Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kafayat Aderonke Yusuf | -- | 2498 | 2023-04-25 16:35:05 | | | |
| 2 | Lindsay Dong | Meta information modification | 2498 | 2023-04-26 10:48:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yusuf, K.; Sampath, V.; Umar, S. Association of Bacterial Infections and Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/43470 (accessed on 07 February 2026).
Yusuf K, Sampath V, Umar S. Association of Bacterial Infections and Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/43470. Accessed February 07, 2026.
Yusuf, Kafayat, Venkatesh Sampath, Shahid Umar. "Association of Bacterial Infections and Cancer" Encyclopedia, https://encyclopedia.pub/entry/43470 (accessed February 07, 2026).
Yusuf, K., Sampath, V., & Umar, S. (2023, April 25). Association of Bacterial Infections and Cancer. In Encyclopedia. https://encyclopedia.pub/entry/43470
Yusuf, Kafayat, et al. "Association of Bacterial Infections and Cancer." Encyclopedia. Web. 25 April, 2023.
Copy Citation
Several critical associations were found between bacterial infections and cancer, of which some are causative: Porphyromonas gingivalis and Fusobacterium nucleatum are associated with periodontal disease, Salmonella spp., Clostridium perfringens, Escherichia coli, Campylobacter spp., and Shigella are associated with gastroenteritis. Helicobacter pylori infection is implicated in the etiology of gastric cancer, and persistent Chlamydia infections present a risk factor for the development of cervical carcinoma, especially in patients with the human papillomavirus (HPV) coinfection. Salmonella typhi infections are linked with gallbladder cancer, Chlamydia pneumoniae infection is implicated in lung cancer, etc.
bacterial infections
cancer
antibiotics
antibiotic resistance
adaptation
dormancy
microbiome
dysbiosis
1. Introduction
Cancer is a disabling, challenging, and frightening disease that can affect any body part [1]. The global cancer epidemic remains a public health concern, as cancer is established as the second foremost cause of death in the United States, with close to 2 million new cases and a little over 600,000 deaths expected to occur in 2022 [2]. Mortality from lung cancer remains the most common type of cancer-related death, accounting for approximately 350 deaths daily [2]. The public health burden associated with these high numbers has fueled massive research efforts to uncover the preventable causes of cancer [3]. In a series of excellent reviews published recently, expert analysts reflect on the fact that the global burden of cancer is growing, especially in the most vulnerable sociodemographic populations, and that significant global effort is required not only to reduce the incidence of cancer but also to provide more equally distributed cancer control [4][5][6].
Cancer results from a series of genetic and epigenetic changes that disrupt regular cell growth, control, and survival. A wide range of intrinsic and extrinsic factors influence these changes. Intrinsic factors may include genetic mutations, random errors in DNA replication, immune and inflammatory responses along with other modifiable factors, including aging; external factors may include diet type, smoking/tobacco use, radiation, and infectious organisms [7].
Infectious pathogens such as bacteria and viruses are modifiable causes of cancer, accounting for 20% of all human tumors [8][9]. Pathogens associated with cancer can exhibit mechanisms that include persistent infection, evasion of the immune response, chronic inflammation leading to continued cell proliferation, and an increased risk of oncogenic transformation, even in immune-competent individuals [10].
The human body is home to many microbes that form complex ecological habitats and influence the physiology of human health and disease, the totality of which may be summarized as the human microbiome [11][12]. The most effective way to describe the human microbiome is as a complex collection of microorganisms found in different body parts, including the skin, the oral cavity and saliva, the respiratory system, the reproductive tract, and the gastrointestinal system. These microorganisms include bacteria, eukaryotes, archaea, fungi, and viruses [12][13]. Since the population of bacteria in the microbiome vastly outnumbers that of other microorganisms, researchers sometimes simply refer to the microbiome as bacteria [13]. According to Curtis and Sperandio, there are 100 trillion bacteria in the human body, with 500–1000 different bacterial species living mostly (but not exclusively) in the gut [14]. The resulting interactions are beneficial to our homeostasis and well-being [15].
Commensal bacteria colonize the host shortly after birth, forming at first a small community that progressively transforms into a diversified ecosystem. Over time, the host-bacterial associations develop into a mutually beneficial relationship [14][16]. The gut, for instance, provides nutrients to resident bacteria, which in turn assists food digestion, the absorption of nutrients, and the metabolism of indigestible substrates. This coexistence can also help to modulate immune system activity, maintain the intestinal architecture, and prevent the colonization of pathogenic microorganisms [14][16]. Despite this, an imbalance in host-bacterial interactions (dysbiosis) can change the physiological equilibrium in the host cells. This discrepancy can facilitate the progression of many conditions including inflammatory bowel disease, malnutrition, obesity, diabetes, and cancer [15][17].
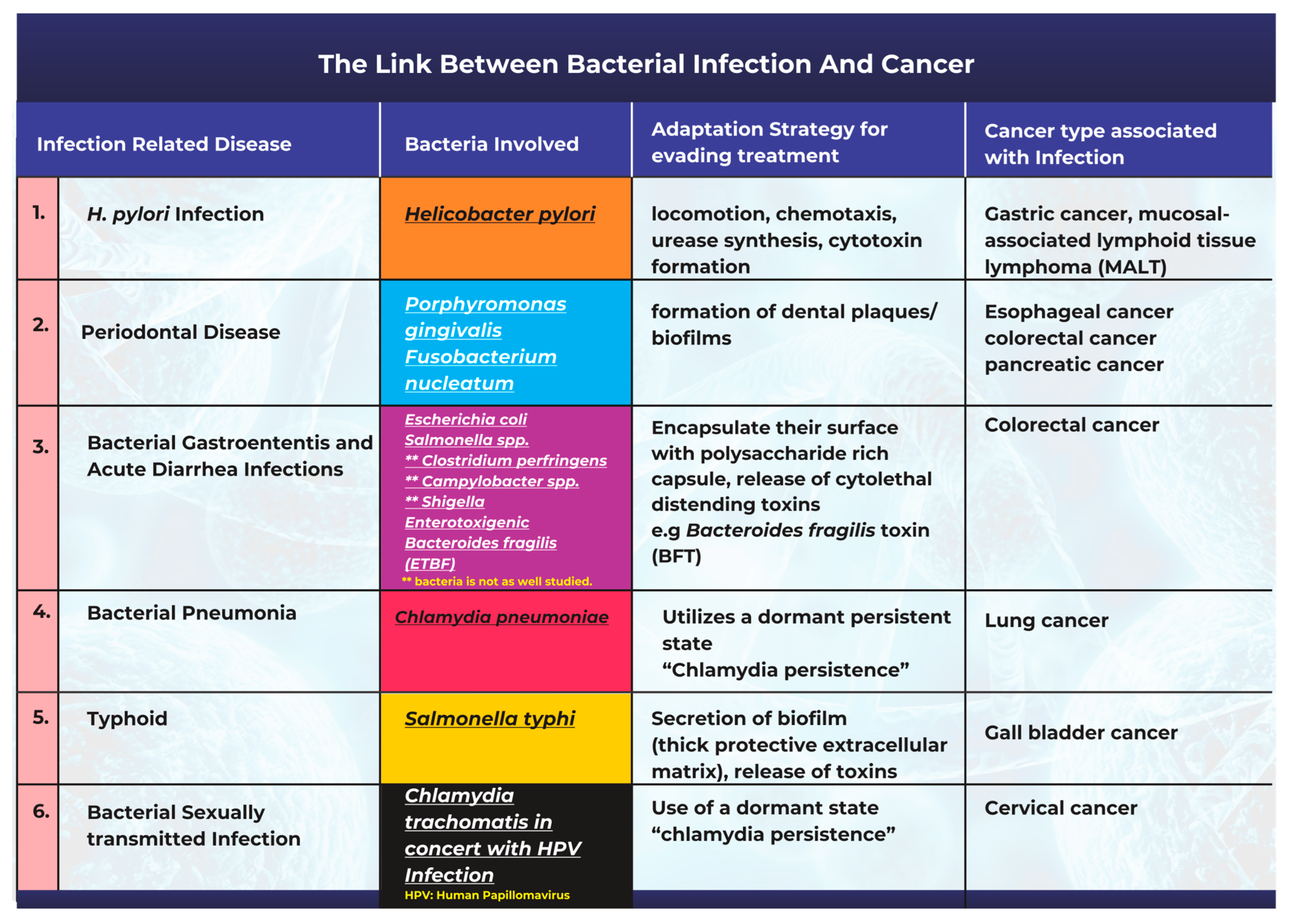
2. The Link between Bacterial Infection and the Onset of Cancer
Bacteria and bacterial infections can act as potential carcinogens and tumor promoters [18]. The production of bacterial toxins, enzymes, and oncogenic peptides can all significantly contribute to tumor development by promoting inflammation, interfering with cell cycle control, and disrupting cell signaling pathways [18][19]. Other studies have also corroborated the fact that microbiota-mediated infection stimulates cancer cell proliferation by targeting host cell DNA, altering the immune system, and promoting epithelial-to-mesenchymal transition [17]. A description of the most frequently studied cases highlighting the link between bacterial infections and cancer is summarized in Figure 1.

Figure 1. An illustration of the links between common bacterial infections and cancer.
2.1. Helicobacter pylori Infection and Gastric Cancer
One of the most well-known and researched connections associating cancer with a bacterial origin is the link between Helicobacter pylori infection and gastric cancer [8][20][21]. H. pylori has been extensively studied in this context with comprehensive epidemiological data supporting the existence of its causal relationship with carcinogenesis [20][22]. H. pylori is a gram-negative spiral-shaped bacterium that lives in a neutral pH niche between the stomach mucus layer and the gastric epithelium [23][24][25]. Studies have opined that there are two potential explanations for the association between H. pylori and gastric cancer. The first of these is that H. pylori infection causes persistent gastric mucosal inflammation, resulting in atrophy and eventual intestinal metaplasia; the second is that H. pylori can create, alter, or release bacterial virulence factors that play a significant role in cancer progression [20][23][25]. Studies have also shown that H. pylori infection contributes to an increase in the bacteria populations from the Proteobacteria, Spirochaetes, and Acidobacteria phyla, alongside a decline in the abundance of Bacteroidetes, Actinobacteria, and Firmicutes [26]. H. pylori infection promotes persistent inflammation of the gastric mucosa and facilitates the progression of both mucosal-associated lymphoid tissue lymphoma and gastric cancer [27][28].
2.2. Periodontal Disease Caused by Bacterial Infections Can Promote Tumorigenesis
Fusobacterium nucleatum is an anaerobic, gram-negative, disease-causing bacterium in the oral cavity [29]. Studies have suggested that dysbiosis of the oral microbiome allows F. nucleatum to become an opportunistic bacterium that can cause gum disease and human cancers [29][30]. A research study revealed that the abundance of F. nucleatum in the tissues of patients with esophageal squamous carcinoma was linked to shorter patient survival times [29].
Porphyromonas gingivalis is a gram-negative bacterium that is an apparent pathogen in periodontal diseases and other systemic conditions [31]. In 2011, a detailed study found that the concentration of P. gingivalis was higher in cancer cells than in normal mouth tissues. T
Causes and incidence: Periodontal disease is a well-established and common oral condition in the human population [32]. Periodontal diseases are associated with several risk factors: smoking habits, poor oral hygiene, medication regimens, age, heredity, and stress [32]. These factors can hasten the dysbiosis of the oral microbiome and the multiplication of pathogenic bacteria, which drive disease progression.
Adaptation strategies for survival and the evasion of treatment: One major mechanism related to the persistence and adaptability observed in oral pathogenic bacteria is their ability to form dental plaques or biofilms (Figure 1). These structures make them resistant to mechanical stress or antibiotic treatment [32][33]. The plaque biofilm acts as a protective barrier by isolating them from harmful agents and maintaining distinct phenotypic characteristics that promote their survival. Once the niche is established, the bacteria can release toxins such as lipopolysaccharide (LPS) and produce proinflammatory cytokines that promote chronic inflammation in periodontal tissues [34].
2.3. Bacterial Pathogenesis in the Onset of Gastroenteritis, Acute Diarrhea, and Colon Cancer
Bacterial gastroenteritis is a kind of inflammation in the stomach and small intestine caused by a bacterial infection. In affected patients, the disease is often accompanied by severe diarrhea [35]. Although gastroenteritis can be caused by viruses, fungi, or parasites, most cases are caused by bacterial pathogens [35]. Bacteria associated with gastroenteritis include Escherichia coli, Salmonella spp., Clostridium perfringens, Campylobacter spp., and Shigella [35]. Salmonella and E. coli are the most frequently studied regarding their potential role in carcinogenesis.
Salmonella is an intracellular infection that affects a variety of animals and humans. The results of a Salmonella infection can range significantly from a moderate, self-limiting gastroenteritis to a severe and potentially fatal systemic infection [36]. Salmonella enterica serovar Enteritidis has been implicated in several gastroenteritis outbreaks linked to contaminated food [37][38].
E. coli is a gram-negative bacterium, ubiquitous in nature, that is found in the human gut microbiome [39]. Several studies have found that patients with colorectal cancer have higher levels of colon mucosa-associated E. coli colonization than those found in healthy individuals [39][40]. Pathogenic E. coli strains have been shown to produce toxins (Figure 1) such as cyclomodulin, cytotoxic necrosis factor (CNF), circulation inhibitory factor (Cif), colibactin, and cytolethal distending toxins (CDT). By disrupting the cell cycle and/or promoting DNA damage, these toxins can affect cell differentiation, apoptosis, and cell proliferation [39][41][42].
Enterotoxigenic Bacteroides fragilis (ETBF) is another common bacterium associated with acute diarrhea infections [43]. Bacteroides fragilis (B. fragilis) is an obligate anaerobic gram-negative bacillus bacterium [44] that makes up 0.1% of the normal flora of the colon [25]. However, ETBF levels are elevated in CRC patients’ feces and colonic mucosal tissues [25]. According to the “alpha-bug” theory, a critical pathogenic species, such as (ETBF), remodels the microbiota to promote CRC, most probably through an IL-17 and Th17-mediated inflammatory response [45].
2.4. Chlamydia pneumoniae Infection and Lung Cancer
Chlamydia pneumoniae (Cpn) is an obligate intracellular bacterium that causes various respiratory diseases in humans, including pneumonia [46]. Chronic obstructive pulmonary disease, asthma, and lung cancer may result from repeated or prolonged exposure to Chlamydia antigens [47].
Previous research has found serological evidence of a link between Chlamydia pneumoniae infection and lung cancer [48]. A report also reviewed previous epidemiological studies on the link between C. pneumoniae and lung cancer. It concluded that earlier investigations supported a causative relationship between C. pneumoniae infection and lung cancer [49]. Correspondingly, a group of researchers conducted a meta-analysis on 12 published articles that studied the link between C. pneumoniae infection and lung cancer. The study concluded that C. pneumoniae infection is correlated with an elevated risk of lung cancer, implying that a higher serological titer may be an efficient predictor of lung cancer risk [50].
2.5. Salmonella typhi Infections and Gallbladder Cancer
Salmonella typhi is a gram-negative, rod-shaped, flagellated bacterium that is well documented as being responsible for typhoid infections [51][52][53]. After infection, S. typhi invades the gallbladder, causing persistent infection in susceptible carriers that serve as a reservoir for the spread of the disease; this persistent chronic infection has also been linked to cancer [52][54]. The ability of the pathogen to survive and persist for an extended time in the gallbladders of patients after infection creates a suitable environment for its tumor-promoting effect [54]. It has also been reported that S. typhi produces typhoid toxins and carcinogenic toxins such as nitroso-chemical compounds. These compounds play a crucial role in the progression of cancer [53].
Causes and incidence: Infection by S. typhi is usually acquired through the ingestion of food or water contaminated with the feces of a person carrying the organism [51]. Salmonella typhi is suspected to be responsible for approximately 22 million cases of typhoid fever, slightly more than 5 million cases of paratyphoid fever, and approximately 200,000 deaths worldwide each year [52].
Adaptation strategies for survival and the evasion of treatment: Through the secretion of biofilm, S. typhi appears to survive and become well adapted to thrive in the gallbladder. Biofilm formation (Figure 1) is a pathological adaptive response that allows the bacteria to aggregate, adhere to surfaces, and develop antimicrobial resistance through the utilization of a thick protective extracellular matrix [52][55].
2.6. Bacterial Infection and Cervical Cancer
Chlamydia trachomatis is a gram-negative bacterium and a prevalent cause of curable sexually-transmitted bacterial infections (STIs) worldwide [56]. The infection presents as urethritis in men and endocervicitis in women [57]. Persistent Chlamydia infections have also been identified as a risk factor for developing cervical carcinoma [57], especially in patients with the human papillomavirus (HPV) coinfection [56][58].
The association between C. trachomatis and cervical cancer is usually seen in the presence of HPV infections [56]. Since HPV infections have long been implicated in the etiology of cervical cancer [59], it is difficult to directly classify the bacterial infection as solely causative, even though it contributes significantly to an increased incidence.
3. Mechanism of Cancer Development after Bacterial Infection
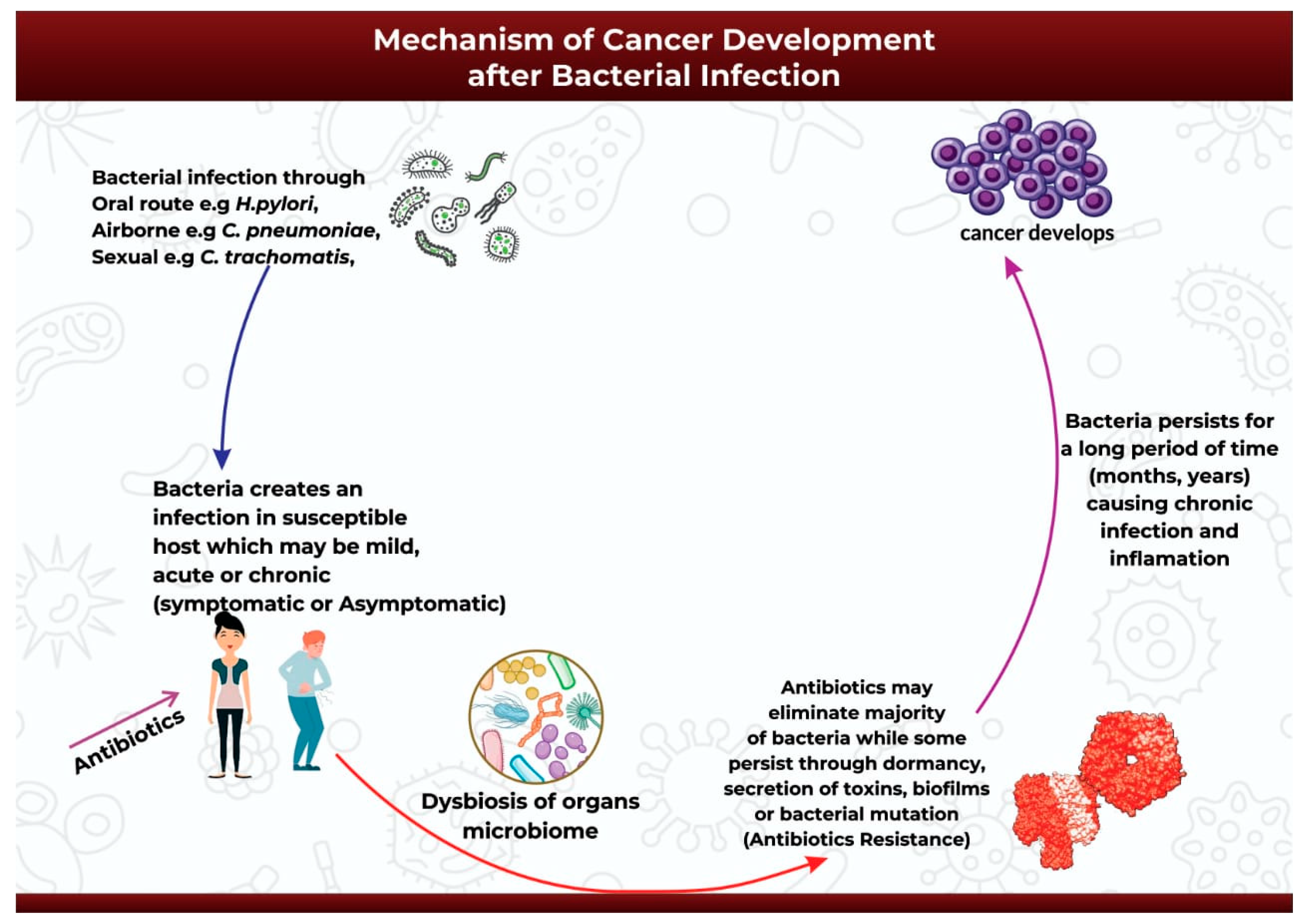
The onset of carcinogenesis after exposure to bacterial infection occurs over an extended period and depends on a wide range of factors such as host susceptibility, genetic background, and suitable environmental conditions [60].
After the successful infection of the host either through oral, airborne, or sexually-transmitted routes (Figure 2), the infection may start as mild, acute, or chronic and may be symptomatic or asymptomatic [61]. Antibiotics might be introduced at this point to help ease the infection [61]. The initial infection and subsequent antibiotic use may then initiate bacterial dysbiosis in the microbiome of the infected organ, in this case promoting the bloom of opportunistic and pathogenic bacteria species [62][63][64]. Antibiotic use may completely eradicate the infection in some cases, or it may be ineffective against the application of alternative survival strategies by the bacteria to facilitate its continued existence over extended periods of time (Figure 2). Pathogenic bacteria can survive and persist in the host environment through dormancy, secretion of cyotethal toxins, the formation of protective biofilms, or mutations to form antibiotic resistant strains [17][18]. This adaptive technique enables bacteria to promote persistent chronic inflammation or chronic infection, and ultimately carcinogenesis [17] (Figure 2).

Figure 2. An illustration of the mechanism of cancer development after bacterial infection.
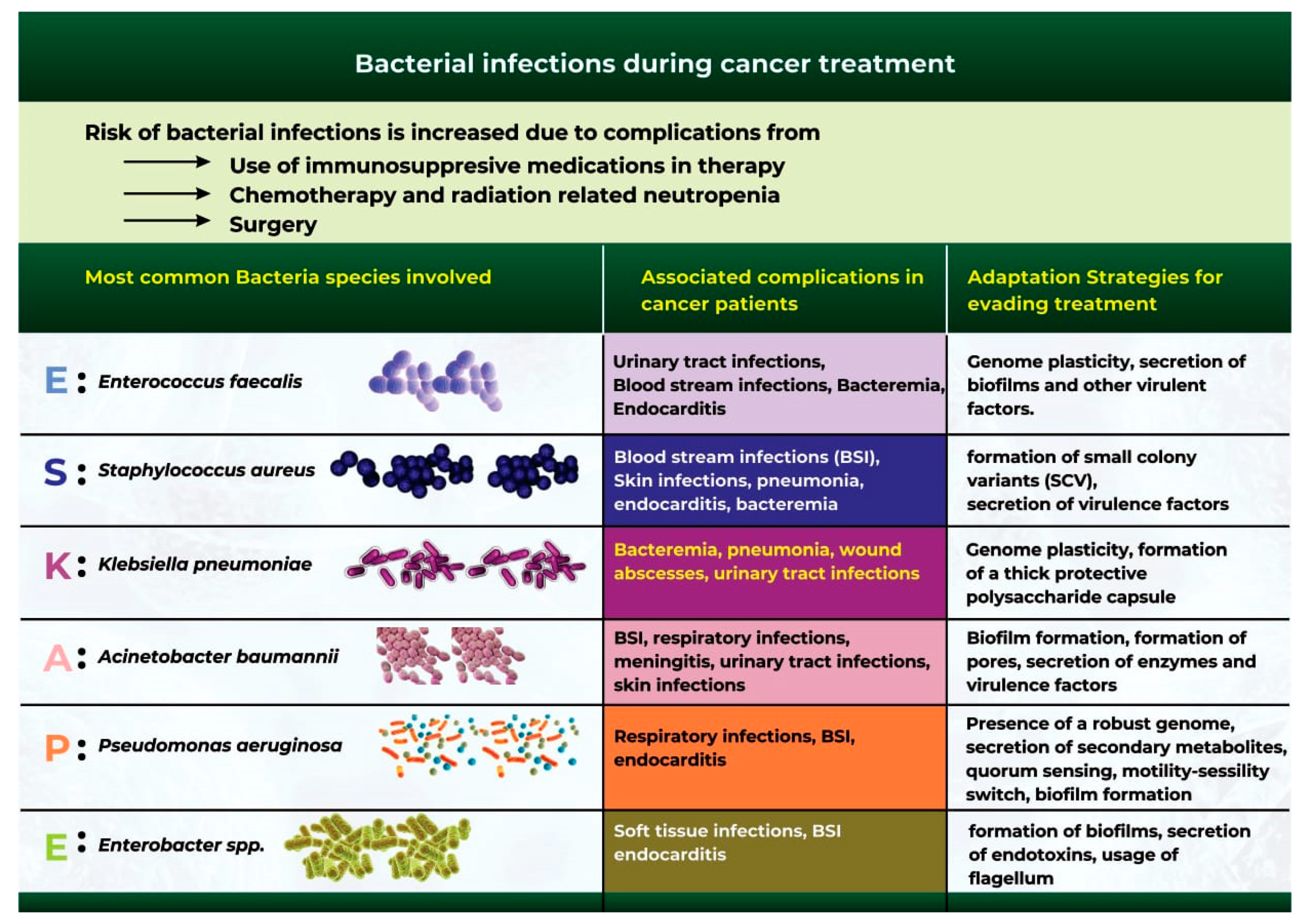
4. Bacterial Infections during Cancer Treatment
Bacterial infection is one of the most common complications during cancer treatment [65]. Although cancer mortality rates have continued to decline in recent years, bacterial infections remain a significant cause of infection-related mortality in patients [66]. Cancer patients are at a high risk of bacterial infection due to surgical complications, chemotherapy and radiotherapy-related neutropenia, and the use of immunosuppressive drugs during cancer treatment [66][67].
Extensive studies have identified a group of six bacterial microbes otherwise classified as the “ESKAPE” pathogens, which are at the forefront of bacterial infection and antibiotic resistance during cancer treatment (Figure 3) [68][69]. These organisms include Acinetobacter baumannii, Staphylococcus aureus, Enterococcus faecalis, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Enterobacter spp. [68][69].

Figure 3. The most common bacterial species that cause bacterial infections during cancer therapy.
5. Antibiotic Use and Antimicrobial Resistance in Cancer Patients
Antibiotics are secondary metabolites produced by microorganisms, higher animals, and plants, and they have antipathogenic effects that can interfere with the growth of other living cells [70]. Antibiotics are increasingly being used to treat cancers, often because of their pro-apoptotic, antiproliferative, and antimetastatic potentials [70]. Since infections are also common in cancer patients, it is essential to use antibiotics to prevent and treat bacterial infections [67].
A major limitation of antibiotic use is the causation of dysbiosis due to the elimination of beneficial bacterial groups, such as Lactobacillus and Bifidobacterium, in addition to the pathogenic bacteria [70]. A further limitation is the ability of pathogenic bacteria to evade killing and induce antibiotic resistance [68].
It is also worth noting that the microbiome plays a crucial role in the development of antibiotic resistance [71][72]. Microbiota dysbiosis induced by antibiotic treatment contributes to the development of resistance that is often due to increased numbers of opportunistic bacteria secreting high levels of antimicrobial resistance genes [73][74].
Antibiotic resistance in cancer patients often correlates with increased susceptibility to infections that eventually reduce the patient’s survival time; it also poses a significant threat to the accomplishments achieved in cancer treatment, and it highlights the importance of monitoring cancer patients and protecting them against antibiotic resistance [68].
References
- Hausman, D.M. What Is Cancer? Perspect. Biol. Med. 2019, 62, 778–784.
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33.
- Golemis, E.A.; Scheet, P.; Beck, T.N.; Scolnick, E.M.; Hunter, D.J.; Hawk, E.; Hopkins, N. Molecular mechanisms of the preventable causes of cancer in the United States. Genes Dev. 2018, 32, 868–902.
- GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 563–591.
- GBD 2019 Adolescent Young Adult Cancer Collaborators. The global burden of adolescent and young adult cancer in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Oncol. 2022, 23, 27–52.
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444.
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116.
- van Elsland, D.; Neefjes, J. Bacterial infections and cancer. EMBO Rep. 2018, 19, e46632.
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190.
- Dalton-Griffin, L.; Kellam, P. Infectious causes of cancer and their detection. J. Biol. 2009, 8, 67.
- Dekaboruah, E.; Suryavanshi, M.V.; Chettri, D.; Verma, A.K. Human microbiome: An academic update on human body site specific surveillance and its possible role. Arch. Microbiol. 2020, 202, 2147–2167.
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75.
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533.
- Curtis, M.M.; Sperandio, V. A complex relationship: The interaction among symbiotic microbes, invading pathogens, and their mammalian host. Mucosal Immunol. 2011, 4, 133–138.
- Conrad, R.; Vlassov, A.S. The Human Microbiota: Composition, Functions, and Therapeutic Potential. Med. Sci. Rev. 2015, 2, 92–103.
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The human microbiota in health and disease. Engineering 2017, 3, 71–82.
- Khatun, S.; Appidi, T.; Rengan, A.K. The role played by bacterial infections in the onset and metastasis of cancer. Curr. Res. Microb. Sci. 2021, 2, 100078.
- Laliani, G.; Sorboni, S.G.; Lari, R.; Yaghoubi, A.; Soleimanpour, S.; Khazaei, M.; Hasanian, S.M.; Avan, A. Bacteria and cancer: Different sides of the same coin. Life Sci. 2020, 246, 117398.
- Lax, A.J. Opinion: Bacterial toxins and cancer—A case to answer? Nat. Rev. Microbiol. 2005, 3, 343–349.
- Parsonnet, J. Bacterial infection as a cause of cancer. Environ. Health Perspect. 1995, 103 (Suppl. 8), 263–268.
- Song, S.; Vuai, M.S.; Zhong, M. The role of bacteria in cancer therapy—Enemies in the past, but allies at present. Infect. Agent Cancer 2018, 13, 9.
- Zella, D.; Gallo, R.C. Viruses and Bacteria Associated with Cancer: An Overview. Viruses 2021, 13, 1039.
- Asaka, M.; Sepulveda, A.R.; Sugiyama, T.; Graham, D.Y. Chapter 40: Gastric Cancer. In Helicobacter pylori: Physiology and Genetics; Mobley, H.L.T., Mendz, G.L., Hazell, S.L., Eds.; ASM Press: Washington, DC, USA, 2001.
- Khosravi, Y.; Dieye, Y.; Poh, B.H.; Ng, C.G.; Loke, M.F.; Goh, K.L.; Vadivelu, J. Culturable bacterial microbiota of the stomach of Helicobacter pylori positive and negative gastric disease patients. Sci. World J. 2014, 2014, 610421.
- Elsalem, L.; Jum’ah, A.A.; Alfaqih, M.A.; Aloudat, O. The Bacterial Microbiota of Gastrointestinal Cancers: Role in Cancer Pathogenesis and Therapeutic Perspectives. Clin. Exp. Gastroenterol. 2020, 13, 151–185.
- Maldonado-Contreras, A.; Goldfarb, K.C.; Godoy-Vitorino, F.; Karaoz, U.; Contreras, M.; Blaser, M.J.; Brodie, E.L.; Dominguez-Bello, M.G. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J. 2011, 5, 574–579.
- Elagan, S.K.; Almalki, S.J.; Alharthi, M.R.; Mohamed, M.S.; El-Badawy, M.F. Role of Bacteria in the Incidence of Common GIT Cancers: The Dialectical Role of Integrated Bacterial DNA in Human Carcinogenesis. Infect. Drug Resist. 2021, 14, 2003–2014.
- Vogiatzi, P.; Cassone, M.; Luzzi, I.; Lucchetti, C.; Otvos, L.; Giordano, A. Helicobacter pylori as a class I carcinogen: Physiopathology and management strategies. J. Cell. Biochem. 2007, 102, 264–273.
- Yamamura, K.; Baba, Y.; Nakagawa, S.; Mima, K.; Miyake, K.; Nakamura, K.; Sawayama, H.; Kinoshita, K.; Ishimoto, T.; Iwatsuki, M.; et al. Human Microbiome Fusobacterium Nucleatum in Esophageal Cancer Tissue Is Associated with Prognosis. Clin. Cancer Res. 2016, 22, 5574–5581.
- Whitmore, S.E.; Lamont, R.J. Oral bacteria and cancer. PLoS Pathog. 2014, 10, e1003933.
- Gao, S.; Liu, Y.; Duan, X.; Liu, K.; Mohammed, M.; Gu, Z.; Ren, J.; Yakoumatos, L.; Yuan, X.; Lu, L.; et al. Porphyromonas gingivalis infection exacerbates oesophageal cancer and promotes resistance to neoadjuvant chemotherapy. Br. J. Cancer 2021, 125, 433–444.
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80.
- Lovegrove, J.M. Dental plaque revisited: Bacteria associated with periodontal disease. J. N. Z. Soc. Periodontol. 2004, 87, 7–21.
- Ishida, N.; Ishihara, Y.; Ishida, K.; Tada, H.; Funaki-Kato, Y.; Hagiwara, M.; Ferdous, T.; Abdullah, M.; Mitani, A.; Michikawa, M.; et al. Periodontitis induced by bacterial infection exacerbates features of Alzheimer’s disease in transgenic mice. Npj Aging Mech. Dis. 2017, 3, 15.
- Sattar, S.B.A.; Singh, S. Bacterial Gastroenteritis; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Sun, J. Impact of bacterial infection and intestinal microbiome on colorectal cancer development. Chin. Med. J. 2022, 135, 400–408.
- Jiang, M.; Zhu, F.; Yang, C.; Deng, Y.; Kwan, P.S.L.; Li, Y.; Lin, Y.; Qiu, Y.; Shi, X.; Chen, H.; et al. Whole-Genome Analysis of Salmonella enterica Serovar Enteritidis Isolates in Outbreak Linked to Online Food Delivery, Shenzhen, China, 2018. Emerg. Infect. Dis. 2020, 26, 789–792.
- Zhang, Y.; Liu, K.; Zhang, Z.; Tian, S.; Liu, M.; Li, X.; Han, Y.; Zhu, K.; Liu, H.; Yang, C.; et al. A Severe Gastroenteritis Outbreak of Salmonella enterica Serovar Enteritidis Linked to Contaminated Egg Fried Rice, China, 2021. Front. Microbiol. 2021, 12, 779749.
- Hernández-Luna, M.A.; López-Briones, S.; Luria-Pérez, R. The Four Horsemen in Colon Cancer. J. Oncol. 2019, 2019, 5636272.
- Pleguezuelos-Manzano, C.; Puschhof, J.; Huber, A.R.; van Hoeck, A.; Wood, H.M.; Nomburg, J.; Gurjao, C.; Manders, F.; Dalmasso, G.; Stege, P.B.; et al. Mutational signature in colorectal cancer caused by genotoxic pks. Nature 2020, 580, 269–273.
- Wassenaar, T.M. E. coli and colorectal cancer: A complex relationship that deserves a critical mindset. Crit. Rev. Microbiol. 2018, 44, 619–632.
- Veziant, J.; Gagnière, J.; Jouberton, E.; Bonnin, V.; Sauvanet, P.; Pezet, D.; Barnich, N.; Miot-Noirault, E.; Bonnet, M. Association of colorectal cancer with pathogenic Escherichia coli: Focus on mechanisms using optical imaging. World J. Clin. Oncol. 2016, 7, 293–301.
- Sears, C.L.; Islam, S.; Saha, A.; Arjumand, M.; Alam, N.H.; Faruque, A.S.; Salam, M.A.; Shin, J.; Hecht, D.; Weintraub, A.; et al. Association of enterotoxigenic Bacteroides fragilis infection with inflammatory diarrhea. Clin. Infect. Dis. 2008, 47, 797–803.
- Elsaghir, H.; Reddivari, A.K.R. Bacteroides Fragilis; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725.
- Premachandra, N.M.; Jayaweera, J.A.A.S. Chlamydia pneumoniae infections and development of lung cancer: Systematic review. Infect. Agents Cancer 2022, 17, 11.
- Xu, X.; Liu, Z.; Xiong, W.; Qiu, M.; Kang, S.; Xu, Q.; Cai, L.; He, F. Combined and interaction effect of Chlamydia pneumoniae infection and smoking on lung cancer: A case-control study in Southeast China. BMC Cancer 2020, 20, 903.
- Laurila, A.L.; Anttila, T.; Läärä, E.; Bloigu, A.; Virtamo, J.; Albanes, D.; Leinonen, M.; Saikku, P. Serological evidence of an association between Chlamydia pneumoniae infection and lung cancer. Int. J. Cancer 1997, 74, 31–34.
- Littman, A.J.; Jackson, L.A.; Vaughan, T.L. Chlamydia pneumoniae and lung cancer: Epidemiologic evidence. Cancer Epidemiol. Biomark. Prev. 2005, 14, 773–778.
- Zhan, P.; Suo, L.J.; Qian, Q.; Shen, X.K.; Qiu, L.X.; Yu, L.K.; Song, Y. Chlamydia pneumoniae infection and lung cancer risk: A meta-analysis. Eur. J. Cancer 2011, 47, 742–747.
- Ashurst, J.V.; Truong, J.; Woodbury, B. Salmonella Typhi; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Di Domenico, E.G.; Cavallo, I.; Pontone, M.; Toma, L.; Ensoli, F. Biofilm Producing Salmonella typhi: Chronic Colonization and Development of Gallbladder Cancer. Int. J. Mol. Sci. 2017, 18, 1887.
- Upadhayay, A.; Pal, D.; Kumar, A. Salmonella typhi induced oncogenesis in gallbladder cancer: Co-relation and progression. Adv. Cancer Biol. Metastasis 2022, 4, 100032.
- Scanu, T.; Spaapen, R.M.; Bakker, J.M.; Pratap, C.B.; Wu, L.E.; Hofland, I.; Broeks, A.; Shukla, V.K.; Kumar, M.; Janssen, H.; et al. Salmonella Manipulation of Host Signaling Pathways Provokes Cellular Transformation Associated with Gallbladder Carcinoma. Cell Host Microbe 2015, 17, 763–774.
- Gunn, J.S.; Marshall, J.M.; Baker, S.; Dongol, S.; Charles, R.C.; Ryan, E.T. Salmonella chronic carriage: Epidemiology, diagnosis, and gallbladder persistence. Trends Microbiol. 2014, 22, 648–655.
- Franchini, A.P.A.; Iskander, B.; Anwer, F.; Oliveri, F.; Fotios, K.; Panday, P.; Hamid, P. The Role of Chlamydia trachomatis in the Pathogenesis of Cervical Cancer. Cureus 2022, 14, e21331.
- Malhotra, M.; Sood, S.; Mukherjee, A.; Muralidhar, S.; Bala, M. Genital Chlamydia trachomatis: An update. Indian J. Med. Res. 2013, 138, 303–316.
- Yang, X.; Siddique, A.; Khan, A.A.; Wang, Q.; Malik, A.; Jan, A.T.; Rudayni, H.A.; Chaudhary, A.A.; Khan, S. Infection: Their potential implication in the Etiology of Cervical Cancer. J. Cancer 2021, 12, 4891–4900.
- Malik, S.; Sah, R.; Muhammad, K.; Waheed, Y. Tracking HPV Infection, Associated Cancer Development, and Recent Treatment Efforts—A Comprehensive Review. Vaccines 2023, 11, 102.
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA Cancer J. Clin. 2017, 67, 326–344.
- Grant, S.S.; Hung, D.T. Persistent bacterial infections, antibiotic tolerance, and the oxidative stress response. Virulence 2013, 4, 273–283.
- Pham, T.A.; Lawley, T.D. Emerging insights on intestinal dysbiosis during bacterial infections. Curr. Opin. Microbiol. 2014, 17, 67–74.
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191.
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39.
- Rolston, K.V. Infections in Cancer Patients with Solid Tumors: A Review. Infect. Dis. Ther. 2017, 6, 69–83.
- Bhat, S.; Muthunatarajan, S.; Mulki, S.S.; Bhat, K.A.; Kotian, K.H. Bacterial Infection among Cancer Patients: Analysis of Isolates and Antibiotic Sensitivity Pattern. Int. J. Microbiol. 2021, 2021, 8883700.
- Perez, K.K.; Olsen, R.J.; Musick, W.L.; Cernoch, P.L.; Davis, J.R.; Peterson, L.E.; Musser, J.M. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J. Infect. 2014, 69, 216–225.
- Nanayakkara, A.K.; Boucher, H.W.; Fowler, V.G.; Jezek, A.; Outterson, K.; Greenberg, D.E. Antibiotic resistance in the patient with cancer: Escalating challenges and paths forward. CA Cancer J. Clin. 2021, 71, 488–504.
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081.
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.G.; Chen, T. Antibiotics for cancer treatment: A double-edged sword. J. Cancer 2020, 11, 5135–5149.
- Pandey, K.; Umar, S. Microbiome in drug resistance to colon cancer. Curr. Opin. Physiol. 2021, 23, 100472.
- Casals-Pascual, C.; Vergara, A.; Vila, J. Intestinal microbiota and antibiotic resistance: Perspectives and solutions. Hum. Microbiome J. 2018, 9, 11–15.
- Baron, S.A.; Diene, S.M.; Rolain, J.-M. Human microbiomes and antibiotic resistance. Hum. Microbiome J. 2018, 10, 43–52.
- Gargiullo, L.; Del Chierico, F.; D’Argenio, P.; Putignani, L. Gut Microbiota Modulation for Multidrug-Resistant Organism Decolonization: Present and Future Perspectives. Front. Microbiol. 2019, 10, 1704.
More
Information
Subjects:
Allergy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
615
Revisions:
2 times
(View History)
Update Date:
26 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




