Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Faouzi Ben Rebah | -- | 1818 | 2023-04-25 18:13:43 | | | |
| 2 | Beatrix Zheng | Meta information modification | 1818 | 2023-04-26 04:51:09 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 1818 | 2023-04-26 04:52:22 | | | | |
| 4 | Wissem mnif | Meta information modification | 1818 | 2023-05-09 09:56:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mnif, W.; Ben Rebah, F. Microbial-Based Flocculants to Enhance Wastewater Sludge Dewaterability. Encyclopedia. Available online: https://encyclopedia.pub/entry/43473 (accessed on 07 February 2026).
Mnif W, Ben Rebah F. Microbial-Based Flocculants to Enhance Wastewater Sludge Dewaterability. Encyclopedia. Available at: https://encyclopedia.pub/entry/43473. Accessed February 07, 2026.
Mnif, Wissem, Faouzi Ben Rebah. "Microbial-Based Flocculants to Enhance Wastewater Sludge Dewaterability" Encyclopedia, https://encyclopedia.pub/entry/43473 (accessed February 07, 2026).
Mnif, W., & Ben Rebah, F. (2023, April 25). Microbial-Based Flocculants to Enhance Wastewater Sludge Dewaterability. In Encyclopedia. https://encyclopedia.pub/entry/43473
Mnif, Wissem and Faouzi Ben Rebah. "Microbial-Based Flocculants to Enhance Wastewater Sludge Dewaterability." Encyclopedia. Web. 25 April, 2023.
Copy Citation
Various microorganisms (fungi, bacteria, and microalgae) are able to produce flocculating materials, such as polysaccharides, proteins, and glycoproteins. The ability of microorganisms to produce these molecules is identified based on many parameters, including the morphology and the existence of slimy extracellular polysaccharides. For this purpose, various methods (colorimetric, 16S rRNA gene sequence, etc.) and reagents (chelating agents, CuSO4 solution crystal violet, etc.) are applied to isolate suitable microorganisms from soil, rivers, seawater, sludge, etc.
bioflocculants
sludge dewatering
microbial-based flocculants
1. Introduction
Because of the intensification of industrial activities and the improvement in people’s living standards, an increasing quantity of wastewater is generated, causing a serious health problem, mainly when it is discharged in the environment without treatment [1]. To manage wastewater from various origins (industrial and urban, etc.), wastewater treatment facilities are designed to remove pollutants using various methods, including physical, chemical, and biological processes. Generally, wastewater treatment processes generate large amounts of sludge, creating a potential threat to the environment and human health [2][3]. The obtained sludge with a lower solid content (under 8%) should be treated for final safe disposal [4][5]. Sludge handling and disposal is a significant step of the whole system, which costs as much as 50% of the total wastewater treatment cost [6][7]. However, sludge management cost is governed mainly by the efficiency of the methods used to separate liquids and solids in sludge [8], allowing the decrease in its volume and enhancing the post-treatment efficiency [9][10]. Generally, after mechanical dehydration, the sludge water content remains more than 70% [11], which should be reduced to meet the subsequent sludge reuse. The performance of sludge dewatering is controlled by various factors related to its composition, the particle size, the surface charge, the presence of extracellular polymers, etc. [12][13]. Because of their composition (mainly hydrophilic proteins and polysaccharides), extracellular polymers bind water molecules, allowing for high water content in sludge and making the sludge dewatering process difficult [14][15][16][17]. In order to enhance the efficiency of sludge dewatering, various methods are applied. These methods are classified into biological, chemical, and physical methods [18][19][20][21][22][23]. The biological methods are based on the use of enzymes that degrade proteins, allowing sludge floc fragmentation [18].
2. Microbial-Based Flocculants
Various microorganisms (fungi, bacteria, and microalgae) are able to produce flocculating materials, such as polysaccharides, proteins, and glycoproteins. The ability of microorganisms to produce these molecules is identified based on many parameters, including the morphology and the existence of slimy extracellular polysaccharides. For this purpose, various methods (colorimetric, 16S rRNA gene sequence, etc.) and reagents (chelating agents, CuSO4 solution crystal violet, etc.) are applied to isolate suitable microorganisms from soil, rivers, seawater, sludge, etc. [24]. The general process of the preparation of microbial-based flocculants is illustrated in Figure 1.
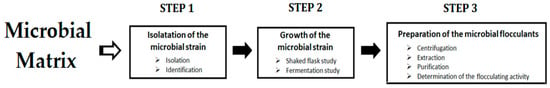
Figure 1. General process of the preparation of the microbial-based flocculants.
Generally, the microbial bioflocculants have been successfully applied for the removal of various pollutants (suspended solids, chemical oxygen demand, heavy metals, dyes, etc.) with high efficiency levels (>90%), allowing a significant flocculating activity (>70%) [24][25]. Interestingly they have the potential to improve sludge dewaterability, as indicated in Table 1.
Table 1. Applications of microbial-based flocculants for sludge dewatering.
| Crude Sludge Characteristics | Sludge Characteristics after Bioflocculation |
References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Sludge | pH | SRF (m/kg) | CST (s) |
MC (%) | DS (%) | Flocculation Conditions | SRF (m/kg) |
CST (s) |
MC (%) | DS (%) | |
| Municipal anaerobically digested sludge | 6.79 | 3.29 × 1013 | 38.70 | Acidithiobacillus ferrooxidans (108 cells/mL, 30 min, 180 rpm) | 0.36 × 1013 | 10.10 | 70.30 | [26] | |||
| Commercial cationic polymer (0.2%) | 1.08 × 1013 | 16.25 | 71.20 | ||||||||
| Municipal secondary sludge | 11.30 × 1012 | 13.20 | Pre-treated sludge flocculant (1.5 g/L), pH 7.5 | 3.40 × 1012 | 22.50 | [27] | |||||
| Al2(SO4)3 (8 g/L, pH 6.5) | 4.70 × 1012 | 15.90 | |||||||||
| PAM (0.15 g/L, pH 7.5) | 3.20 × 1012 | 24.20 | |||||||||
| PAC (4 g/L, pH 7.5) | 3.80 × 1012 | 20.60 | |||||||||
| FeCl3 (8 g/L, pH 6.5) | 4.50 × 1012 | 16.40 | |||||||||
| Municipal secondary sludge | 6.50 | 11.30 × 1012 | 13.20 | Paenibacillus polymyxa flocculant (1.5 g/L, pH 7.5) | 3.60 × 1012 | 21.70 | [28] | ||||
| Secondary sludge | 11.30 × 1012 | 13.20 | Paenibacillus polymyxa flocculant (1.5 g/L, pH 7.5) | 3.90 × 1012 | 20.80 | [29] | |||||
| Secondary sludge | 11.64 × 1012 | Klebsiella pneumoniae (0.1%/wt/v) |
4.66 × 1012 | 59.97 | [30] | ||||||
| Al2(SO4)3 | 6.26 × 1012 | ||||||||||
| PAC | 5.00 × 1012 | ||||||||||
| Secondary sludge | 6.23 | 29.00 × 105 | 3.19 | Proteus mirabilis TJ-1 (7 mg) + CaCl2 (12.5 mg/g Dw), (pH 7.5) | 9.00 × 105 | [31] | |||||
| Chemically treated primary sludge | 6.20 | 71.90 × 1012 | 122.70 | 2.71 | Acidithiobacillus ferrooxidans + Fe2+ (10% v/v) | 5.00 × 1012 | 20.00 | [32] | |||
| Activated sludge | 6.70 | 10.00 × 1012 | 12.60 | 2.08 | Acidithiobacillus ferrooxidans + Fe2+ (10% v/v) | <5.00 × 1012 | 7.90 | [32] | |||
| Anaerobically digested sludge | 7.70 | 8.30 × 1012 | 19.50 | 2.10 | Acidithiobacillus ferrooxidans + Fe2+ (10% v/v) | <3.00 × 1012 | 7.50 | [33] | |||
| Anaerobically digested sludge | 7.45 | 16.10 × 1012 | 30.40 | 2.05 | Acidithiobacillus ferrooxidans + Fe2+ (10% v/v) | <1.00 × 1012 | <20 | [34] | |||
| Chemically treated primary sludge | 6.74 | 111.00 × 1012 | 121.00 | 2.59 | Acidithiobacillus ferrooxidans + Fe2+ (10% v/v) | 11.10 × 1012 | 10.00 | 31.40 | [35] | ||
| Chemically treated primary sludge | 7.03 | 86.90 | 2.00 | Filamentous fungal strains (5% w/v), pH 6.85–7.15 |
35.50 | [36] | |||||
| Secondary sludge | 8.04 | 10.87 × 1012 | 13.10 | Klebsiella sp. (6 mg/g Dw), pH 8 |
3.36 × 1012 | 17.50 | [37] | ||||
| Municipal digested sludge | 7.70 | 339.10 | 82.4 | Acidithiobacillus ferrooxidans ILS-2 + Fe2+ (15% v/v) | 31.30 | 60.10 | [38] | ||||
| Acidithiobacillus ferrooxidans ILS-2 + Fe2+ (21% v/v) | 26.20 | 48.60 | |||||||||
| Secondary activated sludge | 6.40 | 11.30 × 1012 | 12.10 | MBF10 Rhodococcus erythropolis (12 g/kg dry sludge) |
4.80 × 1012 | 19.30 | [39] | ||||
| MBF10 Rhodococcus erythropolis (10.5 g/kg + PAC (19.4 g/kg)) |
3.20 × 1012 | 23.60 | |||||||||
| Municipal activated sludge | 7.43 | 2.76 × 1012 | 21.00 | Talaromyces flavus S1 | 0.83 × 1012 | 12.40 | [40] | ||||
The bioflocculant produced by Rhodococcus erythropolis in alkaline thermal pre-treated sludge allowed a significant increase in both SRF and DS, reaching 3.4 × 1012 m/kg and 22.5%, respectively [27]. In the same research, the use of R. erythropolis supplemented with synthetic polymers (PAC and Al2(SO4)3) increased the charge neutralization and bridging effect, allowing the enlargement of the flocs and, consequently, improving the sludge dewaterability [27]. However, for specific microbial strains there is a need for an energy substance (Fe2+) for efficient production of biogenic flocculants [32][33][34][35]. For example, Acidithiobacillus ferrooxidans in the presence of Fe2+ (10% v/v) significantly improved the dewaterability of anaerobically digested sludge, and the values of SRF and CST passed from 16.1 × 1012 m/kg to less than 1 × 1012 m/kg and from 30.4 s to less than 20 s [34]. The same strain improved the dewaterability of various sludges (chemically treated primary sludge, activated sludge, and anaerobically digested sludge) and the highest reduction was observed for chemically treated primary sludge, with final values for SRF and CST of 5 × 1012 m/kg and 20 s, respectively [33]. Moreover, the biopolymer produced by the same strain (Acidithiobacillus ferrooxidans) reduced the SRF and the CST of municipal anaerobically digested sludge with an interesting reduction rate of MC (70.3%), SRF, and CST. The SRF and CST values passed from 3.29 × 1013 m/kg to 0.36 × 1013 m/kg and from 38.7 s to 10.1 s, respectively. The obtained reduction rates are higher than those reported for polyacrylamide (PAM) [26]. Similarly, the use of filamentous fungal strains for the dewatering of chemically treated primary sludge allowed the decrease in CST from 86.9 to 35.5 s in the presence of metal cations [36]. More recently, the strain A. ferrooxidans ILS-2 was added to municipal digested sludge in the presence of ferrous iron (10–21%), allowing a significant reduction in CST and MC values. However, this reduction increased when increasing Fe2+ loading, and the highest reduction was obtained with ferrous iron at 21%. Fe2+ loading at 21% reduced CST from 339.1 s (without strain and ferrous addition) to 26 s, and MC from 82.4% (without strain and ferrous addition) to 84.6% [38]. Therefore, higher loading of ferrous iron could improve the growth of A. ferrooxidans in sludge, and this strain transforms ferrous iron to biogenic ferric iron that acts as bioflocculant, allowing the enhancement of sludge dewaterability by the release of bound/stagnant water in extracellular polymeric substances in sludge.
A bioflocculant TJ-F1 obtained by growing P. mirabilis was tested for the dewaterability of a secondary sludge showing a higher reduction in SRF compared to a synthetic polymer P(AM-DMC). In the presence of 7 mg of the bioflocculant supplemented with 12.5 mg/g dw (dry weight) of the synthetic polymer and at pH 7.5, the SRT of the sludge reduced by 69% which is significantly higher than that obtained by P(AM-DMC) [31]. In the same context, the exopolysaccharide Klebsiella sp. at a dosage of 6 mg/g dw and at pH 8 allowed a reduction in the secondary sludge SRF by 69%, giving a final DS of about 17.5% [37]. In the same research, the use of the bioflocculant supplemented with alum reduced the SRF by 84.2% and achieved a DS of 21.3% [37]. In this context, Serratia flocculant used for sludge dewatering allowed for a sludge volume index of 54 mg/L, obtained at a dosage of 0.3 g/L of the bioflocculant. However, with a synthetic flocculant, such as cationic polymers, a sludge volume index of 56 mg/L at a dosage of 0.3 g/L was achieved [41]. Similarly, the polysaccharidic bioflocculant produced by Rhodococcus erythropolis cultivated in rice stover hydrolysate showed better sludge dewaterability performances than synthetic polymer in terms of DS and SRF [39]. More recently, the spores of the filamentous fungus Talaromyces flavus S1 were used to inoculate activated sludge. This inoculation improved the dewaterability by 48% [40]. This improvement may be related to the polysaccharides produced by the fungal mycelium [42]. It was reported in the literature that extracellular polymeric substances have the ability to enhance the formation of biofloc, allowing higher settleability of sludge [43]. The content of the extracellular polymeric substances significantly affects their role in sludge dewaterability. Thus, higher carbohydrate content and lower protein content may increase sludge dewatering [44][45]. Likewise, it is very important to point out that sludge characteristics (sludge origin, pH, organic content, cationic content, etc.) affect the facility of extracellular polymeric substances to act in sludge conditioning [46]. Indeed, the use of microbial flocculant could increase the sludge calorific value, as reported by Kurade et al. [26][35]. Moreover, microbial flocculants act at lower dosages when compared to synthetic polymers, such as FeCl3 and Al2(SO4)3 [27].
According to the literature, sludge dewatering can be achieved by adding the microbial strain into sludge and the bioflocculant will be produced during the growth or by the application of a pure bioflocculant purified after its production by a selected microbial strain growing in an appropriate growth medium [47]. However, the microbial bioflocculant production is controlled by various factors including the culture medium and the operating conditions (C/N ratio, oligoelements, pH, temperature, aeration etc.) [24][48]. For large-scale production, optimization studies should be carried out in order to maximize the bioflocculant production. Moreover, the purification process and the preservation method should be taken into consideration in bioflocculant recovery. The optimization of the growth media and the purification process are considered as the main factors that control the product commercialization. For economical production, a low-cost medium should be developed and/or high-yield strains should be selected. In this context, various agricultural and industrial wastes (molasses, poultry processing waste, corn, rice, peanut, potato, corn, etc.) [49][50] and wastewaters generated by many industries (potato starch, brewery, corn ethanol, swine, palm oil mill, livestock, ramie biodegumming, etc.) have demonstrated their ability to replace standard microbial growth media for bioflocculant production [25]. This may considerably reduce the microbial flocculant production cost, as reported by Siddeeg et al. (2019) [25]. In the same way, another strategy was developed based on the screening of new microbial strains able to grow and produce flocculant in a culture medium low in nutrients [51]. Is also important to promote the selection of strains with the ability to produce bioflocculants that act without metal activation [51][52][53]. Furthermore, the microbial bioflocculant yield could be improved using genetic engineering [54]. The microbial diversity and the variability of the carbon sources may affect the nature and the characteristics of the produced bioflocculant (structure, composition, flocculating activity, etc.) [25]. Although these variations may limit the universal use of the produced microbial bioflocculant, these biopolymers seem suitable to replace synthetic polymers in the coagulation/flocculation process in wastewater treatment and sludge dewatering [24]. Generally, the research activities reported for sludge conditioning are limited and more investigations are needed to evaluate the flocculating activity at a large scale for sludge from various origins. A techno-economic feasibility should be conducted, taking into consideration the various parameters, such as the growth conditions (culture medium composition, operating parameters, extraction and purification of bioflocculants, etc.).
References
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S.; Tripathi, P.; Lal, J.A.; Jha, N.K.; Siddiqui, M.H.; Kumar, P.; Tripathi, V.; Ruokolainen, J. Wastewater treatment and reuse: A review of its applications and health implications. Water Air Soil Pollut. 2021, 232, 208.
- Elmi, A.; AlOlayan, M. Sewage sludge land application: Balancing act between agronomic benefits and environmental concerns. J. Clean. Prod. 2020, 250, 119512.
- Ekane, N.; Barquet, K.; Rosemarin, A. Resources and risks: Perceptions on the application of sewage sludge on agricultural land in Sweden, a case study. Front. Sustain. Food Syst. 2021, 5, 647780.
- Anjum, M.; Al-Makishah, N.H.; Barakat, M.A. Wastewater sludge stabilization using pre-treatment methods. Process Saf. Environ. Prot. 2016, 102, 615–632.
- Castellanos-Rozo, J.; Galvis-López, J.A.; Castellanos, N.A.M.; Manjarres-Hernández, E.H.; Rojas, A.L. Assessment of two sludge stabilization methods in a wastewater treatment plant in Sotaquirá, Colombia. Univ. Sci. 2020, 25, 17–36.
- Roldán, M.; Bouzas, A.; Seco, A.; Mena, E.; Mayor, Á.; Barat, R. An integral approach to sludge handling in a WWTP operated for EBPR aiming phosphorus recovery: Simulation of alternatives, LCA and LCC analyses. Water Res. 2020, 175, 115647.
- Flores-Alsina, X.; Ramin, E.; Ikumi, D.; Harding, T.; Batstone, D.; Brouckaert, C.; Sotemann, S.; Gernaey, K.V. Assessment of sludge management strategies in wastewater treatment systems using a plant-wide approach. Water Res. 2021, 190, 116714.
- Mowla, D.; Tran, H.N.; Allen, D.G. A review of the properties of biosludge and its relevance to enhanced dewatering processes. Biomass Bioenergy 2013, 58, 365–378.
- Zhang, L.; Xu, C.; Champagne, P.; Mabee, W. Overview of current biological and thermo-chemical treatment technologies for sustainable sludge management. Waste Manag. Res. 2014, 32, 586–600.
- Wu, B.; Dai, X.; Chai, X. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations. Water Res. 2020, 180, 115912.
- Zhen, Z.; Jinxiang, Y.; Renhui, D. A review on the physical dewatering methods of sludge pretreatment in recent ten years. IOP Conf. Ser. Earth Environ. Sci. 2020, 455, 012189.
- Wang, H.F.; Hu, H.; Wang, H.J.; Bai, Y.N.; Shen, X.F.; Zhang, W.; Zeng, R.J. Comprehensive investigation of the relationship between organic content and waste activated sludge dewaterability. J. Hazard. Mater. 2020, 394, 122547.
- Xiao, K.; Li, N.; Yang, C.; Zhu, Y.; Yu, Z.; Yu, W.; Liang, S.; Hou, H.; Liu, B.; Hu, J.; et al. Deciphering the impacts of composition of extracellular polymeric substances on sludge dewaterability: An often overlooked role of amino acids. Chemosphere 2021, 284, 131297.
- Chen, C.; Zhang, T.; Lv, L.; Chen, Y.; Tang, W.; Tang, S. Destroying the structure of extracellular polymeric substance to improve the dewatering performance of waste activated sludge by ionic liquid. Water Res. 2021, 199, 117161.
- Meyer, T.; Amin, P.; Allen, D.G.; Tran, H. Dewatering of pulp and paper mill biosludge and primary sludge. J. Environ. Chem. Eng. 2018, 6, 6317–6321.
- Yin, X.; Han, P.; Lu, X.; Wang, Y. A review on the dewaterability of bio-sludge and ultrasound pretreatment. Ultrason. Sonochem. 2004, 11, 337–348.
- Li, Y.B.; Song, J.L.; Yao, Q.J.; Chen, Z.X.; Wei, Y.; Li, H.L.; Wang, M.X.; Wang, B.J.; Zhou, J.M. Effects of dissolved oxygen on the sludge dewaterability and extracellular polymeric substances distribution by bioleaching. Chemosphere 2021, 281, 130906.
- Kang, X.; Li, C.; Ding, W.; Ma, Y.; Gao, S.; Zhou, X.; Chen, Y.; Liu, W.; Jiang, G. Optimization of operating conditions in the biological enzymes for efficient waste activated sludge dewatering. Process Saf. Environ. Prot. 2023, 170, 545–552.
- Zhang, X.; Ye, P.; Wu, Y. Enhanced technology for sewage sludge advanced dewatering from an engineering practice perspective: A review. J. Environ. Manag. 2022, 321, 115938.
- Cao, B.; Zhang, T.; Zhang, W.; Wang, D. Enhanced technology based for sewage sludge deep dewatering: A critical review. Water Res. 2021, 189, 116650.
- Guo, J.; Wen, X. Performances and mechanisms of sludge dewatering by a biopolymer from piggery wastewater and application of the dewatered sludge in remediation of Cr (VI)-contaminated soil. J. Environ. Manag. 2020, 259, 109678.
- Hyrycz, M.; Ochowiak, M.; Krupińska, A.; Włodarczak, S.; Matuszak, M. A review of flocculants as an efficient method for increasing the efficiency of municipal sludge dewatering: Mechanisms, performances, influencing factors and perspectives. Sci. Total Environ. 2022, 820, 153328.
- Yan, Y.; Qin, L.; Gao, J.; Nan, R.; Gao, J. Protein extraction and sludge dewatering performance of ultrasound-assisted enzymatic hydrolysis of excess sludge. Environ. Sci. Pollut. Res. 2020, 27, 18317–18328.
- Ben Rebah, F.; Mnif, W.; Siddeeg, S.M. Microbial flocculants as an alternative to synthetic polymers for wastewater treatment: A review. Symmetry 2018, 10, 556.
- Siddeeg, S.M.; Tahoon, M.A.; Rebah, F.B. Agro-industrial waste materials and wastewater as growth media for microbial bioflocculants production: A review. Mater. Res. Express 2019, 7, 012001.
- Kurade, M.B.; Murugesan, K.; Selvam, A.; Yu, S.A.M.; Wong, J.W.C. Sludge conditioning using biogenic flocculant produced by Acidithiobacillus ferrooxidans for enhancement in dewaterability. Bioresour. Technol. 2016, 217, 179–185.
- Guo, J.; Ma, J. Bioflocculant from pre-treated sludge and its applications in sludge dewatering and swine wastewater pretreatment. Bioresour. Technol. 2015, 196, 736–740.
- Guo, J.; Nengzi, K.L.; Zhao, J.; Zhang, Y. Enhanced dewatering of sludge with the composite of bioflocculant MBFGA1 and P(AM-DMC) as a conditioner. Appl. Microbiol. Biotechnol. 2015, 99, 2989–2998.
- Guo, J.; Zhang, Y.; Zhao, J.; Zhang, Y.; Xiao, X.; Wang, B.; Shu, B. Characterization of a bioflocculant from potato starch wastewater and its application in sludge dewatering. Appl. Microbiol. Biotechnol. 2015, 99, 5429–5437.
- Liu, J.; Ma, J.; Liu, Y.; Yang, Y.; Yue, D.; Wang, H. Optimized production of a novel bioflocculant M-C11 by Klebsiella sp. and its application in sludge dewatering. J. Environ. Sci. 2014, 26, 2076–2083.
- Zhang, Z.Q.; Xia, S.Q.; Zhang, J. Enhanced dewatering of waste sludge with microbial flocculant TJ-F1 as a novel conditioner. Water Res. 2010, 44, 3087–3092.
- Wong, J.W.C.; Murugesan, K.; Yu, S.M.; Kurade, M.B.; Selvam, A. Improved dewatering of CEPT sludge by biogenic flocculant from Acidithiobacillus ferrooxidans. Water Sci. Technol. 2016, 73, 843–848.
- Wong, J.W.C.; Murugesan, K.; Selvam, A.; Ravindran, B.; Kurade, M.B.; Yu, S.M. Dewatering of saline sewage sludge using iron-oxidizing bacteria: Effect of substrate concentration. Bioresour. Technol. 2016, 213, 31–38.
- Murugesan, K.; Ravindran, B.; Selvam, A.; Kurade, M.B.; Yu, S.M.; Wong, J.W. Enhanced dewaterability of anaerobically digested sewage sludge using Acidithiobacillusferrooxidans culture as sludge conditioner. Bioresour. Technol. 2014, 169, 374–379.
- Kurade, M.B.; Murugesan, K.; Selvam, A.; Yu, S.M.; Wong, J.W.C. Ferric biogenic flocculant produced by Acidithiobacillus ferrooxidans enable rapid dewaterability of municipal sewage sludge: A comparison with commercial cationic polymer. Int. Biodeterior. Biodegrad. 2014, 96, 105–111.
- Murugesan, K.; Selvam, A.; Wong, J.W. Flocculation and dewaterability of chemically enhanced primary treatment sludge by bioaugmentation with filamentous fungi. Bioresour. Technol. 2014, 168, 198–203.
- Yang, Q.; Luo, K.; Liao, D.X.; Li, X.M.; Wang, D.B.; Liu, X.; Zeng, G.M.; Li, X. A novel bioflocculant produced by Klebsiella sp. and its application to sludge dewatering. Water Environ. J. 2012, 26, 560–566.
- Cai, G.; Ebrahimi, M.; Zheng, G.; Kaksonen, A.H.; Morris, C.; O’Hara, I.M.; Zhang, Z. Effect of ferrous iron loading on dewaterability, heavy metal removal and bacterial community of digested sludge by Acidithiobacillus ferrooxidans. J. Environ. Manag. 2021, 295, 113114.
- Guo, J.; Chen, C. Sludge conditioning using the composite of a bioflocculant and PAC for enhancement in dewaterability. Chemosphere 2017, 185, 277–283.
- Liu, H.; Shi, J.; Xu, X.; Zhan, X.; Fu, B.; Li, Y. Enhancement of sludge dewaterability with filamentous fungi Talaromyces flavus S1 by depletion of extracellular polymeric substances or mycelium entrapment. Bioresour. Technol. 2017, 245, 977–983.
- Subramanian, S.B.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances (EPS) producing bacterial strains of municipal wastewater sludge: Isolation, molecular identification, EPS characterization and performance for sludge settling and dewatering. Water Res. 2010, 44, 2253–2266.
- Liu, W.; Hao, Y.; Jiang, J.; Zhu, A.; Zhu, J.; Dong, Z. Production of a bioflocculant from Pseudomonas veronii L918 using the hydrolyzate of peanut hull and its application in the treatment of ash-flushing wastewater generated from coal fired power plant. BioresourTechnol. 2016, 218, 318.
- Houghton, J.J.; Quarmby, J.; Stephenson, T. Municipal wastewater sludge dewaterability and the presence of microbial extracellular polymer. Water Sci.Technol. 2001, 44, 373–379.
- Cetin, S.; Erdincler, A. The role of carbohydrate and protein parts of extracellular polymeric substances on the dewaterability of biological sludges. Water Sci. Technol. 2004, 50, 49–56.
- Sponza, D.T. Extracellular polymer substances and physicochemical properties of flocs in steady and unsteady-state activated sludge systems. Process Biochem. 2002, 37, 983–998.
- Faye, M.C.A.S.; Zhang, K.K.; Sun, P.; Zhang, Y. Sludge dewaterability: The variation of extracellular polymeric substances during sludge conditioning with two natural organic conditioners. J. Environ. Manag. 2019, 251, 109559.
- Nkosi, N.C.; Basson, A.K.; Ntombela, Z.G.; Maliehe, T.S.; Pullabhotla, R.V.S.R. Isolation, Identification and Characterization of Bioflocculant-Producing Bacteria from Activated Sludge of Vulindlela Wastewater Treatment Plant. Appl. Microbiol. 2021, 1, 586–606.
- Li, H.; Wu, S.; Du, C.; Zhong, Y.; Yang, C. Preparation, performances, and mechanisms of microbial flocculants for wastewater treatment. Int. J. Environ. Res. Public Health 2020, 17, 1360.
- Qi, Z.; Zhu, Y.; Guo, H.; Wang, X.; Zhao, Y.; Zhou, Y.; Chen, Y.; Yang, Y.; Qin, W.; Shao, Q. Production of glycoprotein bioflocculant from untreated rice straw by a cazyme-rich bacterium, Pseudomonas sp. HP2. J. Biotechnol. 2019, 306, 185–192.
- Sam, S.; Kucukasik, F.; Yenigun, O.; Nicolaus, B.; Oner, E.T.; Yukselen, M.A. Flocculating performances of exopolysaccharides produced by a halophilic bacterial strain cultivated on agro-industrial waste. Bioresour. Technol. 2011, 102, 1788–1794.
- Liu, W.; Wang, K.; Li, B.; Yuan, H.; Yang, J. Production and characterization of an intracellular bioflocculant by Chryseobacterium daeguense W6 cultured in low nutrition medium. Bioresour. Technol. 2010, 101, 1044–1048.
- Tang, W.; Song, L.; Li, D.; Qiao, J.; Zhao, T.; Zhao, H. Production, characterization, and flocculation mechanism of cation independent, pH tolerant, and thermally stable bioflocculant from Enterobacter sp. ETH-2. PLoS ONE 2014, 9, e114591.
- Yin, Y.J.; Tian, Z.M.; Tang, W.; Li, L.; Song, L.Y.; Mcelmurry, S.P. Production and characterization of high efficiency bioflocculant isolated from Klebsiella sp. ZZ-3. Bioresour. Technol. 2014, 171, 336–342.
- Chen, Z.; Liu, P.; Li, Z.; Yu, W.; Wang, Z.; Yao, H.; Wang, Y.; Li, Q.; Deng, X.; He, N. Identification of key genes involved in polysaccharide bioflocculant synthesis in Bacillus licheniformis. Biotechnol. Bioeng. 2017, 114, 645–655.
More
Information
Subjects:
Green & Sustainable Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Wastewater Treatment
Revisions:
4 times
(View History)
Update Date:
09 May 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




