Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, Z.; Guan, R.; Li, J.; Sun, Y. The Design Principle of SERS Nanotags. Encyclopedia. Available online: https://encyclopedia.pub/entry/43426 (accessed on 08 February 2026).
Zhang Z, Guan R, Li J, Sun Y. The Design Principle of SERS Nanotags. Encyclopedia. Available at: https://encyclopedia.pub/entry/43426. Accessed February 08, 2026.
Zhang, Zhipeng, Rui Guan, Junrong Li, Yao Sun. "The Design Principle of SERS Nanotags" Encyclopedia, https://encyclopedia.pub/entry/43426 (accessed February 08, 2026).
Zhang, Z., Guan, R., Li, J., & Sun, Y. (2023, April 25). The Design Principle of SERS Nanotags. In Encyclopedia. https://encyclopedia.pub/entry/43426
Zhang, Zhipeng, et al. "The Design Principle of SERS Nanotags." Encyclopedia. Web. 25 April, 2023.
Copy Citation
Surface-enhanced Raman scattering (SERS) is an emerging spectroscopic technology. By integrating with nanotechnology (e.g., noble metal nanoparticles), SERS allows 106–1015 Raman signal amplification and thus sensitive sensing down to single molecules. In addition, SERS possesses extremely narrow Raman spectral line widths (i.e., ~1 nm), which are about 50 times narrower than the commonly used fluorescence bands.
SERS
Raman
nanotages
cancer diagnosis
1. Introduction
As the leading cause of death worldwide, cancer is responsible for nearly 10 million deaths in 2020 [1]. Fortunately, emerging findings suggested that precision medicine can significantly reduce cancer mortality through introducing timely and effective medical interference [2][3][4][5]. To assist precision medicine, biomarkers circulating in body fluids (e.g., blood or urine) are able to noninvasively provide a complete cancer profile to enable early detection and guide personalized treatment management [6][7][8]. Currently, a variety of cancer circulating biomarkers has been investigated as surrogates to indicate cancer occurrence, progression, and treatment response, including proteins, circulating tumor cells (CTCs), nucleic acids (NAs), and extracellular vehicles (EVs) [9][10][11][12].
Despite the significant role of circulating biomarkers in cancer detection, their practical use for precision medicine is largely challenged by two issues: (1) the extremely low abundance of cancer-associated circulating biomarkers in the presence of large amounts of interference molecules. For instance, only 1–100 CTCs are found in one milliliter of blood with 1–2 million peripheral blood mononuclear cells, in which CTCs may experience further loss during the isolation and purification procedures [13][14], and (2) the inaccurate reflection of cancer status by considering only one relevant biomarker. Accumulating evidence shows that the mere use of prostate-specific antigen (PSA) for prostate cancer screening may not produce an improved survival benefit but comes with overtreatments and life-alerting side effects [15][16]. As such, new technologies that enable highly sensitive, specific, and parallel analysis of multiple circulating biomarkers are expected to assist accurate decision-making [2][17].
Surface-enhanced Raman scattering (SERS) is an emerging spectroscopic technology that has witnessed rapid developments in the past decade [18]. By integrating with nanotechnology (e.g., noble metal nanoparticles), SERS allows 106–1015 Raman signal amplification and thus sensitive sensing down to single molecules [19]. In addition, SERS possesses extremely narrow Raman spectral line widths (i.e., ~1 nm), which are about 50 times narrower than the commonly used fluorescence bands [19]. The intrinsic narrow Raman peaks particularly benefit multiplexed labeling with the potential to analyze 31 targets in parallel [20]. Taken together, with the advantages of high sensitivity and multiplexing capability, SERS is a good candidate to implement circulating biomarker detection for early and accurate cancer detection.
2. The Design Principle of SERS Nanotags
Figure 1 depicts the electromagnetic field-related SERS principle, which involves the use of surface plasmonic resonance (SPR) surrounding nanostructure surfaces to enhance the Raman signals of Raman reporters. To allow effective biomarker detection, SERS nanotags are expected to identify the targets as well as readout specific signals. Typically, the design of SERS nanotags should consider four key components, as illustrated in Figure 2a: (1) plasmonic nanostructure, (2) Raman reporter, (3) protective shell, and (4) targeting unit. The integration of these four parts together constitutes the functional SERS nanotags. Table 1 summarizes the roles of each component, typical examples, and working principles.
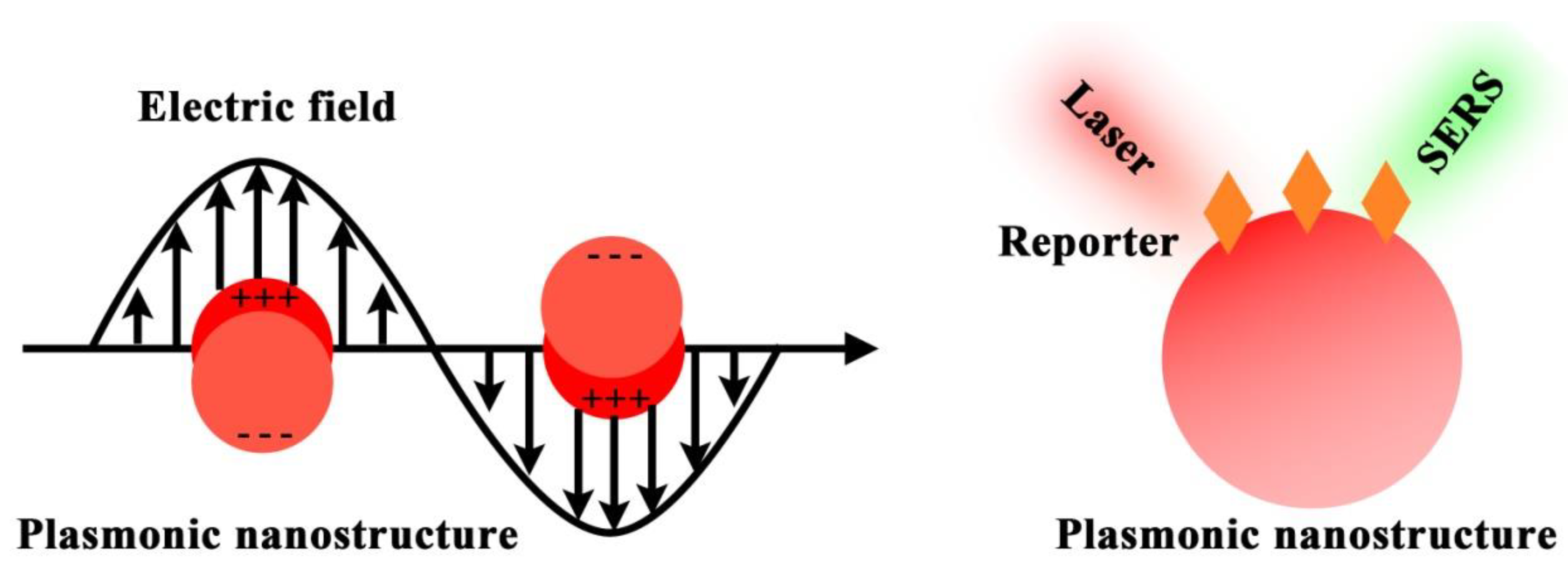
Figure 1. SERS principle of using plasmonic nanostructure to enhance the Raman reporter signals.
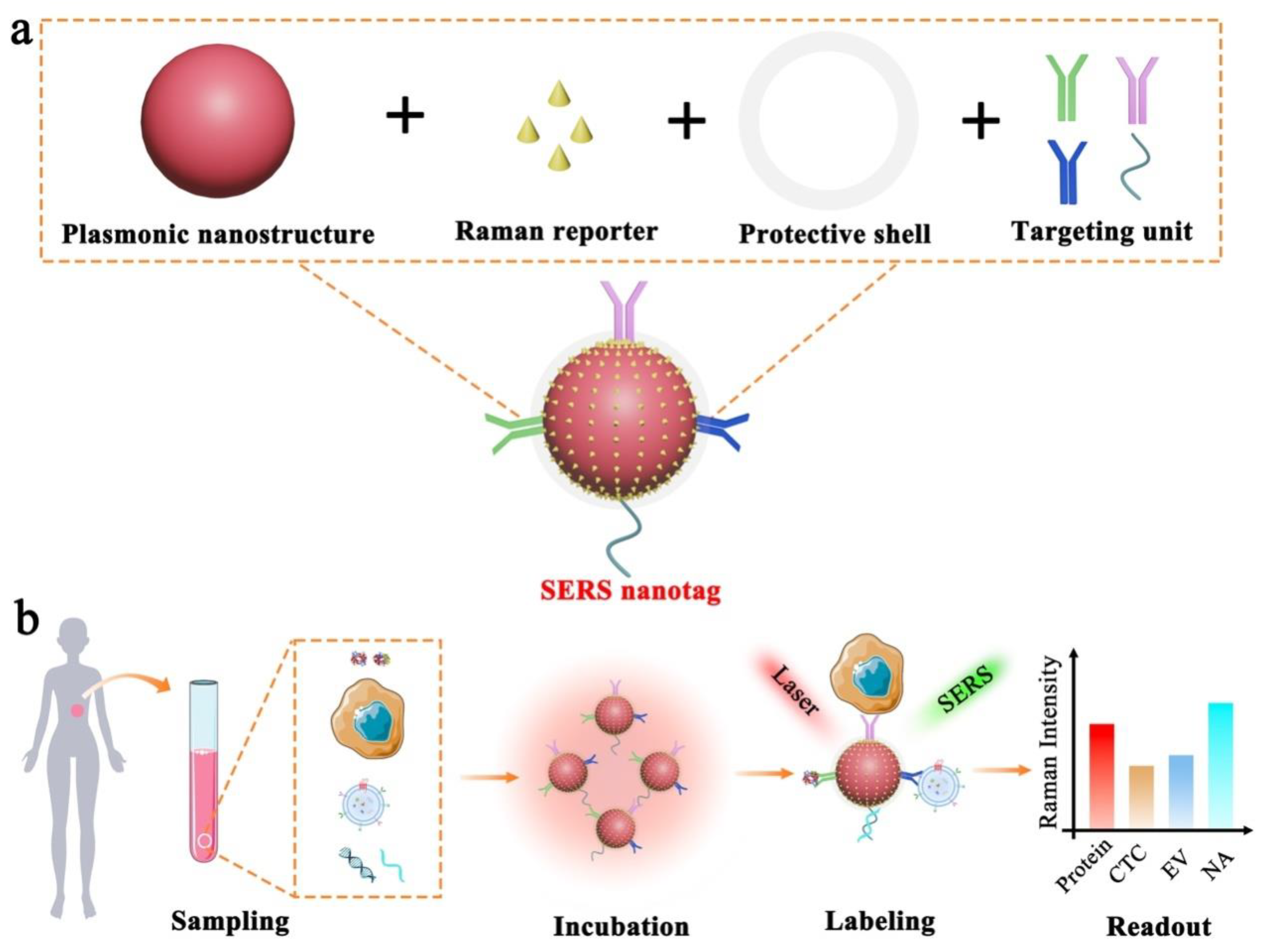
Figure 2. Schematic illustration of SERS nanotag enabled highly sensitive and multiplexed detection of cancer circulating biomarkers. (a) The design of a typical SERS nanotag with the use of four key components. (b) The application of SERS nanotags in the detection of protein, CTC, EV, and NA.
Table 1. Typical components of functional SERS nanotags.
| Component | Role | Example | Working Principle |
|---|---|---|---|
| Plasmonic nanostructure | Enhancing Raman signals |
|
Localized surface plasmon resonance (LSPR) |
| Raman reporter | Generating Raman signals |
|
Intrinsic molecular vibration and rotation |
| Protective shell |
|
|
|
| Targeting unit | Providing required specificity |
|
Specific molecular interactions |
As the foundation of SERS nanotags, the plasmonic nanostructure plays a paramount role in amplifying the weak Raman signals, which underpins the feasibility of single-molecule detection. Briefly, the plasmonic nanostructure utilizes the localized surface plasma resonance (LSPR) to enhance the surrounding electromagnetic field and thus enlarge the molecular vibrational and rotational information [21][22]. Due to the electromagnetic field damping with the distance away from the nanostructures, this LSPR-based Raman enhancement shows the distance-dependent feature with effective signal enhancement limited to around 10 nm near the nanostructure surfaces [23]. Typically, plasmonic nanostructure consists of noble metals (e.g., gold, silver, and copper) that show strong LSPR properties. Importantly, plasmonic nanostructure with different morphology shows variable electromagnetic field distributions and thus quite diverse LSPR-related Raman signal enhancement [24][25][26]. For instance, plasmonic nanostructure that is isotropic (e.g., nanospheres) or anisotropic (e.g., nanostars and nanoflowers) performs differently in enhancing Raman signals. Based on these different morphology, scientists could design promising Raman probes for in vitro and vivo imaging.
Raman reporter provides the intrinsic fingerprint molecular information, which can be used as characteristic signals to indicate targets. As Raman peaks are extremely narrow, the use of different Raman reporters with non-overlapping characteristic bands is capable of labeling multiple targets in parallel. Typically, the thiolated small molecules (e.g., 4-mercaptobenzoic acid, 2,7-mercapto-4-methylcoumarin, 4-mercaptopyridine, and 2-mercapto-4-methyl-5-thiazoleacetic acid) are preferred Raman reporters in multi-biomarker analysis due to their easy functionalization on nanostructures through sulfur and gold/silver interaction and few characteristic peaks to minimize Raman peak overlaps [19]. However, this type of molecules suffers from a relatively low Raman signal enhancement, which is largely related to their small Raman-scattering cross-section. By contrast, the dye-based Raman reporters (e.g., crystal violet and rhodamine B) feature high Raman enhancement but have the limitation of serious Raman peak overlap due to the complex molecular structures with rich vibration and rotation [19]. Thus, these dyes as Raman reporters are recommended to conduct the highly sensitive detection of an individual biomarker instead of the simultaneous analysis of multi-biomarkers. In addition, the emerging triple bond-modulated molecules are attracting an increasing attention as the next generation of Raman reporters for multiplexed biomarker detection. These triple bond-based molecules show unique and simple Raman signals beyond 1800 cm−1, which locate in the Raman silent region without potential interferences from biological samples [27].
A protective shell is used to prevent the dissociation of Raman reporters on nanostructure surfaces, provide sufficient colloidal stability to the nanostructures, and block the exposed free nanostructure surfaces to avoid nonspecific binding events. The representative protective materials include bovine serum albumin (BSA), SH-PEG, SiO2, and liposomes [28][29][30][31].
The targeting unit (e.g., antibodies, aptamers, peptides, small ligands, and nucleic acids) imparts the specificity to SERS nanotags for recognizing desired targets. The incorporation of targeting units on SERS nanotags can be performed through a simple physical adsorption using electrostatic interactions or covalent binding with the assistance of bifunctional linker molecules (e.g., ortho-pyridyldisulfide-polyethylene glycol-N-succinimidyl propionate, dithiobis(succinimidyl propionate) and succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate) [19].
To perform SERS measurements, the targets are typically isolated/purified from the samples first and identified through SERS nanotags for signal readout. Experimentally, under the laser illumination with specific wavelength (e.g., 632.8 nm and 785 nm), the generated Raman spectra are recorded with either a portable Raman instrument in a cuvette or confocal Raman microscope using user-defined integration time [32][33].
References
- World Health Organization (WHO). Cancer. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 3 February 2022).
- Borrebaeck, C.A.K. Precision siagnostics: Moving towards protein biomarker signatures of clinical utility in cancer. Nat. Rev. Cancer 2017, 17, 199.
- Koo, K.M.; Wee, E.J.H.; Mainwaring, P.N.; Wang, Y.; Trau, M. Toward precision medicine: A cancer molecular subtyping nano-strategy for RNA biomarkers in tumor and urine. Small 2016, 12, 6233–6242.
- Xu, Y.; Li, C.; Ma, X.; Tuo, W.; Tu, L.; Li, X.; Sun, Y.; Stang, P.J.; Sun, Y. Long wavelength-emissive Ru(II) metallacycle-based photosensitizer assisting in vivo bacterial diagnosis and antibacterial treatment. Proc. Natl. Acad. Sci. 2022, 119, e2209904119.
- Shi, G.; Zhong, M.; Ye, F.; Zhang, X. Low-frequency HIFU induced cancer immunotherapy: Tempting challenges and potential opportunities. Cancer Bio. Med. 2019, 16, 714.
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472.
- Li, J.; Wuethrich, A.; Dey, S.; Lane, R.E.; Sina, A.A.I.; Wang, J.; Wang, Y.; Puttick, S.; Koo, K.M.; Trau, M. The growing impact of micro/nanomaterial-based systems in precision oncology: Translating “multiomics” technologies. Adv. Funct. Mater. 2020, 30, 1909306.
- Wang, T.; Li, J.; Yu, G.; Yu, K. Effects of siRNA interference and over-expression of HMGA2 on proliferation and apoptosis of colorectal cancer cells. Int. J. Clin. Exp. Pathol. 2017, 10, 4611.
- Li, W.; Wang, H.; Zhao, Z.; Gao, H.; Liu, C.; Zhu, L.; Wang, C.; Yang, Y. Emerging nanotechnologies for liquid biopsy: The detection of circulating tumor cells and extracellular vesicles. Adv. Mater. 2018, 31, 1805344.
- Duan, R.; Zhang, Z.; Zheng, F.; Wang, L.; Guo, J.; Zhang, T.; Dai, X.; Zhang, S.; Yang, D.; Kuang, R.; et al. Combining protein and miRNA quantification for bladder cancer analysis. ACS Appl. Mater. Interfaces 2017, 9, 23420–23427.
- Liu, C.; Xu, X.; Li, B.; Situ, B.; Pan, W.; Hu, Y.; An, T.; Yao, S.; Zheng, L. Single-exosome-counting immunoassays for cancer diagnostics. Nano Lett. 2018, 18, 4226–4232.
- Ning, Z.; Gan, J.; Chen, C.; Zhang, D.; Zhang, H. Molecular functions and significance of the MTA family in hormone-independent cancer. Cancer Metast. Rev. 2014, 33, 901.
- Li, Y.Q.; Chandran, B.K.; Lim, C.T.; Chen, X. Rational design of materials interface for efficient capture of circulating tumor cells. Adv. Sci. 2015, 2, 1500118.
- Li, J.; Wang, J.; Wang, Y.; Trau, M. Simple and rapid colorimetric detection of melanoma circulating tumor cells using bifunctional magnetic nanoparticles. Analyst 2017, 142, 4788–4793.
- Bostwick, D.G. Prostate-specific antigen. Current role in diagnostic pathology of prostate cancer. Am. J. Clin. Pathol. 1994, 102, S31–S37.
- Ankerst, D.P.; Thompson, I.M. Sensitivity and specificity of prostate-specific antigen for prostate cancer detection with high rates of biopsy verification. Arch. Ital. Urol. Androl. 2006, 78, 125–129.
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171.
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117.
- Wang, Y.; Yan, B.; Chen, L. SERS tags: Novel optical nanoprobes for bioanalysis. Chem. Rev. 2013, 113, 1391–1428.
- Mir-Simon, B.; Reche-Perez, I.; Guerrini, L.; Pazos-Perez, N.; Alvarez-Puebla, R.A. Universal one-pot and scalable synthesis of SERS encoded nanoparticles. Chem. Mater. 2015, 27, 950–958.
- Tan, T.; Tian, C.; Ren, Z.; Yang, J.; Chen, Y.; Sun, L.; Li, Z.; Wu, A.; Yin, J.; Fu, H. LSPR-dependent SERS performance of silver nanoplates with highly stable and broad tunable LSPR prepared through an improved seed-mediated strategy. Phys. Chem. Chem. Phys. 2013, 15, 21034–21042.
- Im, H.; Bantz, K.C.; Lee, S.H.; Johnson, T.W.; Haynes, C.L.; Oh, S.-H. Self-assembled plasmonic nanoring cavity arrays for SERS and LSPR biosensing. Adv. Mater. 2013, 25, 2678–2685.
- Li, J.; Koo, K.M.; Wang, Y.; Trau, M. Native microRNA targets trigger self-assembly of nanozyme-patterned hollowed nanocuboids with optimal interparticle gaps for plasmonic-activated cancer detection. Small 2019, 15, 1904689.
- Jeon, T.Y.; Park, S.-G.; Lee, S.Y.; Jeon, H.C.; Yang, S.-M. Shape control of Ag nanostructures for practical sers substrates. ACS Appl. Mater. Interfaces 2013, 5, 243–248.
- Benz, F.; Chikkaraddy, R.; Salmon, A.; Ohadi, H.; de Nijs, B.; Mertens, J.; Carnegie, C.; Bowman, R.W.; Baumberg, J.J. SERS of individual nanoparticles on a mirror: Size does matter, but so does shape. J. Phys. Chem. Lett. 2016, 7, 2264–2269.
- Shen, X.S.; Wang, G.Z.; Hong, X.; Zhu, W. Nanospheres of silver nanoparticles: Agglomeration, surface morphology control and application as SERS substrates. Phys. Chem. Chem. Phys. 2009, 11, 7450–7454.
- Wei, L.; Chen, Z.; Shi, L.; Long, R.; Anzalone, A.V.; Zhang, L.; Hu, F.; Yuste, R.; Cornish, V.W.; Min, W. Super-multiplex vibrational imaging. Nature 2017, 544, 465–470.
- Zheng, X.-S.; Hu, P.; Cui, Y.; Zong, C.; Feng, J.-M.; Wang, X.; Ren, B. Bsa-coated nanoparticles for improved SERS-based intracellular ph sensing. Anal. Chem. 2014, 86, 12250–12257.
- Indrasekara, A.S.D.S.; Paladini, B.J.; Naczynski, D.J.; Starovoytov, V.; Moghe, P.V.; Fabris, L. Dimeric gold nanoparticle assemblies as tags for SERS-based cancer detection. Adv. Healthc. Mater. 2013, 2, 1370–1376.
- Fales, A.M.; Yuan, H.; Vo-Dinh, T. Silica-coated gold nanostars for combined surface-enhanced Raman scattering (SERS) detection and singlet-oxygen generation: A potential nanoplatform for theranostics. Langmuir 2011, 27, 12186–12190.
- Farahavar, G.; Abolmaali, S.S.; Nejatollahi, F.; Safaie, A.; Javanmardi, S.; Khajeh Zadeh, H.; Yousefi, R.; Nadgaran, H.; Mohammadi-Samani, S.; Tamaddon, A.M.; et al. Single-chain antibody-decorated Au layer nanoprobes for targeted SERS imaging and remote-controlled photothermal therapy of melanoma cancer cells. Mater. Sci. Eng. C 2021, 124, 112086.
- Kumar, A.R.; Shanmugasundaram, K.B.; Li, J.; Zhang, Z.; Ibn Sina, A.A.; Wuethrich, A.; Trau, M. Ultrasensitive melanoma biomarker detection using a microchip sers immunoassay with anisotropic au–ag alloy nanoboxes. RSC Adv. 2020, 10, 28778–28785.
- Farokhinejad, F.; Li, J.; Hugo, L.E.; Howard, C.B.; Wuethrich, A.; Trau, M. Detection of dengue virus 2 with single infected mosquito resolution using yeast affinity bionanofragments and plasmonic sers nanoboxes. Anal. Chem. 2022, 94, 14177–14184.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
956
Revisions:
2 times
(View History)
Update Date:
25 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




