Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yao Sun and Version 2 by Camila Xu.
Surface-enhanced Raman scattering (SERS) is an emerging spectroscopic technology. By integrating with nanotechnology (e.g., noble metal nanoparticles), SERS allows 106–1015 Raman signal amplification and thus sensitive sensing down to single molecules. In addition, SERS possesses extremely narrow Raman spectral line widths (i.e., ~1 nm), which are about 50 times narrower than the commonly used fluorescence bands.
- SERS
- Raman
- nanotages
- cancer diagnosis
1. Introduction
As the leading cause of death worldwide, cancer is responsible for nearly 10 million deaths in 2020 [1]. Fortunately, emerging findings suggested that precision medicine can significantly reduce cancer mortality through introducing timely and effective medical interference [2][3][4][5][2,3,4,5]. To assist precision medicine, biomarkers circulating in body fluids (e.g., blood or urine) are able to noninvasively provide a complete cancer profile to enable early detection and guide personalized treatment management [6][7][8][6,7,8]. Currently, a variety of cancer circulating biomarkers has been investigated as surrogates to indicate cancer occurrence, progression, and treatment response, including proteins, circulating tumor cells (CTCs), nucleic acids (NAs), and extracellular vehicles (EVs) [9][10][11][12][9,10,11,12].
Despite the significant role of circulating biomarkers in cancer detection, their practical use for precision medicine is largely challenged by two issues: (1) the extremely low abundance of cancer-associated circulating biomarkers in the presence of large amounts of interference molecules. For instance, only 1–100 CTCs are found in one milliliter of blood with 1–2 million peripheral blood mononuclear cells, in which CTCs may experience further loss during the isolation and purification procedures [13][14][13,14], and (2) the inaccurate reflection of cancer status by considering only one relevant biomarker. Accumulating evidence shows that the mere use of prostate-specific antigen (PSA) for prostate cancer screening may not produce an improved survival benefit but comes with overtreatments and life-alerting side effects [15][16][15,16]. As such, new technologies that enable highly sensitive, specific, and parallel analysis of multiple circulating biomarkers are expected to assist accurate decision-making [2][17][2,17].
Surface-enhanced Raman scattering (SERS) is an emerging spectroscopic technology that has witnessed rapid developments in the past decade [18]. By integrating with nanotechnology (e.g., noble metal nanoparticles), SERS allows 106–1015 Raman signal amplification and thus sensitive sensing down to single molecules [19]. In addition, SERS possesses extremely narrow Raman spectral line widths (i.e., ~1 nm), which are about 50 times narrower than the commonly used fluorescence bands [19]. The intrinsic narrow Raman peaks particularly benefit multiplexed labeling with the potential to analyze 31 targets in parallel [20]. Taken together, with the advantages of high sensitivity and multiplexing capability, SERS is a good candidate to implement circulating biomarker detection for early and accurate cancer detection.
2. The Design Principle of SERS Nanotags
Figure 12 depicts the electromagnetic field-related SERS principle, which involves the use of surface plasmonic resonance (SPR) surrounding nanostructure surfaces to enhance the Raman signals of Raman reporters. To allow effective biomarker detection, SERS nanotags are expected to identify the targets as well as readout specific signals. Typically, the design of SERS nanotags should consider four key components, as illustrated in Figure 21a: (1) plasmonic nanostructure, (2) Raman reporter, (3) protective shell, and (4) targeting unit. The integration of these four parts together constitutes the functional SERS nanotags. Table 1 summarizes the roles of each component, typical examples, and working principles.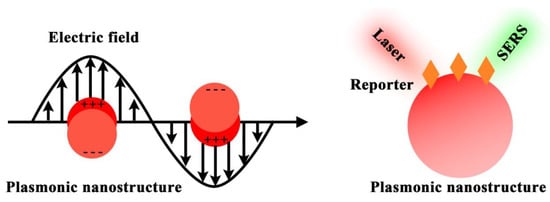
Figure 1. SERS principle of using plasmonic nanostructure to enhance the Raman reporter signals.
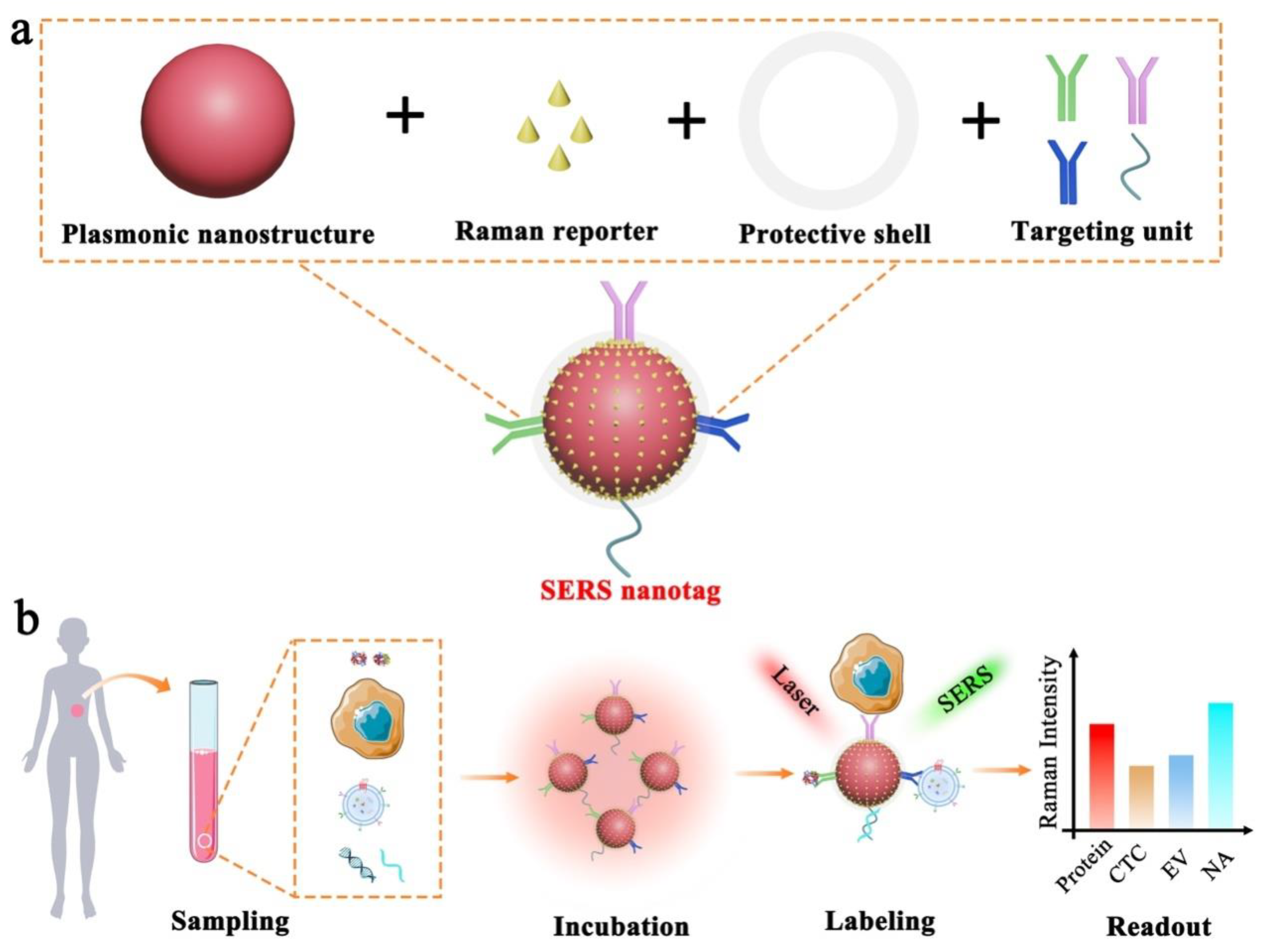
Figure 2. Schematic illustration of SERS nanotag enabled highly sensitive and multiplexed detection of cancer circulating biomarkers. (a) The desigprinciple of using plasmon of a typical SERS nanotag with the use of four key components. (b) T nanostructure to enhance the application of SERS nanotags in the detection of protein, CTC, EV, and NAaman reporter signals.
Table 1.
Typical components of functional SERS nanotags.
| Component | Role | Example | Working Principle |
|---|---|---|---|
| Plasmonic nanostructure | Enhancing Raman signals |
|
Localized surface plasmon resonance (LSPR) |
| Raman reporter | Generating Raman signals |
|
Intrinsic molecular vibration and rotation |
| Protective shell |
|
|
|
| Targeting unit | Providing required specificity |
|
Specific molecular interactions |
