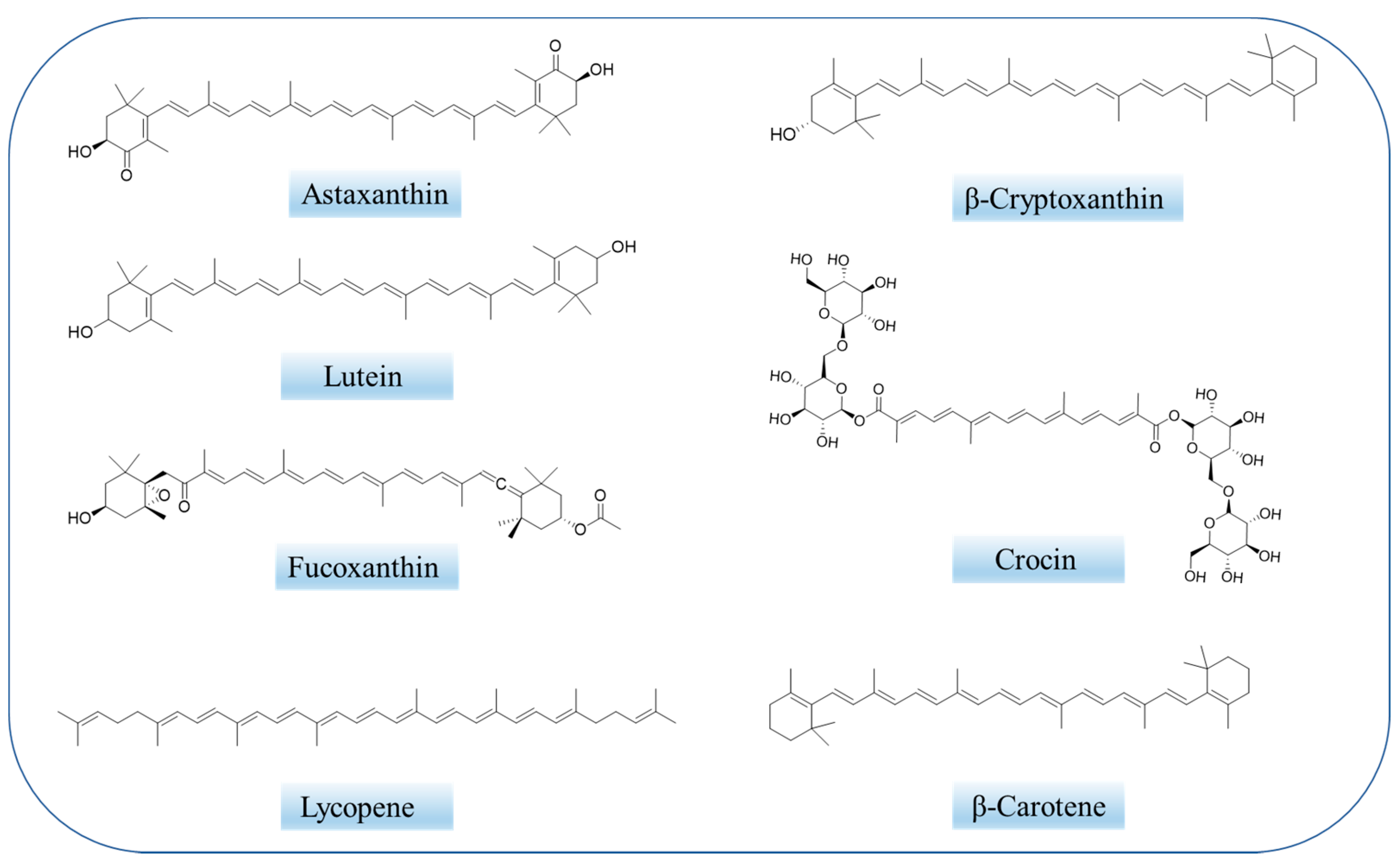
2.3. Therapeutic Mechanisms of Carotenoids on Alzheimer’s Disease
3. Carotenoid-Loaded Nanocarriers for Alzheimer’s Disease Therapy
3.1. Polymeric Nanocarriers
3.1.1. Polymeric Micelles
3.1.2. Polymeric Nanoparticles
3.1.3. Dendrimers
3.2. Lipid-Based Nanocarriers
3.2.1. Liposomes
3.2.2. Solid Lipid Nanoparticles (SLNs)
3.2.3. Nanostructured Lipid Carriers (NLCs)
3.3. Inorganic Nanocarriers
3.4. Hybrid Nanocarriers
4. Different Nano-Encapsulated Carotenoids in Alzheimer’s Disease Therapy
4.1. Crocin and Crocetin
4.2. Astaxanthin
4.3. Lycopene
4.4. Lutein
4.5. Fucoxanthin
5. Conclusions and Perspectives
Nowadays, it is clear that carotenoids have many benefits for health and positive nutritional effects and can reduce the risk of many diseases. However, there are some critical points to be considered: (1) Most carotenoids play a synergistic role when combined with other compounds, and the single form of carotenoids may not be effective, but from another perspective if two or more carotenoids are put together inside the nanocarrier there may be competition for absorption, which leads to lower bioavailability. (2) Carotenoids are unstable, and easy to transform into different compounds; therefore, the safety of carotenoids needs additional research. (3) The therapeutic effect varies from person to person, thus the effective dose is an unknown problem.
Carotenoid-based nano-drug delivery systems are feasible for effective disease prevention and treatment. This entry presents a series of examples of carotenoid nano-delivery systems for Alzheimer’s disease. Each technology has its own strengths and limitations. To some extent, nano-delivery systems can improve the loading capacity, bioavailability, bioactivity, stability and solubility of carotenoids. In the author’s opinion, polymeric micelles are more suitable for the delivery of carotenoids. First, the polymer micelles can be adjusted to a suitable size to accommodate carotenoids of different sizes. Second, the modifiability and ease of modification of the polymer surface increases the functional properties of carotenoids. Finally, carotenoid polymers can make it easier to pass through the blood–brain barrier by adjusting the hydrophilic–lipophilic balance. However, industrial production of nanomedicines is still in its early stages. Safety and health concerns need to be explored in depth before widespread consumption. First, each process or material must be formally approved by regulatory authorities. However, the regulatory framework for the inclusion of nano-carriers in pharmaceutical products is still in flux. State agencies are expected to add initiatives and some legislation to regulate and monitor the proper development and application of nanoparticles in food and drug formulations.
In modern medicine, the idea of “synergy” between drug and carrier has attracted increased attention, seeking to preserve and improve the health benefits of various drugs in prevention and the treatment of many diseases. In the case of carotenoids, drug delivery systems can assist these bioactive compounds in exerting greater biological activity and stability. On the other hand, some nano-delivery systems can also play more functional roles, including targeted delivery to the brain or other organs, or overcome the blood–brain-barrier. Thirdly, since most of the nanocarriers are natural protein or polysaccharide components, they can provide the body with some needed nutrients to a certain extent and improve the efficiency of disease prevention and treatment.
References
- Paris, D.; Beaulieu-Abdelahad, D.; Bachmeier, C.; Reed, J.; Ait-Ghezala, G.; Bishop, A.; Chao, J.; Mathura, V.; Crawford, F.; Mullan, M. Anatabine lowers Alzheimer’s A beta production in vitro and in vivo. Eur. J. Pharmacol. 2011, 670, 384–391.
- Fratiglioni, L.; Launer, L.J.; Andersen, K.; Breteler, M.M.B.; Copeland, J.R.M.; Dartigues, J.F.; Lobo, A.; Martinez-Lage, J.; Soininen, H.; Hofman, A.; et al. Incidence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurology 2000, 54, S10–S15.
- Lobo, A.; Launer, L.J.; Fratiglioni, L.; Andersen, K.; Di Carlo, A.; Breteler, M.M.B.; Copeland, J.R.M.; Dartigues, J.F.; Jagger, C.; Martinez-Lage, J.; et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurology 2000, 54, S4–S9.
- Jonsson, L.; Jonhagen, M.E.; Kilander, L.; Soininen, H.; Hallikainen, M.; Waldemar, G.; Nygaard, H.; Andreasen, N.; Winblad, B.; Wimo, A. Determinants of costs of care for patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2006, 21, 449–459.
- Citron, M. Strategies for disease modification in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 677–685.
- Chon, S.-H.; Yang, E.-J.; Lee, T.; Song, K.-S. Beta-Secretase (BACE1) inhibitory and neuroprotective effects of p-terphenyls from Polyozellus multiplex. Food Funct. 2016, 7, 3834–3842.
- Golde, T.E. The A beta hypothesis: Leading us to rationally-designed therapeutic strategies for the treatment or prevention of Alzheimer disease. Brain Pathol. 2005, 15, 84–87.
- Hardy, J.; Selkoe, D.J. Medicine-The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356.
- Kabir, M.T.; Uddin, M.S.; Setu, J.R.; Ashraf, G.M.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Exploring the Role ofPSENMutations in the Pathogenesis of Alzheimer’s Disease. Neurotox. Res. 2020, 38, 833–849.
- Kabir, M.T.; Uddin, M.S.; Zaman, S.; Begum, Y.; Ashraf, G.M.; Bin-Jumah, M.N.; Bungau, S.G.; Mousa, S.A.; Abdel-Daim, M.M. Molecular Mechanisms of Metal Toxicity in the Pathogenesis of Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 1–20.
- Cores, A.; Abril, S.; Michalska, P.; Duarte, P.; Olives, A.I.; Martin, M.A.; Villacampa, M.; Leon, R.; Menendez, J.C. Bisavenathramide Analogues as Nrf2 Inductors and Neuroprotectors in In Vitro Models of Oxidative Stress and Hyperphosphorylation. Antioxidants 2021, 10, 941.
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044.
- Loi, M.; Paciolla, C. Plant Antioxidants for Food Safety and Quality: Exploring New Trends of Research. Antioxidants 2021, 10, 972.
- Rodriguez-Yoldi, M.J. Anti-Inflammatory and Antioxidant Properties of Plant Extracts. Antioxidants 2021, 10, 921.
- Bolhassani, A.; Khavari, A.; Bathaie, S.Z. Saffron and natural carotenoids: Biochemical activities and anti-tumor effects. Biochim. Biophys. Acta-Rev. Cancer 2014, 1845, 20–30.
- Cong, L.; Wang, C.; Chen, L.; Liu, H.; Yang, G.; He, G. Expression of phytoene synthase1 and Carotene Desaturase crtl Genes Result in an Increase in the Total Carotenoids Content in Transgenic Elite Wheat (Triticum aestivum L.). J. Agric. Food Chem. 2009, 57, 8652–8660.
- Prakash, D.; Gupta, C. Carotenoids: Chemistry and Health Benefits. Phytochem. Nutraceut. Importance 2014, 181–195.
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Carotenoids: Importance in Daily Life-Insight Gained from EPR and ENDOR. Appl. Magn. Reson. 2021, 52, 1093–1112.
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488.
- Widomska, J.; Zareba, M.; Subczynski, W.K. Can Xanthophyll-Membrane Interactions Explain Their Selective Presence in the Retina and Brain? Foods 2016, 5, 7.
- Lakey-Beitia, J.; Doens, D.; Kumar, D.J.; Murillo, E.; Fernandez, P.L.; Rao, K.S.; Durant-Archibold, A.A. Anti-amyloid aggregation activity of novel carotenoids: Implications for Alzheimer’s drug discovery. Clin. Interv. Aging 2017, 12, 815–822.
- Bohn, T. Carotenoids and Markers of Oxidative Stress in Human Observational Studies and Intervention Trials: Implications for Chronic Diseases. Antioxidants 2019, 8, 179.
- Iddir, M.; Yaruro, J.F.P.; Cocco, E.; Hardy, E.M.; Appenzeller, B.M.R.; Guignard, C.; Larondelle, Y.; Bohn, T. Impact of Protein-Enriched Plant Food Items on the Bioaccessibility and Cellular Uptake of Carotenoids. Antioxidants 2021, 10, 1005.
- Young, A.J.; Lowe, G.L. Carotenoids-Antioxidant Properties. Antioxidants 2018, 7, 28.
- Kim, S.H.; Kim, M.S.; Lee, B.Y.; Lee, P.C. Generation of structurally novel short carotenoids and study of their biological activity. Sci. Rep. 2016, 6, 21987.
- Polyakov, N.E.; Leshina, T.V.; Salakhutdinov, N.F.; Konovalova, T.A.; Kispert, L.D. Antioxidant and redox properties of supramolecular complexes of carotenoids with beta-glycyrrhizic acid. Free Radic. Biol. Med. 2006, 40, 1804–1809.
- Polyakov, N.E.; Leshina, T.V.; Salakhutdinov, N.F.; Kispert, L.D. Host-guest complexes of carotenoids with beta-glycyrrhizic acid. J. Phys. Chem. B 2006, 110, 6991–6998.
- Polyakov, N.E.; Leshina, T.V.; Meteleva, E.S.; Dushkin, A.V.; Konovalova, T.A.; Kispert, L.D. Water Soluble Complexes of Carotenoids with Arabinogalactan. J. Phys. Chem. B 2009, 113, 275–282.
- Polyakov, N.E.; Kispert, L.D. Water soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid delivery. Carbohydr. Polym. 2015, 128, 207–219.
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Supramolecular Carotenoid Complexes of Enhanced Solubility and Stability-The Way of Bioavailability Improvement. Molecules 2019, 24, 3947.
- Apanasenko, I.E.; Selyutina, O.Y.; Polyakov, N.E.; Suntsova, L.P.; Meteleva, E.S.; Dushkin, A.V.; Vachali, P.; Bernstein, P.S. Solubilization and stabilization of macular carotenoids by water soluble oligosaccharides and polysaccharides. Archives Biochem. Biophys. 2015, 572, 58–65.
- Neve, R.L.; Robakis, N.K. Alzheimer’s disease: A re-examination of the amyloid hypothesis. Trends Neurosci. 1998, 21, 15–19.
- Dominiak, K.; Jarmuszkiewicz, W. The Relationship between Mitochondrial Reactive Oxygen Species Production and Mitochondrial Energetics in Rat Tissues with Different Contents of Reduced Coenzyme Q. Antioxidants 2021, 10, 533.
- Kolodziej, F.; O’Halloran, K.D. Re-Evaluating the Oxidative Phenotype: Can Endurance Exercise Save the Western World? Antioxidants 2021, 10, 609.
- Olowe, R.; Sandouka, S.; Saadi, A.; Shekh-Ahmad, T. Approaches for Reactive Oxygen Species and Oxidative Stress Quantification in Epilepsy. Antioxidants 2020, 9, 990.
- Picazo, C.; Molin, M. Impact of Hydrogen Peroxide on Protein Synthesis in Yeast. Antioxidants 2021, 10, 952.
- Ullah, H.; Di Minno, A.; Santarcangelo, C.; Khan, H.; Daglia, M. Improvement of Oxidative Stress and Mitochondrial Dysfunction by beta-Caryophyllene: A Focus on the Nervous System. Antioxidants 2021, 10, 546.
- Woo, J.; Cho, H.; Seol, Y.; Kim, S.H.; Park, C.; Yousefian-Jazi, A.; Hyeon, S.J.; Lee, J.; Ryu, H. Power Failure of Mitochondria and Oxidative Stress in Neurodegeneration and Its Computational Models. Antioxidants 2021, 10, 229.
- Kaur, I.; Behl, T.; Aleya, L.; Rahman, M.H.; Kumar, A.; Arora, S.; Akter, R. Role of metallic pollutants in neurodegeneration: Effects of aluminum, lead, mercury, and arsenic in mediating brain impairment events and autism spectrum disorder. Environ. Sci. Pollut. Res. 2021, 28, 8989–9001.
- Shichiri, M. The role of lipid peroxidation in neurological disorders. J. Clin. Biochem. Nutr. 2014, 54, 151–160.
- Lima, L.W.; Nardi, S.; Santoro, V.; Schiavon, M. The Relevance of Plant-Derived Se Compounds to Human Health in the SARS-CoV-2 (COVID-19) Pandemic Era. Antioxidants 2021, 10, 1031.
- Ofosu, F.K.; Mensah, D.-J.F.; Daliri, E.B.-M.; Oh, D.-H. Exploring Molecular Insights of Cereal Peptidic Antioxidants in Metabolic Syndrome Prevention. Antioxidants 2021, 10, 518.
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324.
- Huang, J.J.; Lin, S.; Xu, W.; Cheung, P.C.K. Occurrence and biosynthesis of carotenoids in phytoplankton. Biotechnol. Adv. 2017, 35, 597–618.
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 2018, 120, 681–699.
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313.
- Meyers, K.J.; Mares, J.A.; Igo, R.P., Jr.; Truitt, B.; Liu, Z.; Millen, A.E.; Klein, M.; Johnson, E.J.; Engelman, C.D.; Karki, C.K.; et al. Genetic Evidence for Role of Carotenoids in Age-Related Macular Degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS). Investig. Ophthalmol. Vis. Sci. 2014, 55, 587–599.
- Arvanitakis, Z.; Fleischman, D.A.; Arfanakis, K.; Leurgans, S.E.; Barnes, L.L.; Bennett, D.A. Association of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairment. Brain Struct. Funct. 2016, 221, 2135–2146.
- Neuwelt, E.A.; Bauer, B.; Fahlke, C.; Fricker, G.; Iadecola, C.; Janigro, D.; Leybaert, L.; Molnar, Z.; O’Donnell, M.E.; Povlishock, J.T.; et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat. Rev. Neurosci. 2011, 12, 169–182.
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx J. Am. Soc. Exp. NeuroTherapeutics 2005, 2, 3–14.
- Guiot, C.; Zullino, S.; Priano, L.; Cavalli, R. The physics of drug-delivery across the blood-brain barrier. Ther. Deliv. 2016, 7, 153–156.
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed.-Nanotechnol. Biol. Med. 2010, 6, 714–729.
- Wang, Y.C.; Shim, M.S.; Levinson, N.S.; Sung, H.W.; Xia, Y.N. Stimuli-Responsive Materials for Controlled Release of Theranostic Agents. Adv. Funct. Mater. 2014, 24, 4206–4220.
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561.
- Rempe, R.; Cramer, S.; Huewel, S.; Galla, H.-J. Transport of Poly(n-butylcyano-acrylate) nanoparticles across the blood-brain barrier in vitro and their influence on barrier integrity. Biochem. Biophys. Res. Commun. 2011, 406, 64–69.
- Kim, H.R.; Andrieux, K.; Gil, S.; Taverna, M.; Chacun, H.; Desmaele, D.; Taran, F.; Georgin, D.; Couvreur, P. Translocation of poly(ethylene glycol-co-hexadecyl)cyanoacrylate nanoparticles into rat brain endothelial cells: Role of apolipoproteins in receptor-mediated endocytosis. Biomacromolecules 2007, 8, 793–799.
- Tian, X.-H.; Lin, X.-N.; Wei, F.; Feng, W.; Huang, Z.-C.; Wang, P.; Ren, L.; Diao, Y. Enhanced brain targeting of temozolomide in polysorbate-80 coated polybutylcyanoacrylate nanoparticles. Int. J. Nanomed. 2011, 6, 445–452.
- Svenson, S. Dendrimers as versatile platform in drug delivery applications. Eur. J. Pharm. Biopharm. 2009, 71, 445–462.
- He, H.; Li, Y.; Jia, X.-R.; Du, J.; Ying, X.; Lu, W.-L.; Lou, J.-N.; Wei, Y. PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials 2011, 32, 478–487.
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185.
- Webb, M.S.; Rebstein, P.; Lamson, W.; Bally, M.B. Liposomal drug delivery: Recent patents and emerging opportunities. Recent Pat. Drug Deliv. Formul. 2007, 1, 185–194.
- Zhang, Q.-Z.; Zha, L.-S.; Zhang, Y.; Jiang, W.-M.; Lu, W.; Shi, Z.-Q.; Jiang, X.-G.; Fu, S.-K. The brain targeting efficiency following nasally applied MPEG-PLA nanoparticles in rats. J. Drug Target. 2006, 14, 281–290.
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Li, Y.Q.; Wu, X.Y. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 491–504.
- Goppert, T.M.; Muller, R.H. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: Comparison of plasma protein adsorption patterns. J. Drug Target. 2005, 13, 179–187.
- Kerwin, B.A. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: Structure and degradation pathways. J. Pharm. Sci. 2008, 97, 2924–2935.
- Muller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128.
- Katouzian, I.; Esfanjani, A.F.; Jafari, S.M.; Akhavan, S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017, 68, 14–25.
- Iqbal, M.A.; Md, S.; Sahni, J.K.; Baboota, S.; Dang, S.; Ali, J. Nanostructured lipid carriers system: Recent advances in drug delivery. J. Drug Target. 2012, 20, 813–830.
- Rizwanullah, M.; Ahmad, J.; Amin, S. Nanostructured Lipid Carriers: A Novel Platform for Chemotherapeutics. Curr. Drug Deliv. 2016, 13, 4–26.
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313.
- Alam, T.; Pandit, J.; Vohora, D.; Aqil, M.; Ali, A.; Sultana, Y. Optimization of nanostructured lipid carriers of lamotrigine for brain delivery: In vitro characterization and in vivo efficacy in epilepsy. Expert Opin. Drug Deliv. 2015, 12, 181–194.
- Li, F.; Wang, Y.; Liu, Z.; Lin, X.; He, H.; Tang, X. Formulation and characterization of bufadienolides-loaded nanostructured lipid carriers. Drug Dev. Ind. Pharm. 2010, 36, 508–517.
- Weber, S.; Zimmer, A.; Pardeike, J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: A review of the state of the art. Eur. J. Pharm. Biopharm. 2014, 86, 7–22.
- Burton, G.W.; Ingold, K.U. Beta-Carotene: An unusual type of lipid antioxidant. Science 1984, 224, 569–573.
- Palozza, P.; Serini, S.; Maggiano, N.; Angelini, M.; Boninsegna, A.; Di Nicuolo, F.; Ranelletti, F.O.; Calviello, G. Induction of cell cycle arrest and apoptosis in human colon adenocarcinoma cell lines by beta-carotene through down-regulation of cyclin A and Bcl-2 family proteins. Carcinogenesis 2002, 23, 11–18.
- Moratalla-Lopez, N.; Jose Bagur, M.; Lorenzo, C.; Martinez-Navarro, M.E.; Rosario Salinas, M.; Alonso, G.L. Bioactivity and Bioavailability of the Major Metabolites of Crocus sativus L. Flower. Molecules 2019, 24, 2827.
- Batarseh, Y.S.; Bharate, S.S.; Kumar, V.; Kumar, A.; Vishwakarma, R.A.; Bharate, S.B.; Kaddoumi, A. Crocus sativus Extract Tightens the Blood-Brain Barrier, Reduces Amyloid beta Load and Related Toxicity in 5XFAD Mice. Acs Chem. Neurosci. 2017, 8, 1756–1766.
- Donoso, A.; Gonzalez-Duran, J.; Agurto Munoz, A.; Gonzalez, P.A.; Agurto-Munoz, C. Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials. Pharmacol. Res. 2021, 166, 105479.
- Routray, W.; Dave, D.; Cheema, S.K.; Ramakrishnan, V.V.; Pohling, J. Biorefinery approach and environment-friendly extraction for sustainable production of astaxanthin from marine wastes. Crit. Rev. Biotechnol. 2019, 39, 469–488.
- Kidd, P. Astaxanthin, Cell Membrane Nutrient with Diverse Clinical Benefits and Anti-Aging Potential. Altern. Med. Rev. 2011, 16, 355–364.
- Liu, X.; Shibata, T.; Hisaka, S.; Osawa, T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009, 1254, 18–27.
- Kong, K.W.; Ismail, A. Lycopene content and lipophilic antioxidant capacity of by-products from Psidium guajava fruits produced during puree production industry. Food Bioprod. Process. 2011, 89, 53–61.
- Perkins-Veazie, P.; Collins, J.K.; Pair, S.D.; Roberts, W. Lycopene content differs among red-fleshed watermelon cultivars. J. Sci. Food Agric. 2001, 81, 983–987.
- Chuacharoen, T.; Sabliov, C.M. Stability and controlled release of lutein loaded in zein nanoparticles with and without lecithin and pluronic F127 surfactants. Colloids Surf. A-Physicochem. Eng. Asp. 2016, 503, 11–18.
- Dembitsky, V.M.; Maoka, T. Allenic and cumulenic lipids. Prog. Lipid Res. 2007, 46, 328–375.
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B-Biol. 2009, 95, 101–107.
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs 2011, 9, 1806–1828.
- Yang, M.; Jin, L.; Wu, Z.; Xie, Y.; Zhang, P.; Wang, Q.; Yan, S.; Chen, B.; Liang, H.; Naman, C.B.; et al. PLGA-PEG Nanoparticles Facilitate In Vivo Anti-Alzheimer’s Effects of Fucoxanthin, a Marine Carotenoid Derived from Edible Brown Algae. J. Agric. Food Chem. 2021, 69, 9764–9777.
- Xiang, S.; Liu, F.; Lin, J.; Chen, H.; Huang, C.; Chen, L.; Zhou, Y.; Ye, L.; Zhang, K.; Jin, J.; et al. Fucoxanthin Inhibits beta-Amyloid Assembly and Attenuates beta-Amyloid Oligomer-Induced Cognitive Impairments. J. Agric. Food Chem. 2017, 65, 4092–4102.
- Oliyaei, N.; Moosavi-Nasab, M.; Tanideh, N.; Iraji, A. Multiple roles of fucoxanthin and astaxanthin against Alzheimer’s disease: Their pharmacological potential and therapeutic insights. Brain Res. Bull. 2022, 193, 11–21.
- Li, N.; Gao, X.; Zheng, L.; Huang, Q.; Zeng, F.; Chen, H.; Farag, M.A.; Zhao, C. Advances in fucoxanthin chemistry and management of neurodegenerative diseases. Phytomedicine 2022, 105, 154352.
- Lee, A.H.; Hong, S.-C.; Park, I.; Yoon, S.; Kim, Y.; Kim, J.; Yang, S.-H. Validation of Fucoxanthin from Microalgae Phaeodactylum tricornutum for the Detection of Amyloid Burden in Transgenic Mouse Models of Alzheimer’s Disease. Appl. Sci. 2021, 11, 5878.




